Rapid Differentiation of Unfrozen and Frozen-Thawed Tuna with Non-Destructive Methods and Classification Models: Bioelectrical Impedance Analysis (BIA), Near-Infrared Spectroscopy (NIR) and Time Domain Reflectometry (TDR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Tuna Processing
2.1.2. Freezing and Thawing Process
2.1.3. Additives and Reagents
2.2. Destructive Analysis: Physicochemical Characterization
2.3. Data Acquisition
2.3.1. Bioelectrical Impedance Analysis (BIA)
2.3.2. Near-Infrared Spectroscopy (NIR)
2.3.3. Time Domain Reflectometry (TDR)
2.4. Data Analysis
2.4.1. Data Cleaning, Data Preprocessing and Principal Component Analysis (PCA)
2.4.2. Comparison between Unfrozen and Frozen-Thawed Samples
2.4.3. Creation and Descriptive Statistics of Calibration and Validation Datasets
2.4.4. Classification Model Building
3. Results
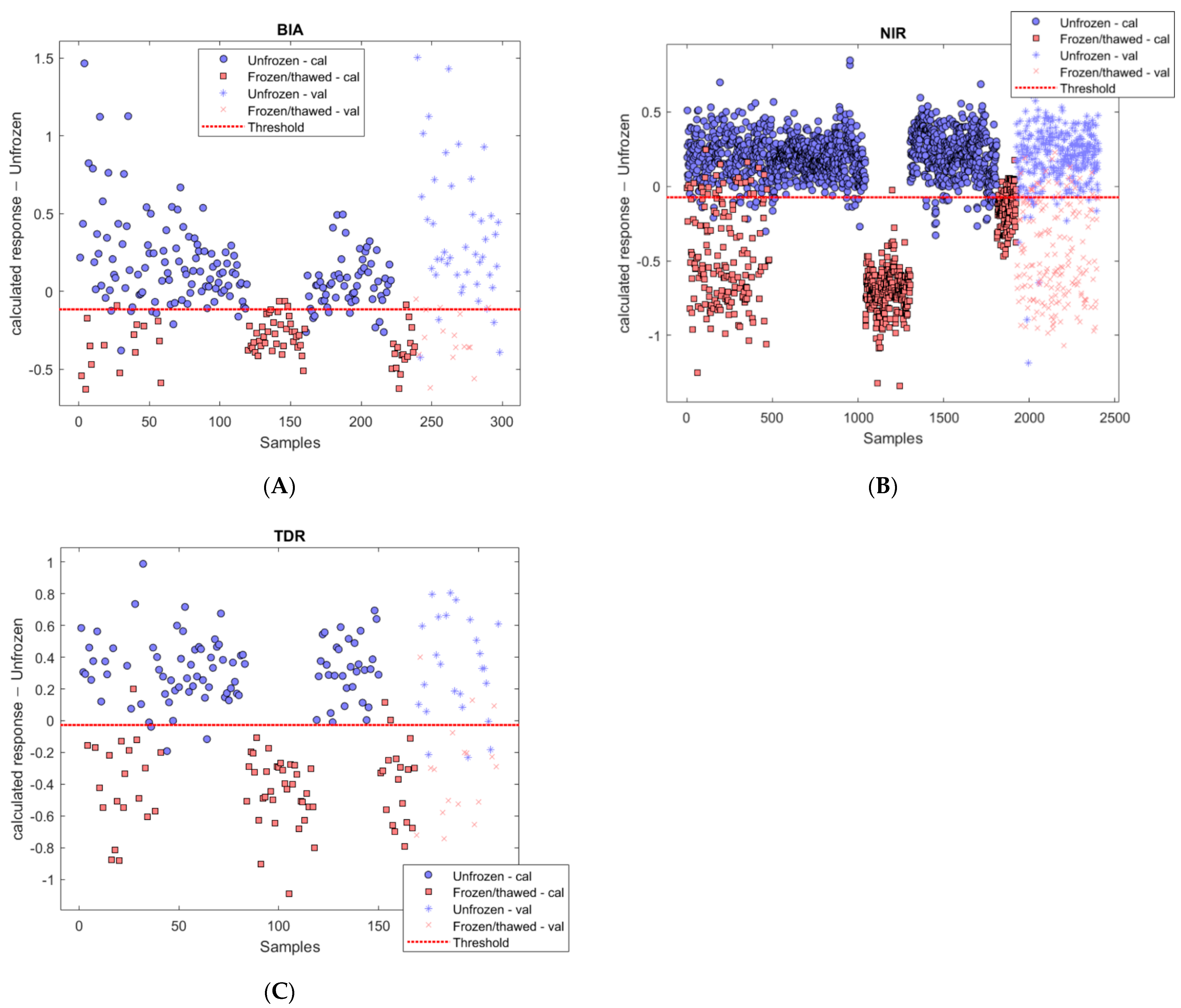
3.1. Differences between Unfrozen and Frozen-Thawed Samples
3.2. Descriptive Statistics of Calibration and Validation Samples
3.3. Models Building
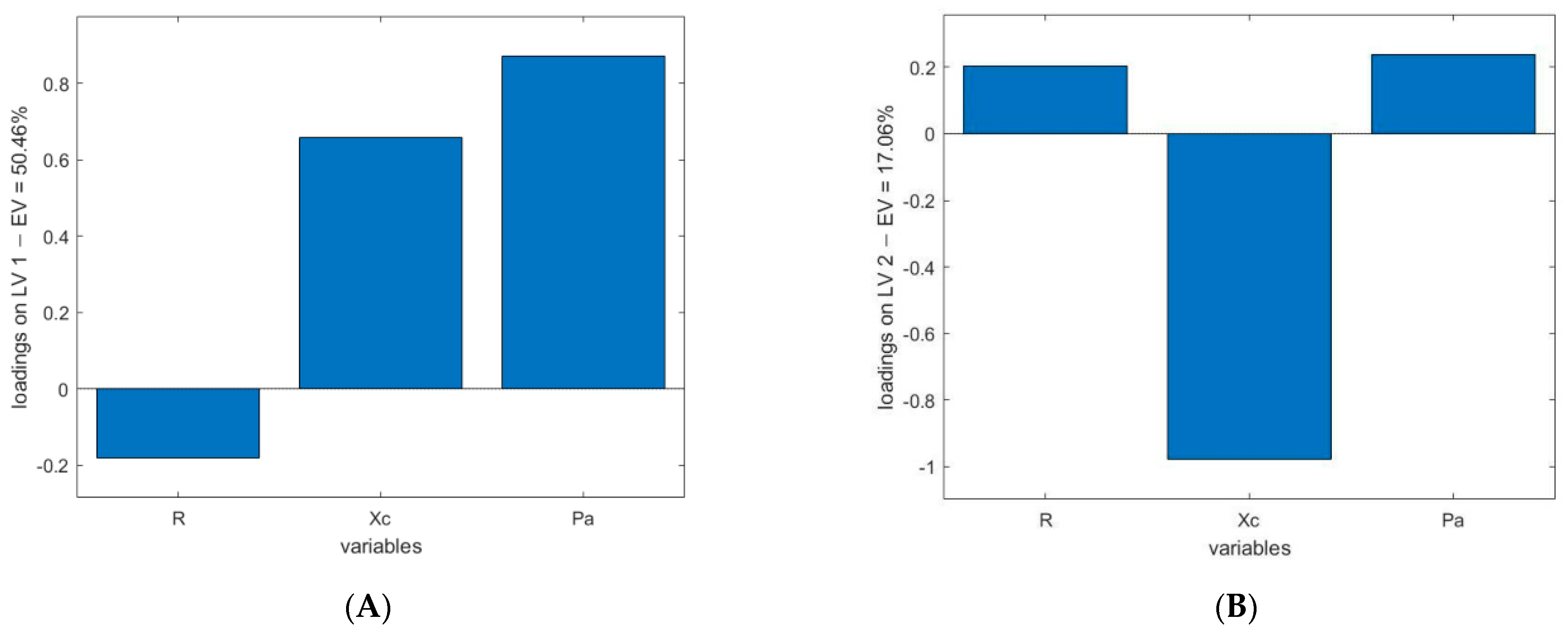
- BIA (Figure 2a,b). Loadings of the first two LVs explain the higher amount of variance (50.46% for latent variable 1 (LV1) and 17.06% for latent variable 2 (LV2)). The loadings of these two LVs reveal that two variables have the higher influence in the model: Pa and Xc.
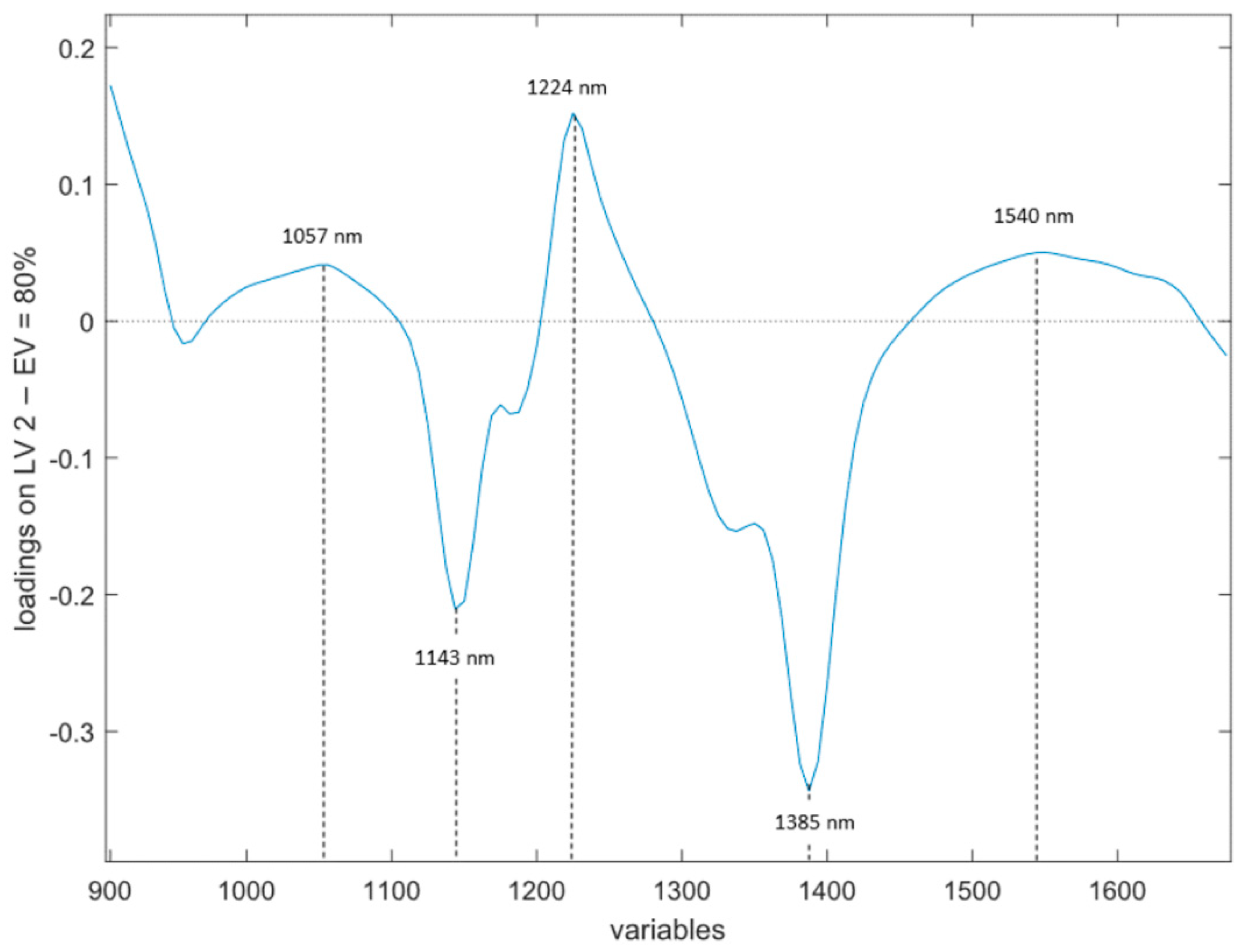
- NIR (Figure 3). In this case, the LV that retains the higher amount of information is LV2, with an 80.0% explained variance. In this case, an alternating positive and negative pattern is found. Three positive groups of wavelengths are contributing to the model at 980–1100 nm, 1200–1280 nm and 1460–1650 nm, with maximum peaks at 1057 nm, 1224 nm and 1540 nm. The spectral ranges contributing with negative signs are at 1100–1200 nm and 1280–1460 nm, with maximum peaks at 1143 nm and 1388 nm.
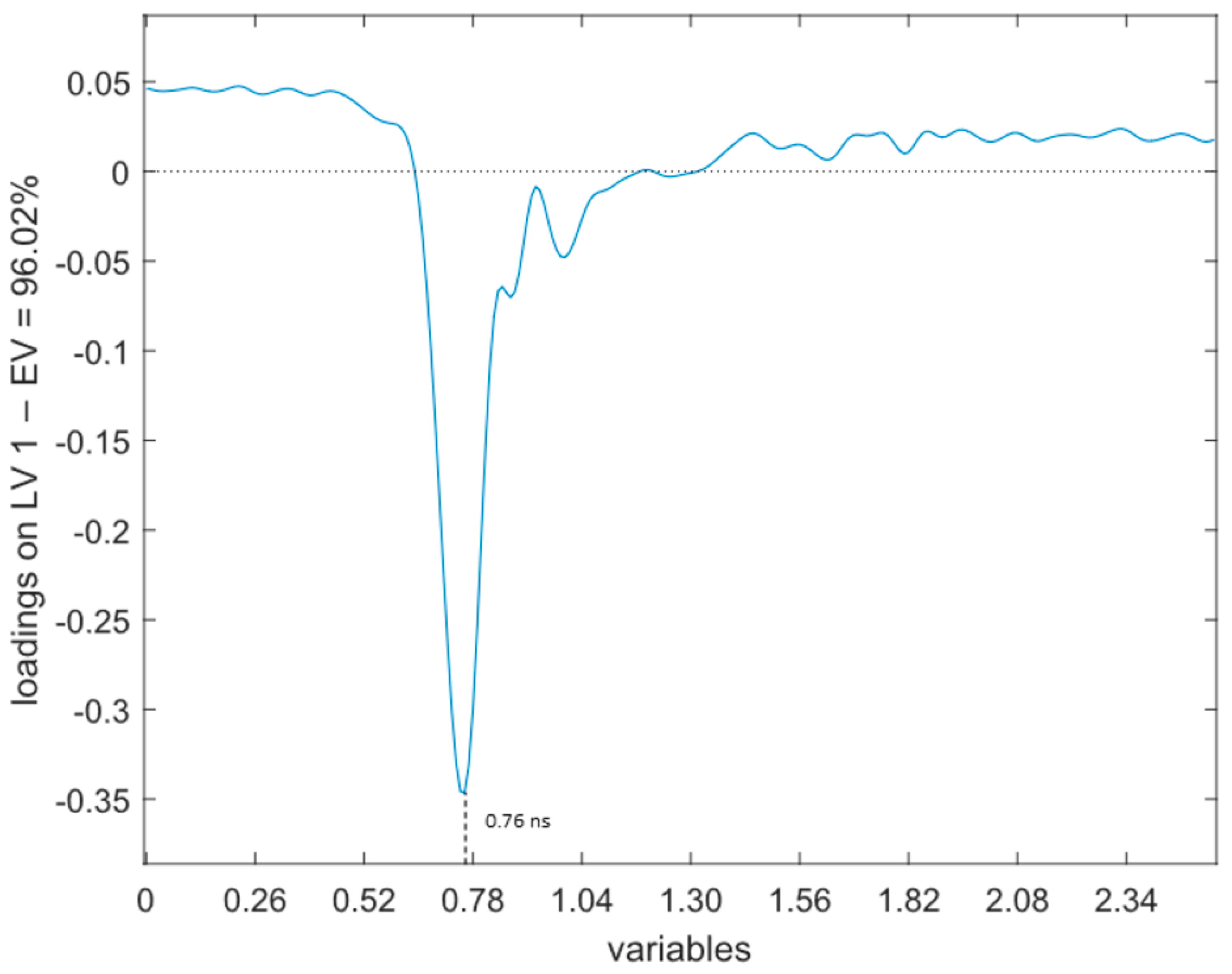
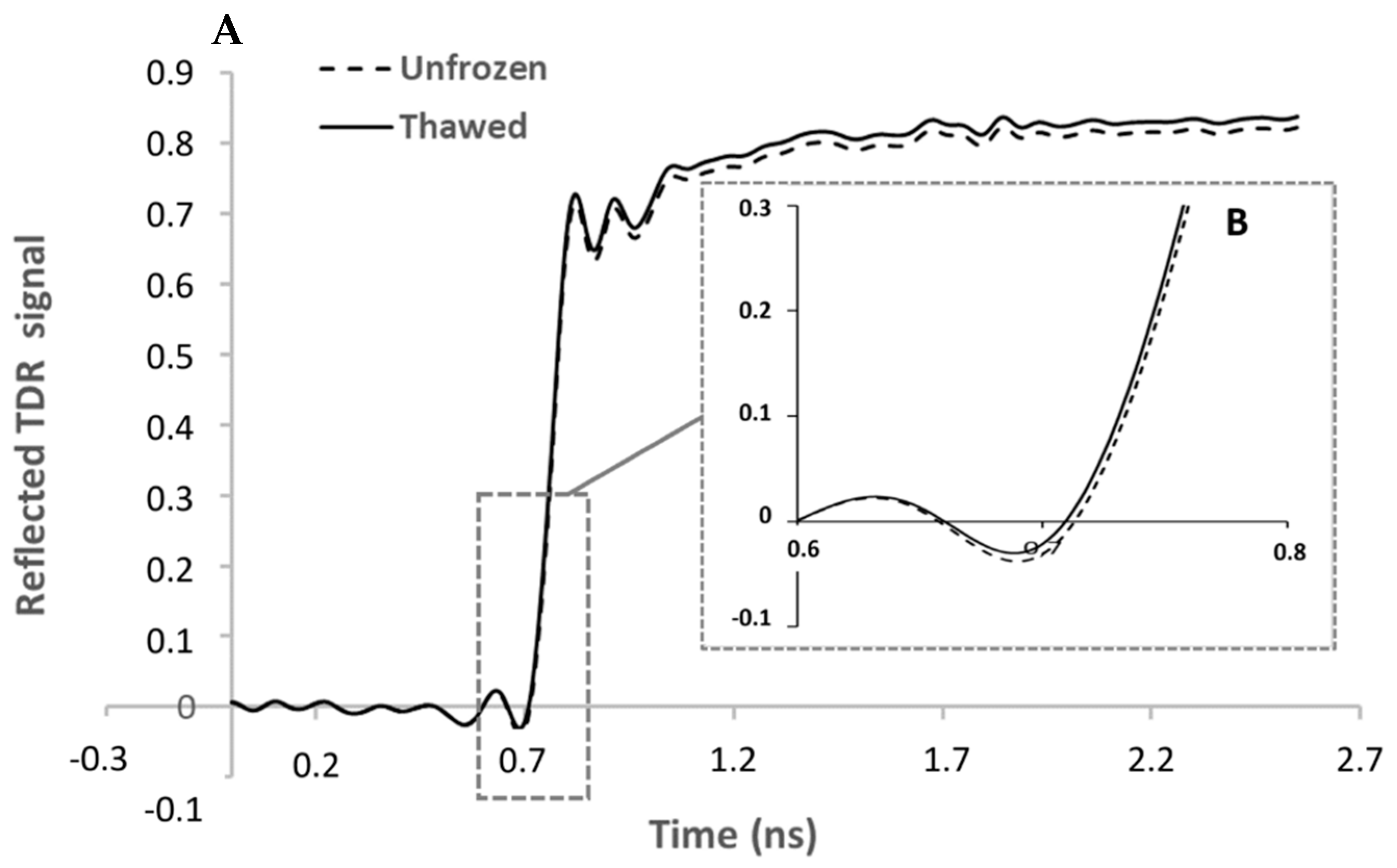
- TDR (Figure 4). The loadings of the first LV explain 96.02% of the variance, showing a relevant peak in the region between 0.61 ns and 1.17 ns and a maximum with negative sign at 0.76 ns.
4. Discussion
4.1. Changes during Frozen-Thawed Process
4.2. BIA
4.3. NIR
4.4. TDR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Market Observatory for Fisheries and Aquaculture Products (EUMOFA). EU Consumer Habits Regarding Fishery and Aquaculture Products. Final Report. January 2017. Available online: https://www.eumofa.eu/documents/20178/84590/EU+consumer+habits_final+report+.pdf/5c61348d-a69c-449e-a606-f5615a3a7e4c (accessed on 24 November 2021).
- European Market Observatory for Fisheries and Aquaculture Products (EUMOFA). The EU Fish Market. December 2019. Available online: https://www.eumofa.eu/documents/20178/314856/EN_The+EU+fish+market_2019.pdf/ (accessed on 24 November 2021).
- Uddin, M. Differentiation of Fresh and Frozen–Thawed fish. In Handbook of Seafood and Seafood Product Analysis; Nollet, L.M.L., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 735–750. [Google Scholar]
- Bozzetta, E.; Pezzolato, M.; Cencetti, E.; Varello, K.; Abramo, F.; Mutinelli, F.; Ingravalle, F.; Teneggi, E. Histology as a Valid and Reliable Tool to Differentiate Fresh from Frozen-Thawed Fish. J. Food Prot. 2012, 75, 1536–1541. [Google Scholar] [CrossRef]
- Kilinc, B.; Cakli, S.; Dincer, T.; Cadun, A. Effects of phosphates treatment on the quality of frozen-thawed fish species. J. Muscle Foods 2009, 20, 377–391. [Google Scholar] [CrossRef]
- van Ruth, S.M.; Brouwer, E.; Koot, A.; Wijtten, M. Seafood and Water Management. Foods 2014, 3, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011. Off. J. Eur. Union. 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1169 (accessed on 24 November 2021).
- FAO. FAO Fisheries Circulars No. 750; Reference Manual to Codes of Practice for Fish and Fishery Products; FAO: Rome, Italy; WHO: Geneva, Switzerland, 1982. [Google Scholar]
- JAS. Notification No. 514 of the Ministry of Agriculture, Forestry and Fisheries; Quality Labelling Standard for Perishable Foods; Japanese Agricultural Standards: Tokyo, Japan, 2000. [Google Scholar]
- Regulation (EU) No 1276/2011 of the European Parliament and of the Council of 8 December 2011. Off. J. Eur. Union. 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1276 (accessed on 24 November 2021).
- Reilly, A. Overview of Food Fraud in the Fisheries Sector. FAO Fisheries and Aquaculture Circular. 2018. Available online: https://www.fao.org/documents/card/en/c/I8791EN/ (accessed on 24 November 2021).
- Karoui, R.; Thomas, E.; Dufour, E. Utilisation of a rapid technique based on front-face fluorescence spectroscopy for differentiating between fresh and frozen–thawed fish fillets. Food Res. Int. 2006, 39, 349–355. [Google Scholar] [CrossRef]
- Nott, K.P.; Evans, S.D.; Hall, L.D. Quantitative magnetic resonance imaging of fresh and frozen-thawed trout. Magn. Reson. Imaging 1999, 17, 445–455. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Pu, H.B.; Chen, X.; Liu, Y.; Zhang, H.; Li, J.L. Integration of classifiers analysis and hyperspectral imaging for rapid discrimination of fresh from cold-stored and frozen-thawed fish fillets. J. Food Eng. 2015, 161, 33–39. [Google Scholar] [CrossRef]
- Oehlenschläger, J. Measurement of freshness quality of fish based on electrical properties. In Quality of Fish from Catch to Consumer: Labelling, Monitoring and Traceability; Luten, J.B., Oehlenschläger, J., Ólafsdóttir, G., Eds.; Wageningen Academic Publishers: Gelderland, The Netherlands, 2003; pp. 237–249. [Google Scholar] [CrossRef]
- Kent, M.; Oehlenschläger, J. Measuring Electrical Properties. In Fishery Products: Quality, Safety and Authenticity; Rehbein, H., Oehlenschläger, J., Eds.; John Wiley & Sons: Chichester, UK, 2009; pp. 286–300. [Google Scholar] [CrossRef]
- Zhao, X.; Zhuang, H.; Yoon, S.C.; Dong, Y.; Wang, W.; Zhao, W. Electrical Impedance Spectroscopy for Quality Assessment of Meat and Fish: A Review on Basic Principles, Measurement Methods, and Recent Advances. J. Food Qual. 2017, 2017, 6370739. [Google Scholar] [CrossRef] [Green Version]
- Karoui, R.; Lefur, B.; Grondin, C.; Thomas, E.; Demeulemester, C.; Baerdemaeker, J.D.; Guillard, A.S. Mid-infrared spectroscopy as a new tool for the evaluation of fish freshness. Int. J. Food Sci. Technol. 2007, 42, 57–64. [Google Scholar] [CrossRef]
- Sivertsen, A.H.; Kimiya, T.; Heia, K. Automatic freshness assessment of cod (Gadus morhua) fillets by Vis/Nir spectroscopy. J. Food Eng. 2011, 103, 317–323. [Google Scholar] [CrossRef]
- Velioğlu, H.M.; Temiz, H.T.; Boyaci, I.H. Differentiation of fresh and frozen-thawed fish samples using Raman spectroscopy coupled with chemometric analysis. Food Chem. 2015, 172, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Karoui, R.; Blecker, C. Fluorescence Spectroscopy Measurement for Quality Assessment of Food Systems—A Review. Food Bioprocess Technol. 2011, 4, 364–386. [Google Scholar] [CrossRef]
- Cox, M.K. Bioelectrical Impedance Analysis Measures of Body Composition and Condition, and Its Sensitivity to the Freezing Process. J. Aquat. Food Prod. Technol. 2015, 24, 368–377. [Google Scholar] [CrossRef]
- Zhou, J.J.; Wu, X.Y.; Chen, Z.; You, J.; Xiong, S.B. Evaluation of freshness in freshwater fish based on near infrared reflectance spectroscopy and chemometrics. LWT Food Sci. Technol. 2019, 106, 145–150. [Google Scholar] [CrossRef]
- Huang, H.; Yu, H.; Xu, H.; Ying, Y. Near infrared spectroscopy for on/in-line monitoring of quality in foods and beverages: A review. J. Food Eng. 2008, 87, 303–313. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Reis, M.M.; Martínez, E.; Saitua, E.; Rodríguez, R.; Pérez, I.; Olabarrieta, I. Non-invasive differentiation between fresh and frozen/thawed tuna fillets using near infrared spectroscopy (Vis-NIRS). LWT Food Sci. Technol. 2017, 78, 129–137. [Google Scholar] [CrossRef]
- Uddin, M.; Okazaki, E. Classification of Fresh and Frozen-thawed Fish by Near-infrared Spectroscopy. J. Food Sci. 2004, 69, C665–C668. [Google Scholar] [CrossRef]
- Kent, M.; Daschner, F. Time domain spectroscopy. In Fishery Products: Quality, Safety and Authenticity; Rehbein, H., Oehlenschläger, J., Eds.; John Wiley & Sons: Chichester, UK, 2009; pp. 273–285. [Google Scholar] [CrossRef]
- Jha, S.N.; Narsaiah, K.; Basediya, A.L.; Sharma, R.; Jaiswal, P.; Kumar, R.; Bhardwaj, R. Measurement techniques and application of electrical properties for nondestructive quality evaluation of foods—A review. J. Food Sci. Technol. 2011, 48, 387–411. [Google Scholar] [CrossRef] [Green Version]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Gudjónsdóttir, M.; Karlsdóttir, M.G.; Arason, S.; Rustad, T. Injection of fish protein solutions of fresh saithe (Pollachius virens) fillets studied by low field Nuclear Magnetic Resonance and physicochemical measurements. J. Food Sci. Technol. 2013, 50, 228–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Denis, T.; Goupy, J. Optimization of a nitrogen analyser based on the Dumas method. Anal. Chim. Acta 2004, 515, 191–198. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists International, 18th ed.; AOAC International: Washington, DC, USA, 2005. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, O.; Knöchel, R. A hand-held TDR-system with a fast system-rise time and a high resolution bandwidth for moisture measurements in the microwave frequency range. In Proceedings of the 5th International Conference on Electromagnetic Wave Interaction with Water and Moist Substances, Rotorua, New Zealand, 23–26 March 2003; Thakur, K., Ed.; Industrial Research Ltd.: Auckland, New Zealand, 2003; pp. 171–179. [Google Scholar]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Snee, R.D. Validation of Regression Models: Methods and Examples. Technometrics 1977, 19, 415–428. [Google Scholar] [CrossRef]
- Ballabio, D.; Consonni, V. Classification tools in chemistry. Part 1: Linear models. PLS-DA. Anal. Methods 2013, 5, 3790–3798. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Davis, J.; Goadrich, M. The relationship between Precision-Recall and ROC curves. In Proceedings of the 23rd International Conference on Machine Learning, Pittsburgh, PA, USA, 25–29 June 2006; Cohen, W., Moore, A., Eds.; Association for Computing Machinery: New York, NY, USA, 2006; pp. 233–240. [Google Scholar] [CrossRef] [Green Version]
- Hassoun, A.; Shumilina, E.; Di Donato, F.; Foschi, M.; Simal-Gandara, J.; Biancolillo, A. Emerging Techniques for Differentiation of Fresh and Frozen–Thawed Seafoods: Highlighting the Potential of Spectroscopic Techniques. Molecules 2020, 25, 4472. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Nakazawa, N.; Okazaki, E. Recent research on factors influencing the quality of frozen seafood. Fish. Sci. 2020, 86, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Vidaček, S.; Medić, H.; Botka-Petrak, K.; Nežak, J.; Petrak, T. Bioelectrical impedance analysis of frozen sea bass (Dicentrarchus labrax). J. Food Eng. 2008, 88, 263–271. [Google Scholar] [CrossRef]
- Davalos, R.; Rubinsky, B. Electrical Impedance Tomography of Cell Viability in Tissue with Application to Cryosurgery. J. Biomech. Eng. 2004, 126, 305–309. [Google Scholar] [CrossRef]
- Pennisi, F.; Giraudo, A.; Cavallini, N.; Esposito, G.; Merlo, G.; Geobaldo, F.; Acutis, P.L.; Pezzolato, M.; Savorani, F.; Bozzetta, E. Differentiation between Fresh and Thawed Cephalopods Using NIR Spectroscopy and Multivariate Data Analysis. Foods 2021, 10, 528. [Google Scholar] [CrossRef]
- Sannia, M.; Serva, L.; Balzan, S.; Segato, S.; Novelli, E.; Fasolato, L. Application of near-infrared spectroscopy for frozen-thawed characterization of cuttlefish (Sepia officinalis). J. Food Sci. Technol. 2019, 56, 4437–4447. [Google Scholar] [CrossRef] [PubMed]
- Laub-Ekgreen, M.H.; Martinez-Lopez, B.; Jessen, F.; Skov, T. Non-destructive measurement of salt using NIR spectroscopy in the herring marinating process. LWT Food Sci. Technol. 2018, 97, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Kamruzzaman, M.; ElMasry, G.; Sun, D.W.; Allen, P. Non-destructive prediction and visualization of chemical composition in lamb meat using NIR hyperspectral imaging and multivariate regression. Innov. Food Sci. Emerg. Technol. 2012, 16, 218–226. [Google Scholar] [CrossRef]
- Morsy, N.; Sun, D.W. Robust linear and non-linear models of NIR spectroscopy for detection and quantification of adulterants in fresh and frozen-thawed minced beef. Meat Sci. 2013, 93, 292–302. [Google Scholar] [CrossRef]
- Ballin, N.Z. Authentication of meat and meat products. Meat Sci. 2010, 86, 577–587. [Google Scholar] [CrossRef]
- Kent, M.; Knöchel, R.; Daschner, F.; Schimmer, O.; Oehlenschläger, J.; Mierke-Klemeyer, S.; Barr, U.K.; Floberg, P.; Tejada, M.; Huidobro, A.; et al. Time domain reflectometry as a tool for the estimation of quality in foods. Int. Agrophys. 2004, 18, 225–229. [Google Scholar]
- Mendes, R.; Schimmer, O.; Vieira, H.; Pereira, J.; Teixeira, B. Control of abusive water addition to Octopus vulgaris with non-destructive methods. J. Sci. Food Agric. 2018, 98, 369–376. [Google Scholar] [CrossRef]
- Fulladosa, E.; Duran-Montgé, P.; Serra, X.; Picouet, P.; Schimmer, O.; Gou, P. Estimation of dry-cured ham composition using dielectric time domain reflectometry. Meat Sci. 2013, 93, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Celorio, M.; Garcia-Gil, N.; Gou, P.; Arnau, J.; Fulladosa, E. Effect of temperature, high pressure and freezing/thawing of dry-cured ham slices on dielectric time domain reflectometry response. Meat Sci. 2015, 100, 91–96. [Google Scholar] [CrossRef] [PubMed]
| Unfrozen | Thawed | |||
|---|---|---|---|---|
| Non-Injected | Injected | Non-Injected | Injected | |
| BIA | 120 | 90 | 20 | 70 |
| NIR | 960 | 720 | 160 | 560 |
| TDR | 30 | 90 | 20 | 70 |
| Pre-Processing | LV | Error-Rate | Accuracy | Sensitivity | Specificity | Precision | ||
|---|---|---|---|---|---|---|---|---|
| Calibration | Unfrozen | Autoscaling | 2 | 0.08 | 0.91 | 0.90 | 0.93 | 0.97 |
| Thawed | 0.93 | 0.90 | 0.81 | |||||
| CV | Unfrozen | 0.10 | 0.90 | 0.90 | 0.90 | 0.95 | ||
| Thawed | 0.90 | 0.90 | 0.80 | |||||
| Validation | Unfrozen | 0.14 | 0.88 | 0.91 | 0.81 | 0.93 | ||
| Thawed | 0.81 | 0.91 | 0.76 |
| Pre-Processing | LV | Error-Rate | Accuracy | Sensitivity | Specificity | Precision | ||
|---|---|---|---|---|---|---|---|---|
| Calibration | Unfrozen | 1st derivative (order 2, window 5) + Mean Center | 9 | 0.08 | 0.94 | 0.96 | 0.88 | 0.95 |
| Thawed | 0.88 | 0.96 | 0.91 | |||||
| CV | Unfrozen | 0.08 | 0.94 | 0.96 | 0.88 | 0.95 | ||
| Thawed | 0.88 | 0.96 | 0.90 | |||||
| Validation | Unfrozen | 0.10 | 0.91 | 0.94 | 0.86 | 0.92 | ||
| Thawed | 0.86 | 0.94 | 0.89 |
| Pre-Processing | LV | Error-Rate | Accuracy | Sensitivity | Specificity | Precision | ||
|---|---|---|---|---|---|---|---|---|
| Calibration | Unfrozen | SNV + Mean Center | 8 | 0.04 | 0.96 | 0.97 | 0.96 | 0.97 |
| Thawed | 0.96 | 0.97 | 0.96 | |||||
| CV | Unfrozen | 0.13 | 0.87 | 0.83 | 0.92 | 0.93 | ||
| Thawed | 0.92 | 0.83 | 0.81 | |||||
| Validation | Unfrozen | 0.15 | 0.86 | 0.88 | 0.82 | 0.88 | ||
| Thawed | 0.82 | 0.88 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto-Ortega, S.; Melado-Herreros, Á.; Foti, G.; Olabarrieta, I.; Ramilo-Fernández, G.; Gonzalez Sotelo, C.; Teixeira, B.; Velasco, A.; Mendes, R. Rapid Differentiation of Unfrozen and Frozen-Thawed Tuna with Non-Destructive Methods and Classification Models: Bioelectrical Impedance Analysis (BIA), Near-Infrared Spectroscopy (NIR) and Time Domain Reflectometry (TDR). Foods 2022, 11, 55. https://doi.org/10.3390/foods11010055
Nieto-Ortega S, Melado-Herreros Á, Foti G, Olabarrieta I, Ramilo-Fernández G, Gonzalez Sotelo C, Teixeira B, Velasco A, Mendes R. Rapid Differentiation of Unfrozen and Frozen-Thawed Tuna with Non-Destructive Methods and Classification Models: Bioelectrical Impedance Analysis (BIA), Near-Infrared Spectroscopy (NIR) and Time Domain Reflectometry (TDR). Foods. 2022; 11(1):55. https://doi.org/10.3390/foods11010055
Chicago/Turabian StyleNieto-Ortega, Sonia, Ángela Melado-Herreros, Giuseppe Foti, Idoia Olabarrieta, Graciela Ramilo-Fernández, Carmen Gonzalez Sotelo, Bárbara Teixeira, Amaya Velasco, and Rogério Mendes. 2022. "Rapid Differentiation of Unfrozen and Frozen-Thawed Tuna with Non-Destructive Methods and Classification Models: Bioelectrical Impedance Analysis (BIA), Near-Infrared Spectroscopy (NIR) and Time Domain Reflectometry (TDR)" Foods 11, no. 1: 55. https://doi.org/10.3390/foods11010055
APA StyleNieto-Ortega, S., Melado-Herreros, Á., Foti, G., Olabarrieta, I., Ramilo-Fernández, G., Gonzalez Sotelo, C., Teixeira, B., Velasco, A., & Mendes, R. (2022). Rapid Differentiation of Unfrozen and Frozen-Thawed Tuna with Non-Destructive Methods and Classification Models: Bioelectrical Impedance Analysis (BIA), Near-Infrared Spectroscopy (NIR) and Time Domain Reflectometry (TDR). Foods, 11(1), 55. https://doi.org/10.3390/foods11010055






