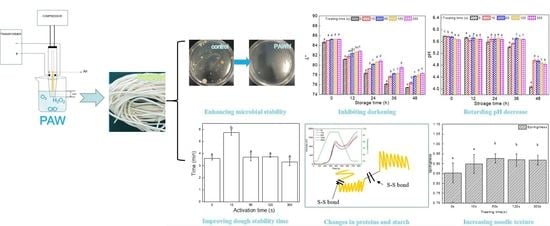
Evaluation of the Storage Stability and Quality Properties of Fresh Noodles Mixed with Plasma-Activated Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
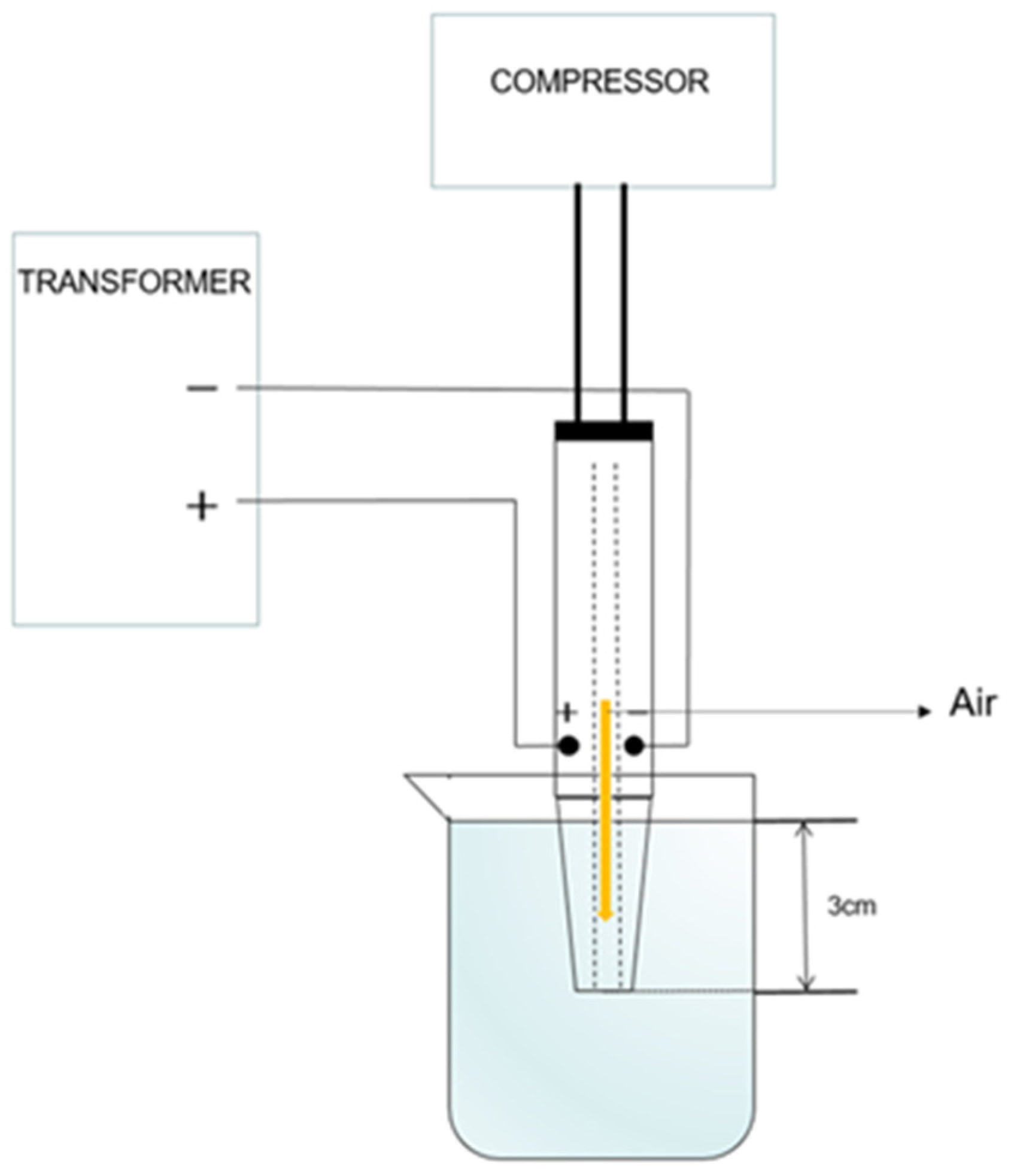
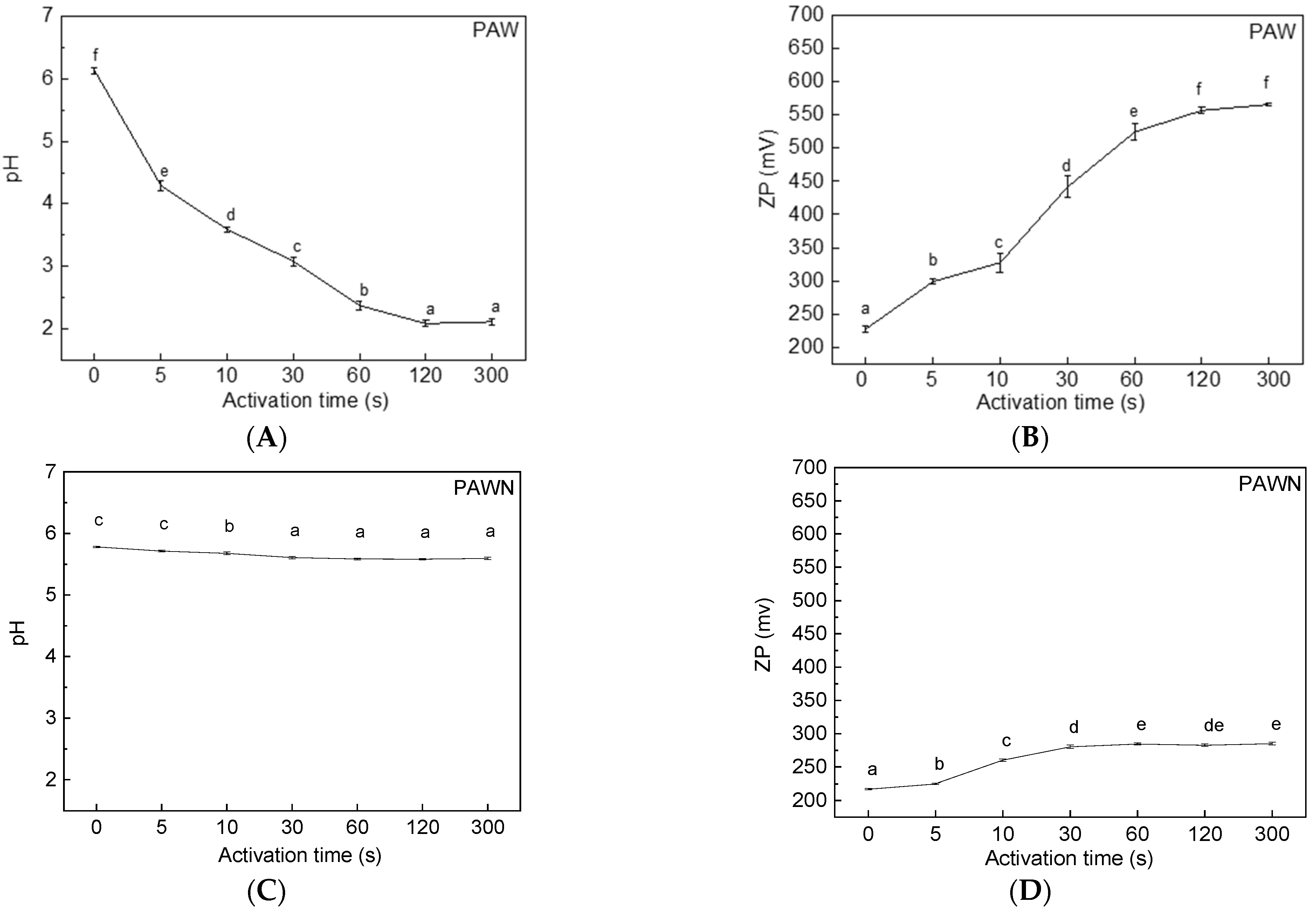
2.2. Preparation of Plasma-Activated Water (PAW)
2.3. Preparation of Fresh Noodles
2.4. Determination of Total Plate Count (TPC)
2.5. Color and pH Measurement of Fresh Noodles
2.6. Analysis of Farinographic Properties of Wheat Dough
2.7. Viscosity Analysis
2.8. Low-Field-1H Nuclear Magnetic Resonance (LF-1H NMR) Analysis
2.9. Size-Exclusion High-Performance Liquid Chromatography (SE-HPLC) Analysis
2.10. Textural Properties
2.11. Statistical Analysis
3. Results and Discussion
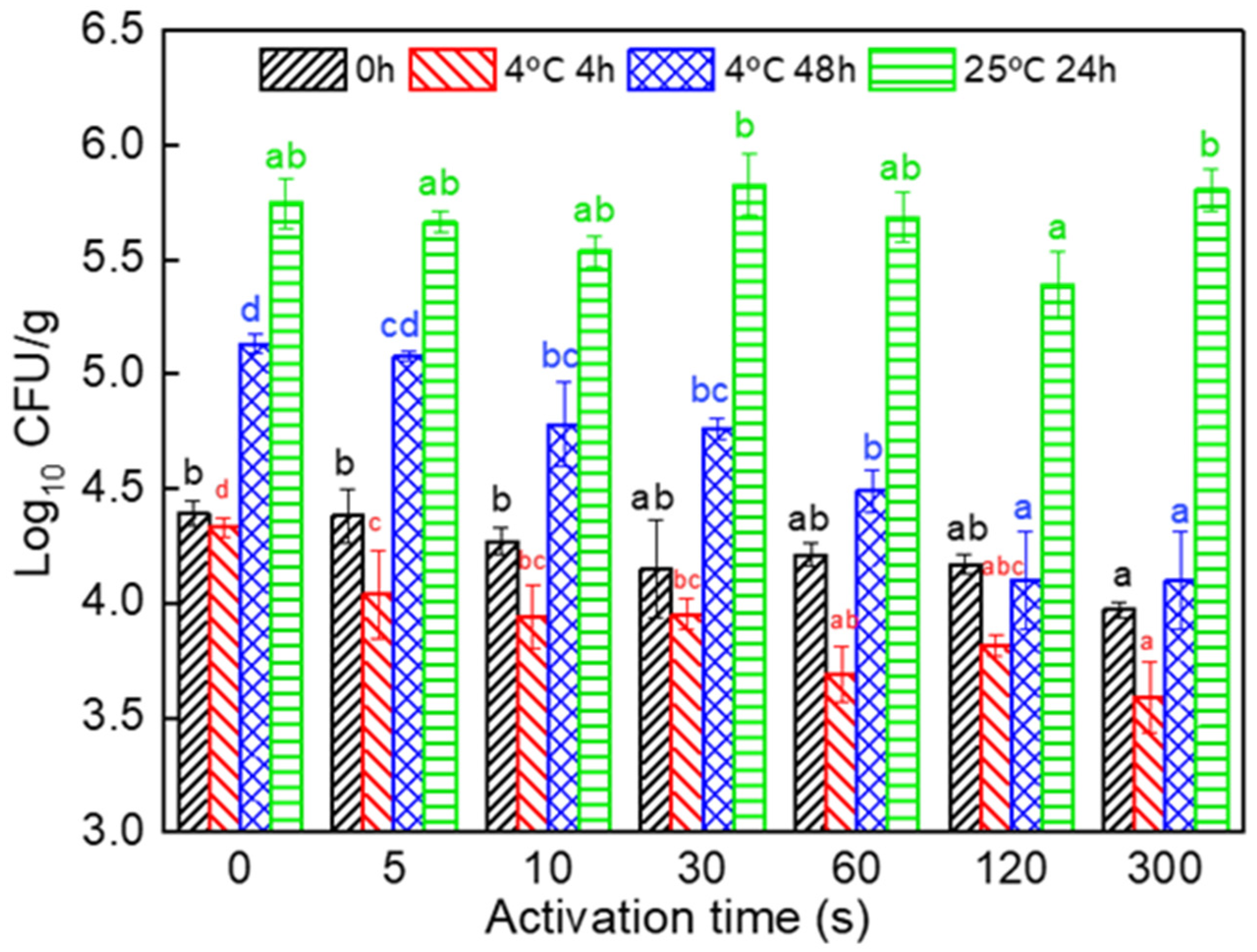
3.1. Microbial Growth in Fresh Noodles Produced with PAW
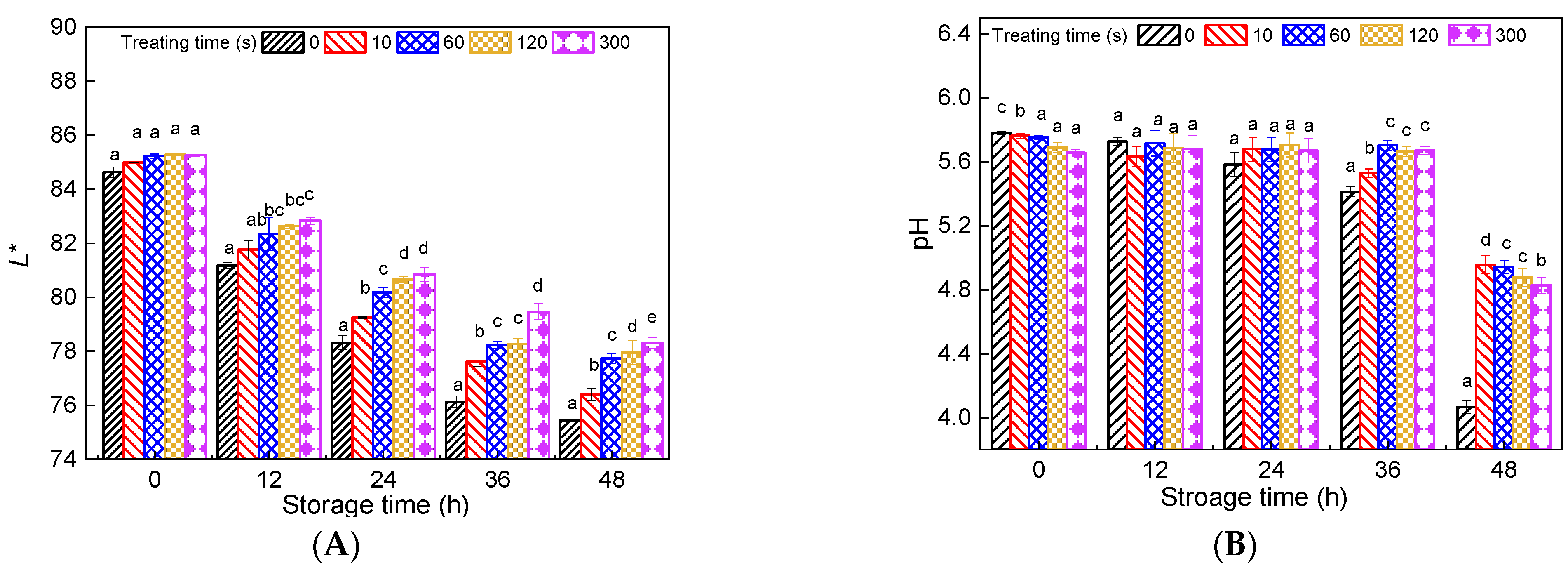
3.2. Effect of PAW on L* Value and pH Changes in Fresh Noodles during Storage
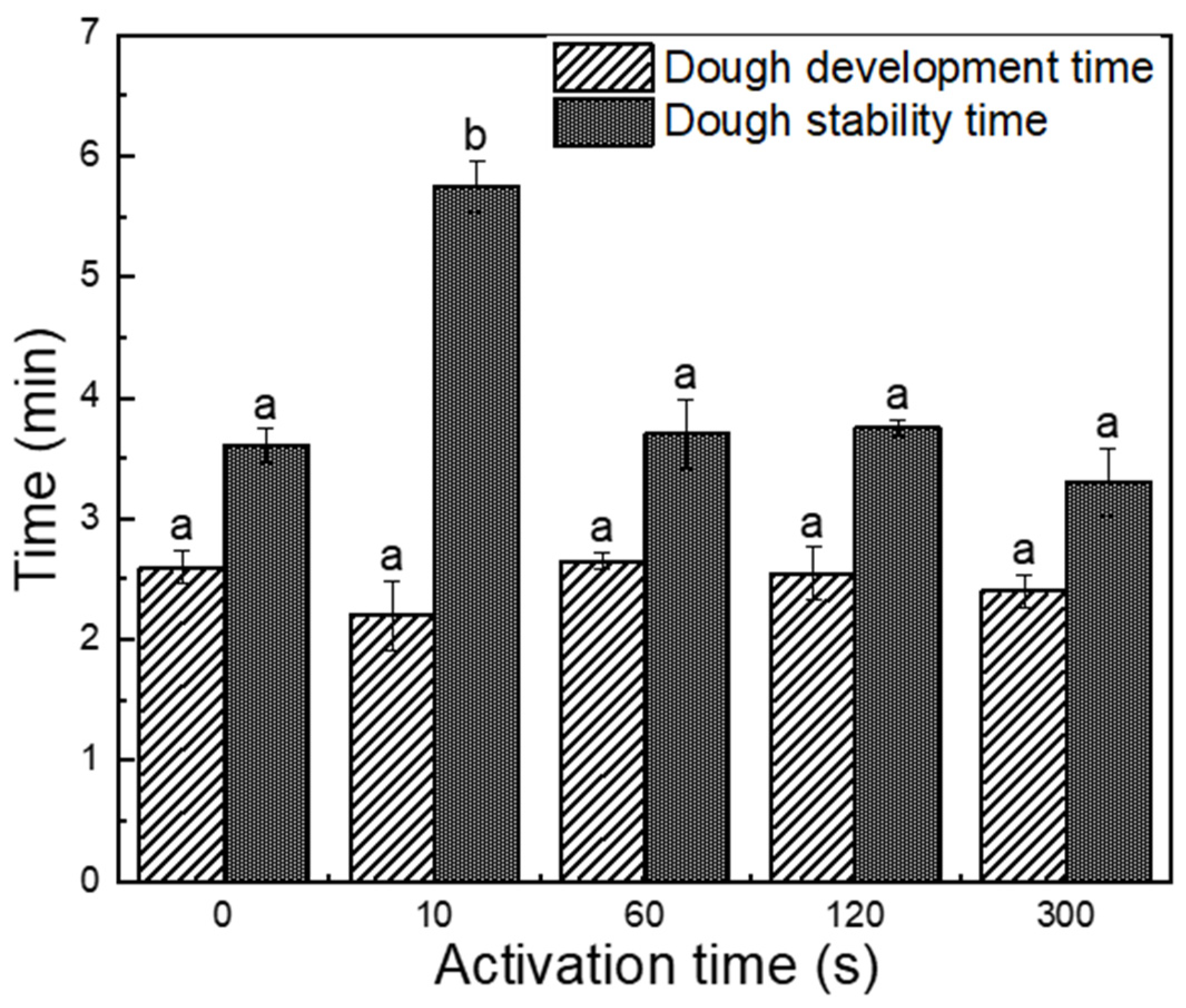
3.3. Effect of PAW on Mixing Properties of Dough
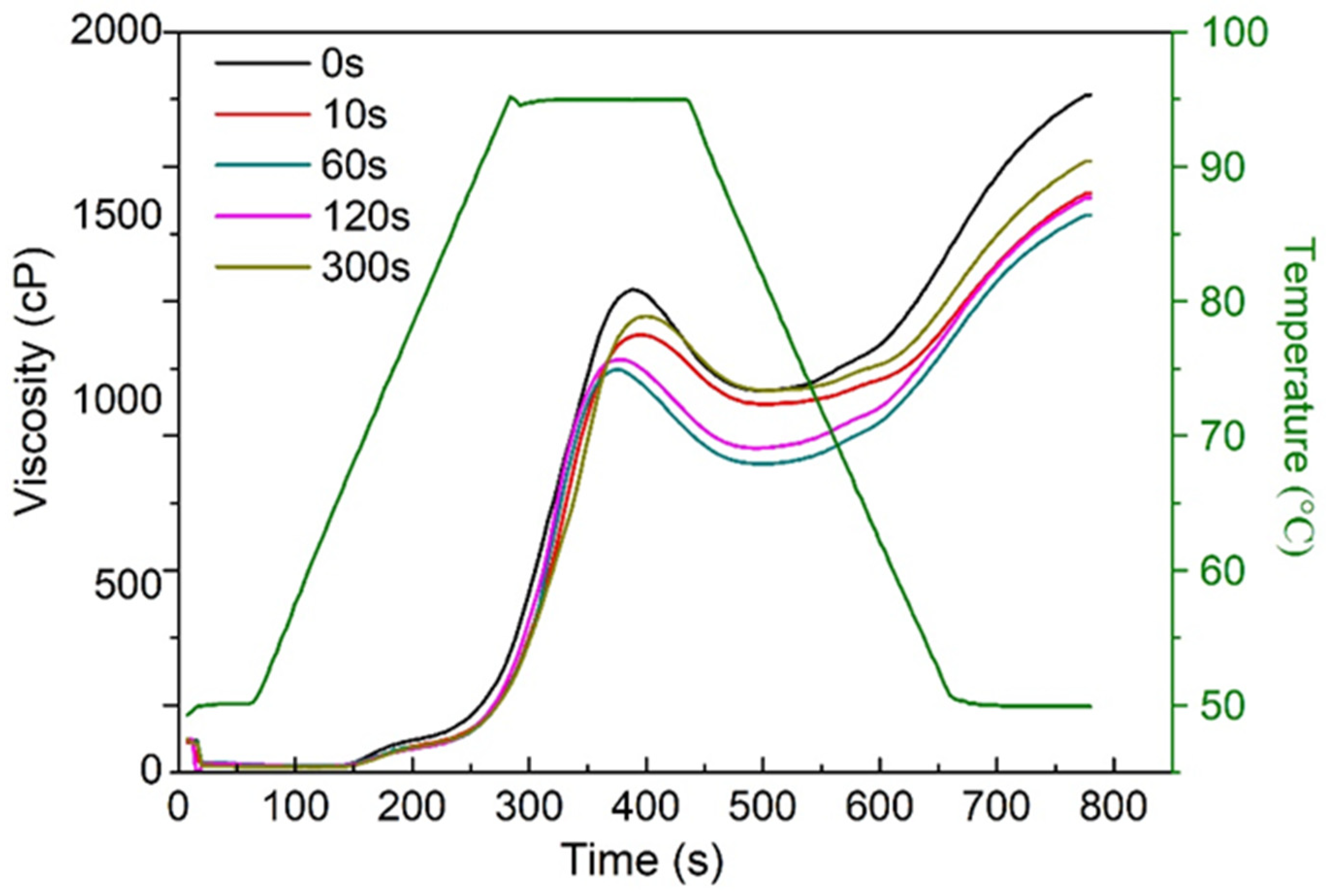
3.4. Effect of PAW on the Changes in Viscosity Properties of Starch in Fresh Noodles
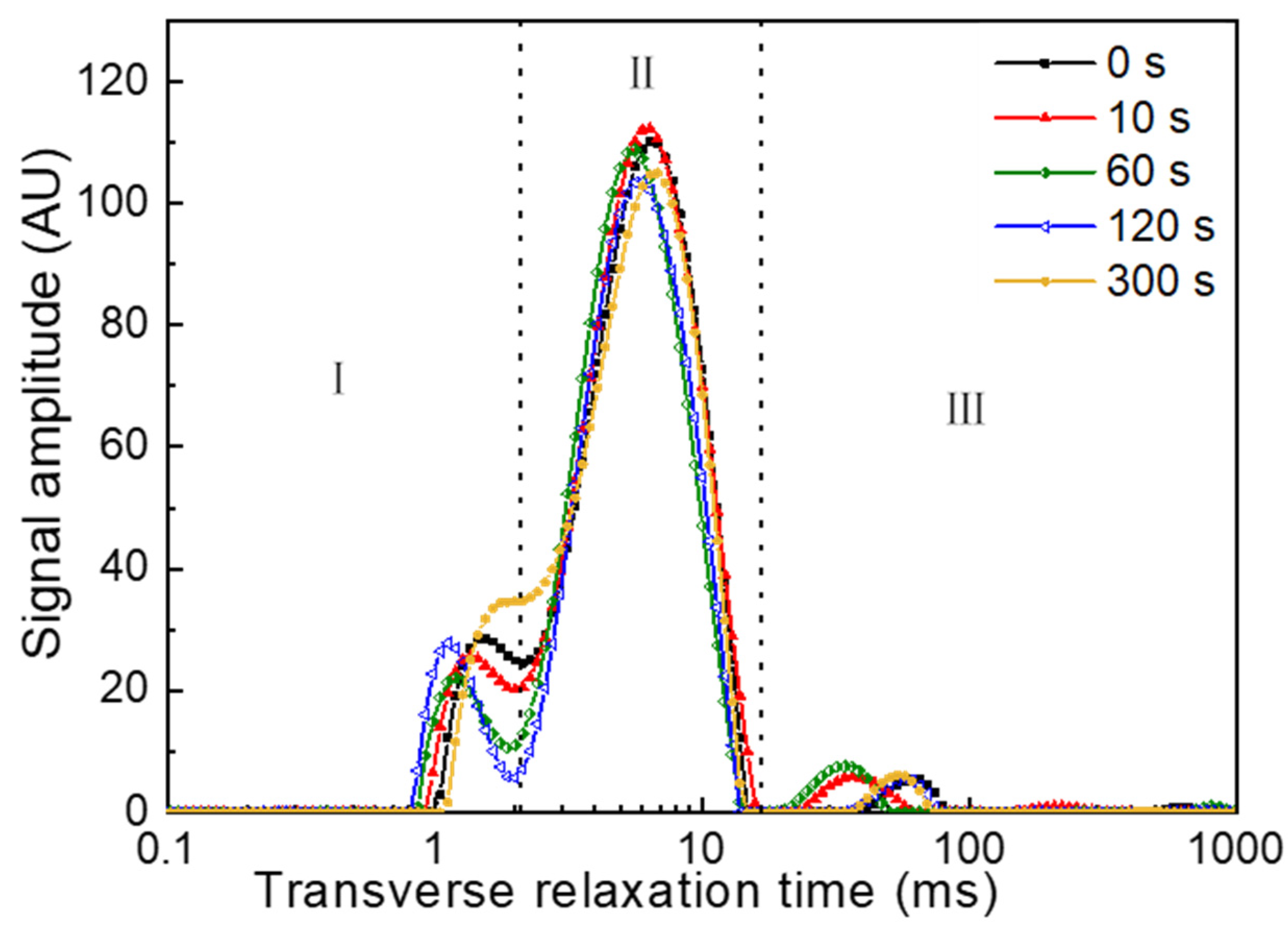
3.5. Changes in Water Status of PAW-Treated Fresh Noodles
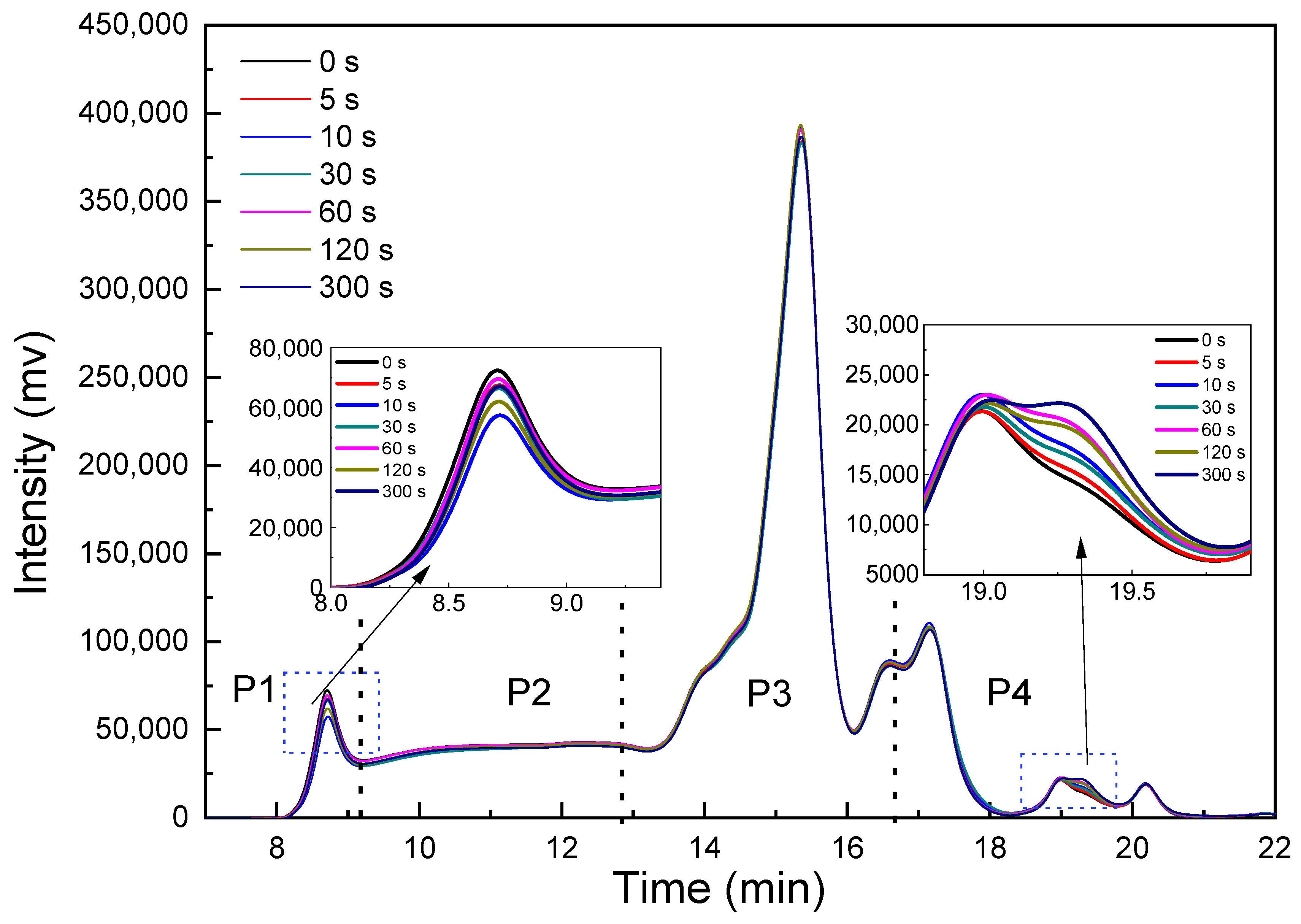
3.6. SDS Extractability and Molecular Weight Distribution Profiles of Proteins
3.7. Effect of PAW on Textural Properties of Fresh Noodles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Ma, M.; Yang, T.; Li, M.; Sun, Q. Dynamic distribution and transition of gluten proteins during noodle processing. Food Hydrocoll. 2022, 123, 107114. [Google Scholar] [CrossRef]
- Fu, B.X. Asian noodles: History, classification, raw materials, and processing. Food Res. Int. 2008, 41, 888–902. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Li, M.; Sun, Q. Inhibitory effects of sorbitol on the collapse and deterioration of gluten network in fresh noodles during storage. Food Chem. 2021, 344, 128638. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-T.; Guo, X.-N.; Zhu, K.-X. Inhibition of L-Cysteine on the Browning of Fresh Wet Noodles. Foods 2021, 10, 1156. [Google Scholar] [CrossRef]
- Huang, J.-R.; Huang, C.-Y.; Huang, Y.-W.; Chen, R.-H. Shelf-life of fresh noodles as affected by chitosan and its Maillard reaction products. LWT—Food Sci. Technol. 2007, 40, 1287–1291. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Guo, X.; Peng, W.; Zhou, H. Effect of water activity (aw) and irradiation on the shelf-life of fresh noodles. Innov. Food Sci. Emerg. Technol. 2011, 12, 526–530. [Google Scholar] [CrossRef]
- Li, M.; Peng, J.; Zhu, K.-X.; Guo, X.-N.; Zhang, M.; Peng, W.; Zhou, H.-M. Delineating the microbial and physical–chemical changes during storage of ozone treated wheat flour. Innov. Food Sci. Emerg. Technol. 2013, 20, 223–229. [Google Scholar] [CrossRef]
- Bai, Y.-P.; Guo, X.-N.; Zhu, K.-X.; Zhou, H.-M. Shelf-life extension of semi-dried buckwheat noodles by the combination of aqueous ozone treatment and modified atmosphere packaging. Food Chem. 2017, 237, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Chaple, S.; Sarangapani, C.; Jones, J.; Carey, E.; Causeret, L.; Genson, A.; Duffy, B.; Bourke, P. Effect of atmospheric cold plasma on the functional properties of whole wheat (Triticum aestivum L.) grain and wheat flour. Innov. Food Sci. Emerg. Technol. 2020, 66, 102529. [Google Scholar] [CrossRef]
- Ma, R.; Yu, S.; Tian, Y.; Wang, K.; Sun, C.; Li, X.; Zhang, J.; Chen, K.; Fang, J. Effect of Non-Thermal Plasma-Activated Water on Fruit Decay and Quality in Postharvest Chinese Bayberries. Food Bioprocess Technol. 2016, 9, 1825–1834. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Jiang, A.; Sun, X.; Guan, Q.; Hu, W. Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innov. Food Sci. Emerg. Technol. 2020, 59, 102256. [Google Scholar] [CrossRef]
- Sifuentes-Nieves, I.; Flores-Silva, P.C.; Gallardo-Vega, C.; Hernández-Hernández, E.; Neira-Velázquez, G.; Mendez-Montealvo, G.; Velazquez, G. Films made from plasma-modified corn starch: Chemical, mechanical and barrier properties. Carbohydr. Polym. 2020, 237, 116103. [Google Scholar] [CrossRef]
- Shen, J.; Tian, Y.; Li, Y.; Ma, R.; Zhang, Q.; Zhang, J.; Fang, J. Bactericidal Effects against S. aureus and Physicochemical Properties of Plasma Activated Water stored at different temperatures. Sci. Rep. 2016, 6, 28505. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, G.; Wei, R.; Zhang, Y.; Li, S.; Chen, Y. Quality characteristics of fresh wet noodles treated with nonthermal plasma sterilization. Food Chem. 2019, 297, 124900. [Google Scholar] [CrossRef]
- GB/T 4789.2; Code of National Food Safety Standard of China. Food Microbiological Examination: Aerobic Plate Count. The State Food and Drug Administration: Beijing, China, 2016.
- Li, M.; Zhang, J.-H.; Zhu, K.-X.; Peng, W.; Zhang, S.-K.; Wang, B.; Zhu, Y.-J.; Zhou, H.-M. Effect of superfine green tea powder on the thermodynamic, rheological and fresh noodle making properties of wheat flour. LWT—Food Sci. Technol. 2012, 46, 23–28. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists. AACC Method 76-21. AACC International Approved Methods, 10th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- Assifaoui, A.; Champion, D.; Chiotelli, E.; Verel, A. Characterization of water mobility in biscuit dough using a low-field 1H NMR technique. Carbohydr. Polym. 2006, 64, 197–204. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; Von Woedtke, T. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Wang, M. Cold storage temperature following pulsed electric fields treatment to inactivate sublethally injured microorganisms and extend the shelf life of green tea infusions. Int. J. Food Microbiol. 2009, 129, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, M.; Zhu, K.-X.; Guo, X.-N.; Zhou, H.-M. Delineating the physico-chemical, structural, and water characteristic changes during the deterioration of fresh noodles: Understanding the deterioration mechanisms of fresh noodles. Food Chem. 2017, 216, 374–381. [Google Scholar] [CrossRef]
- Zaidul, I.M.; Karim, A.A.; Manan, D.; Ariffin, A.; Norulaini, N.N.; Omar, A.M. A farinograph study on the viscoelastic properties of sago/wheat flour dough systems. J. Sci. Food Agric. 2004, 84, 616–622. [Google Scholar] [CrossRef]
- Fu, L.; Tian, J.-C.; Sun, C.-L.; Li, C. RVA and Farinograph Properties Study on Blends of Resistant Starch and Wheat Flour. Agric. Sci. China 2008, 7, 812–822. [Google Scholar] [CrossRef]
- Dias, A.R.G.; Zavareze, E.D.R.; Elias, M.C.; Helbig, E.; da Silva, D.O.; Ciacco, C.F. Pasting, expansion and textural properties of fermented cassava starch oxidised with sodium hypochlorite. Carbohydr. Polym. 2011, 84, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Daris, K.; Wang, Y.J. Characterization of different starches oxidized by hypochlorite. Starch-Starke 2001, 53, 211–218. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wang, L. Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydr. Polym. 2003, 52, 207–217. [Google Scholar] [CrossRef]
- Banura, S.; Thirumdas, R.; Kaur, A.; Deshmukh, R.; Annapure, U. Modification of starch using low pressure radio frequency air plasma. LWT—Food Sci. Technol. 2018, 89, 719–724. [Google Scholar] [CrossRef]
- Ritota, M.; Gianferri, R.; Bucci, R.; Brosio, E. Proton NMR relaxation study of swelling and gelatinisation process in rice starch–water samples. Food Chem. 2008, 110, 14–22. [Google Scholar] [CrossRef]
- Han, C.; Ma, M.; Li, M.; Sun, Q. Further interpretation of the underlying causes of the strengthening effect of alkali on gluten and noodle quality: Studies on gluten, gliadin, and glutenin. Food Hydrocoll. 2020, 103, 105661. [Google Scholar] [CrossRef]
- Tian, C.; Zhen, T.; Ma, M.; Li, M.; Sun, Q. Contribution of catechin monomers in tea polyphenols to the structure and physicochemical properties of wheat gluten and its sub-fractions. J. Cereal Sci. 2021, 101, 103306. [Google Scholar] [CrossRef]
- Bahrami, N.; Bayliss, D.; Chope, G.; Penson, S.; Perehinec, T.; Fisk, I.D. Cold plasma: A new technology to modify wheat flour functionality. Food Chem. 2016, 202, 247–253. [Google Scholar] [CrossRef]
- Liu, J.; Luo, D.; Li, X.; Xu, B.; Zhang, X.; Liu, J. Effects of inulin on the structure and emulsifying properties of protein components in dough. Food Chem. 2016, 210, 235–241. [Google Scholar] [CrossRef]
- Niu, M.; Xiong, L.; Zhang, B.; Jia, C.; Zhao, S. Comparative study on protein polymerization in whole-wheat dough modified by transglutaminase and glucose oxidase. LWT 2018, 90, 323–330. [Google Scholar] [CrossRef]
- Primo-Martín, C.; Valera, R.; Martínez-Anaya, M.A. Effect of Pentosanase and Oxidases on the Characteristics of Doughs and the Glutenin Macropolymer (GMP). J. Agric. Food Chem. 2003, 51, 4673–4679. [Google Scholar] [CrossRef] [PubMed]
- Baik, B.-K.; Lee, M.-R. Effects of Starch Amylose Content of Wheat on Textural Properties of White Salted Noodles. Cereal Chem. 2003, 80, 304–309. [Google Scholar] [CrossRef]
- An, D.; Li, Q.; Li, E.; Obadi, M.; Li, C.; Li, H.; Zhang, J.; Du, J.; Zhou, X.; Li, N.; et al. Structural basis of wheat starch determines the adhesiveness of cooked noodles by affecting the fine structure of leached starch. Food Chem. 2021, 341, 128222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-X.; Li, J.; Li, M.; Guo, X.-N.; Peng, W.; Zhou, H.-M. Functional properties of chitosan–xylose Maillard reaction products and their application to semi-dried noodle. Carbohydr. Polym. 2013, 92, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
| Quality Parameters | Activation Time (s) | ||||
|---|---|---|---|---|---|
| 0 | 10 | 60 | 120 | 300 | |
| Hardness (g) | 6750.89 ± 241.99 b | 5757.49 ± 61.98 a | 5624.21 ± 171.55 a | 5310.20 ± 101.74 ba | 5351.96 ± 185.47 a |
| Chewiness (g) | 3608.09 ± 188.96 c | 3025.74 ± 178.57 ab | 3059.79 ± 64.33 b | 2791.09 ± 310.56 ab | 2726.69 ± 185.59 a |
| Adhesiveness (g·s) | 450.80 ± 71.27 b | 232.82 ± 68.95 a | 215.25 ± 24.95 a | 186.10 ± 27.43 a | 190.29 ± 25.61 a |
| Springiness | 0.85 ± 0.05 a | 0.90 ± 0.05 a | 0.93 ± 0.02 b | 0.92 ± 0.03 b | 0.92 ± 0.03 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Y.; Li, M.; Ma, M.; Sun, Q. Evaluation of the Storage Stability and Quality Properties of Fresh Noodles Mixed with Plasma-Activated Water. Foods 2022, 11, 133. https://doi.org/10.3390/foods11010133
Wang Y, Liu Y, Li M, Ma M, Sun Q. Evaluation of the Storage Stability and Quality Properties of Fresh Noodles Mixed with Plasma-Activated Water. Foods. 2022; 11(1):133. https://doi.org/10.3390/foods11010133
Chicago/Turabian StyleWang, Yawei, Yuchang Liu, Man Li, Meng Ma, and Qingjie Sun. 2022. "Evaluation of the Storage Stability and Quality Properties of Fresh Noodles Mixed with Plasma-Activated Water" Foods 11, no. 1: 133. https://doi.org/10.3390/foods11010133
APA StyleWang, Y., Liu, Y., Li, M., Ma, M., & Sun, Q. (2022). Evaluation of the Storage Stability and Quality Properties of Fresh Noodles Mixed with Plasma-Activated Water. Foods, 11(1), 133. https://doi.org/10.3390/foods11010133