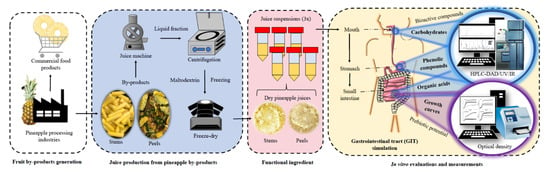
Impact of Simulated Human Gastrointestinal Digestion on the Bioactive Fraction of Upcycled Pineapple By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Consumables
2.2. Raw Materials
2.3. Samples Preparation
Samples Preparation for Prebiotic Evaluation
2.4. Prebiotic Activity
2.4.1. Microorganisms’ Growth Conditions
2.4.2. Inoculum Preparation
2.4.3. In Vitro Fermentation Assay
2.5. HPLC Determinations
2.6. Gastrointestinal Tract Simulation (GIT)
2.7. Antioxidant Capacity Determination (ORAC and ABTS Methods)
2.8. Quantification of Total Polyphenols
2.8.1. Folin–Ciocalteu Method
2.8.2. Individual Phenolic Compound Identification by HPLC
2.9. Statistical Analysis
3. Results
3.1. Structural Features and Chemical Composition of Pineapple By-Products
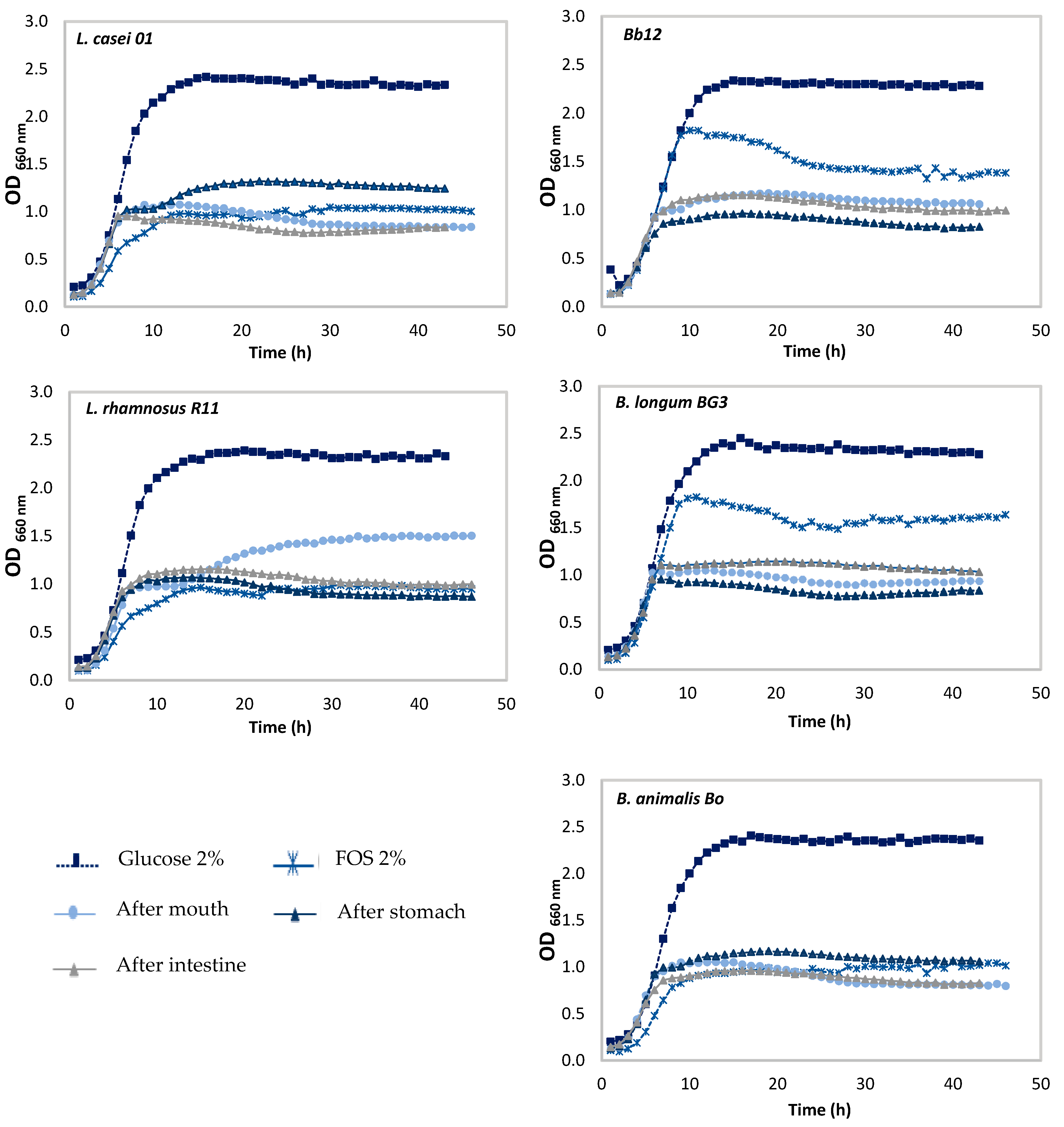
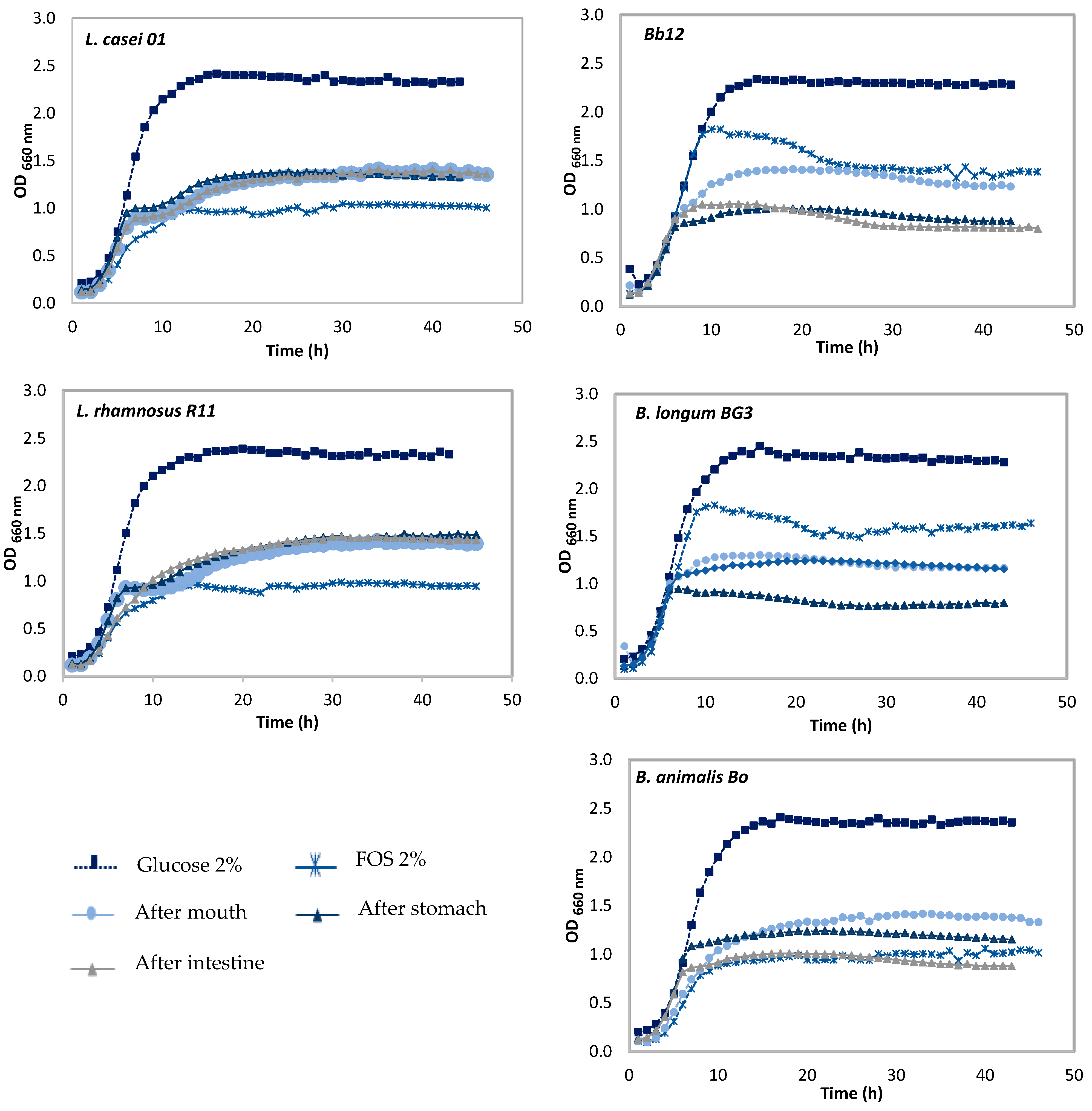
3.2. Effect of Pineapple By-Products on the Prebiotic Growth
3.3. Fermentation Metabolites—Production of Acetic and Lactic Acids
3.4. Phenolic Compounds and Antioxidant Activities
3.5. Effects of the Simulated GIT upon Pineapple Liquid Fractions
3.5.1. Effects of GIT Simulation upon Pineapple By-Products Liquid Fractions
3.5.2. Phenolic Compounds
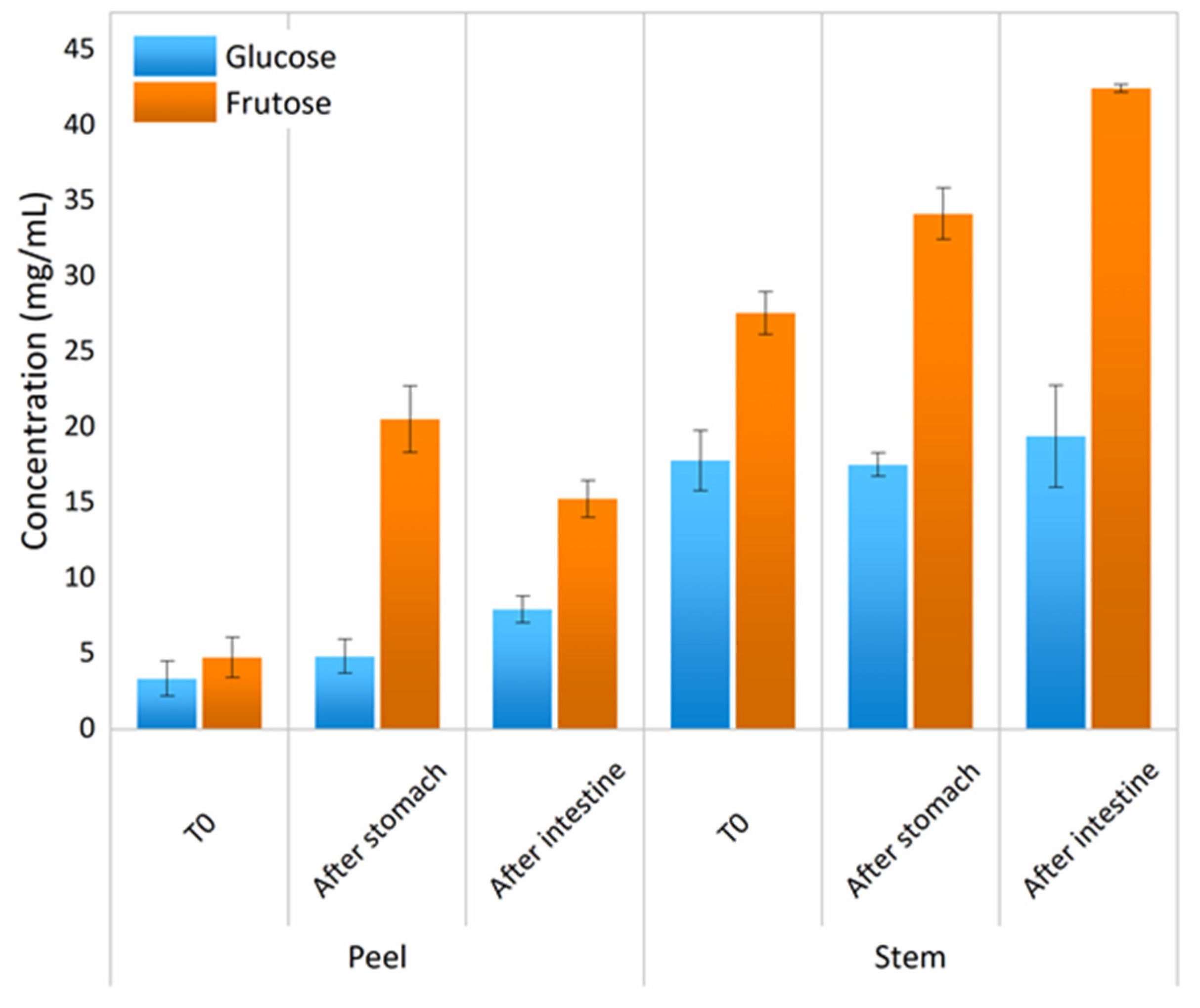
3.6. Simple Sugars and Complex Carbohydrates
Prebiotic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gil, L.S.; Maupoey, P.F. An integrated approach for pineapple waste valorisation. Bioethanol production and bromelain extraction from pineapple residues. J. Clean. Prod. 2018, 172, 1224–1231. [Google Scholar]
- Schieber, A.; Stintzing, F.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Roda, A.; De Faveri, D.M.; Giacosa, S.; Dordoni, R.; Lambri, M. Effect of pre-treatments on the saccharification of pineapple waste as a potential source for vinegar production. J. Clean. Prod. 2016, 112, 4477–4484. [Google Scholar] [CrossRef]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Pineapple wastes: A potential source for bromelain extraction. Food Bioprod. Process. 2012, 90, 385–391. [Google Scholar] [CrossRef]
- Dighe, N.S.; Pattan, S.R.; Merekar, A.N.; Laware, R.B.; Bhawar, S.B.; Nirmal, S.N.; Musmade, D. Bromelain A Wonder Supplement: A Review. Pharmacologyonline 2010, 1, 11–18. [Google Scholar]
- Connolly, M.L.; Lovegrove, J.A.; Tuohy, K.M. Konjac glucomannan hydrolysate beneficially modulates bacterial composition and activity within the faecal microbiota. J. Funct. Foods 2010, 2, 219–224. [Google Scholar] [CrossRef]
- Mohammad, G.; Andreas, D.; Klaus, D. Isolation of polysaccharides from pineapple fruit pulp and their enzymatic liquifaction. Int. Food Res. J. 2010, 17, 193–203. [Google Scholar]
- Omojasola, P.F.; Jilani, O.P.; Ibiyemi, S. Cellulase production by some fungi cultured on pineapple waste. Nat. Sci. 2008, 6, 64–79. [Google Scholar]
- Selani, M.M.; Brazaca, S.G.C.; dos Santos Dias, C.T.; Ratnayake, W.S.; Flores, R.A.; Bianchini, A. Characterisation and potential application of pineapple pomace in an extruded product for fibre enhancement. Food Chem. 2014, 163, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Simas-Tosin, F.F.; de Souza, L.M.; Wagner, R.; Pereira, G.C.; Barraza, R.R.; Wendel, C.F.; Sassaki, G.L.; Iacomini, M.; Gorin, P.A. Structural characterization of a glucuronoarabinoxylan from pineapple (Ananas comosus (L.) Merrill) gum exudate. Carbohydr. Polym. 2013, 94, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Shah, N.P. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int. Food Res. J. 2008, 15, 219–232. [Google Scholar]
- Pruthi, J.; Girdhari, L. Varietal trials in canning of pineapples. Cent. Food Technol. Res. Inst. 1955, 4, 284–285. [Google Scholar]
- Salunkhe, D.K.; Kadam, S. Handbook of Fruit Science and Technology: Production, Composition, Storage, and Processing; CRC Press/Taylor & Frances: Boca Raton, FL, USA, 1995. [Google Scholar]
- Srivastava, M.; Kapoor, V. Seed galactomannans: An overview. Chem. Biodiver. 2005, 2, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Coscueta, E.R.; Valetti, N.W.; Pastrana-Castro, L.M.; Teixeira, J.A.; Picó, G.A.; Pintado, M.M. Optimization of bromelain isolation from pineapple byproducts by polysaccharide complex formation. Food Hydrocoll. 2019, 87, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Campos, D.A.; Woitovich Valetti, N.; Oliveira, A.; Pastrana-Castro, L.M.; Teixeira, J.A.; Pintado, M.M.; Picó, G. Platform design for extraction and isolation of Bromelain: Complex formation and precipitation with carrageenan. Process. Biochem. 2016, 54, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Gullón, P.; Gullón, B.; Cardelle-Cobas, A.; Alonso, J.L.; Pintado, M.; Gomes, A.M. Effects of hemicellulose-derived saccharides on behavior of Lactobacilli under simulated gastrointestinal conditions. Int. Food. Res. J. 2014, 64, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Pinto, J.; Pereira, C.; Malcata, F.X.; Pacheco, M.B.; Gomes, A.M.; Pintado, M. In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food Bioprod. Process. 2015, 95, 96–105. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.; Irwin, J. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef] [Green Version]
- Campos, D.A.; Coscueta, E.R.; Vilas-Boas, A.A.; Silva, S.; Teixeira, J.A.; Pastrana-Castro, L.M.; Pintado, M.M. Impact of functional flours from pineapple by-products on human intestinal microbiota. J. Funct. Foods 2020, 67, 103830. [Google Scholar] [CrossRef]
- Madureira, A.R.; Campos, D.A.; Oliveira, A.; Sarmento, B.; Pintado, M.M.; Gomes, A.M. Insights into the protective role of solid lipid nanoparticles on rosmarinic acid bioactivity during exposure to simulated gastrointestinal conditions. Colloids Surf. B 2016, 139, 277–284. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC−fluorescein) assay. J. Agric. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Campos, D.A.; Madureira, A.R.; Sarmento, B.; Gomes, A.M.; Pintado, M.M. Stability of bioactive solid lipid nanoparticles loaded with herbal extracts when exposed to simulated gastrointestinal tract conditions. Int. Food Res. J. 2015, 78, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Damen, B.; Verspreet, J.; Pollet, A.; Broekaert, W.F.; Delcour, J.A.; Courtin, C.M. Prebiotic effects and intestinal fermentation of cereal arabinoxylans and arabinoxylan oligosaccharides in rats depend strongly on their structural properties and joint presence. Mol. Nutr. Food Res. 2011, 55, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Huebner, J.; Wehling, R.; Hutkins, R. Functional activity of commercial prebiotics. Int. Dairy J. 2007, 17, 770–775. [Google Scholar] [CrossRef]
- Kurdi, P.; Hansawasdi, C. Assessment of the prebiotic potential of oligosaccharide mixtures from rice bran and cassava pulp. LWT Food Sci. Technol. 2015, 63, 1288–1293. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Shah, N.P. Probiotics—From Metchnikoff to bioactives. Int. Dairy J. 2008, 18, 714–728. [Google Scholar] [CrossRef]
- Østlie, H.M.; Helland, M.H.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria in milk. Int. J. Food Microbiol. 2003, 87, 17–27. [Google Scholar] [CrossRef]
- Southgate, D. Digestion and metabolism of sugars. Am. J. Clin. Nutr. 1995, 62, 203S–210S. [Google Scholar] [CrossRef]
- Campos, D.A.; Ribeiro, T.B.; Teixeira, J.A.; Pastrana, L.; Pintado, M. Integral valorization of pineapple (Ananas comosus L.) by-products through a green chemistry approach towards added value ingredients. Foods 2020, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.G.; Harris, P.J. Ferulic acid is esterified to glucuronoarabinoxylans in pineapple cell walls. Phytochemistry 2001, 56, 513–519. [Google Scholar] [CrossRef]
- Steingass, C.B.; Jutzi, M.; Müller, J.; Carle, R.; Schmarr, H.G. Ripening-dependent metabolic changes in the volatiles of pineapple (Ananas comosus (L.) Merr.) fruit: II. Multivariate statistical profiling of pineapple aroma compounds based on comprehensive two-dimensional gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2609–2624. [Google Scholar] [CrossRef]
- Difonzo, G.; Vollmer, K.; Caponio, F.; Pasqualone, A.; Carle, R.; Steingass, C. Characterisation and classification of pineapple (Ananas comosus [L.] Merr.) juice from pulp and peel. Food Control. 2019, 96, 260–270. [Google Scholar] [CrossRef]
- Changpraykaew, S.; Petchlert, C. Digestive effect on antioxidant activity of three commercial fruit juices. In Proceedings of the Burapha University International Conference, Bangsaen Chonburi, Thailand, 10–12 July 2015; Burapha University Chon Buri: Chon Buri, Thailand, 2015; pp. 1064–1070. [Google Scholar]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Pérez-Sacristán, B.; Blanco-Navarro, I.; Blázquez-García, S. Comparative in vitro bioaccessibility of carotenoids from relevant contributors to carotenoid intake. J. Agric. Food Chem. 2007, 55, 6387–6394. [Google Scholar] [CrossRef]
- Sanchez, J.; Marzorati, M.; Grootaert, C.; Baran, M.; Van Craeyveld, V.; Courtin, C.; Broekaert, W.; Delcour, J.; Verstraete, W.; Van de Wiele, T. Arabinoxylan-oligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the Simulator of Human Intestinal Microbial Ecosystem. Microb. Biotechnol. 2009, 2, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]

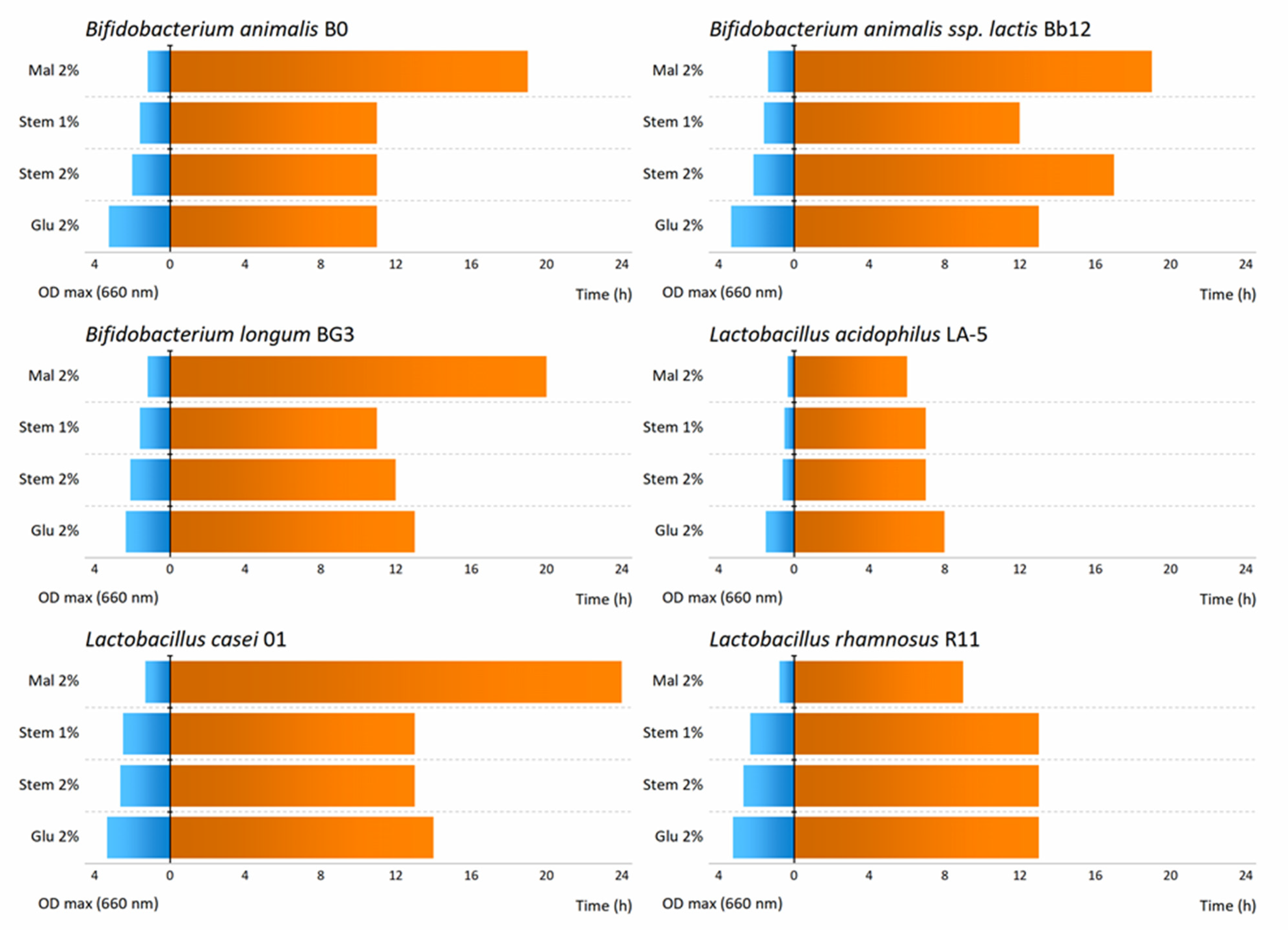

| Probiotic Strains | Maximum Growth Rate (µmax/h) | |||
|---|---|---|---|---|
| Positive Control (w/v) | Pineapple Extract (w/v) | |||
| GLU 2% | MAL 2% | 2% | 1% | |
| Bifidobacterium animalis B0 | 0.4347 | 0.0682 | 0.2587 | 0.2022 |
| Bifidobacterium animalis ssp. lactis Bb12 | 0.4725 | 0.0960 | 0.2425 a | 0.2011 a |
| Bifidobacterium longum BG3 | 0.2511 a | 0.0679 | 0.2646 a | 0.1955 |
| Lactobacillus acidophilus LA-5 | 0.2840 | 0.0340 | 0.0877 a | 0.0664 a |
| Lactobacillus casei 01 | 0.4748 | 0.0952 | 0.3366 a | 0.3120 a |
| Lactobacillus rhamnosus R11 | 0.4069 | 0.0383 | 0.3198 | 0.2652 |
| Probiotic Strains | Parameter | Incubation Time (h) | Positive Controls (w/v) | Stem 2% (w/v) | |
|---|---|---|---|---|---|
| GLU 2% | FOS 2% | ||||
| Bifidobacterium animalis B0 | pH value | 0 | 5.97 ± 0.00 | 6.80 ± 0.03 | 6.78 ± 0.01 |
| 24 | 6.00 ± 0.00 | 6.20 ± 0.01 | 6.08 ± 0.02 | ||
| 48 | 4.23 ± 0.01 | 5.92 ± 0.01 | 5.83 ± 0.00 | ||
| Glucose | 0 | 2.67 ± 0.04 | 0.55 ± 0.01 | 0.53 ± 0.01 | |
| 24 | 3.32 ± 0.01 | 0.85 ± 0.01 | 3.36 ± 0.01 | ||
| 48 | 1.54 ± 0.01 | 0.80 ± 0.00 | 2.12 ± 0.04 | ||
| Fructose | 0 | 0.77 ± 0.01 | 0.00 ± 0.00 | 0.13 ± 0.05 | |
| 24 | 2.60 ± 0.04 | 0.69 ± 0.00 | 2.67 ± 0.04 | ||
| 48 | 2.29 ± 0.01 | 1.00 ± 0.02 | 2.73 ± 0.09 | ||
| Lactic acid | 0 | 0.45 ± 0.03 | 0.24 ± 0.02 | 0.07 ± 0.00 | |
| 24 | 0.46 ± 0.01 | 0.26 ± 0.01 | 0.47 ± 0.01 | ||
| 48 | 5.82 ± 0.08 | 0.31 ± 0.02 | 5.41 ± 0.02 | ||
| Acetic acid | 0 | 1.64 ± 0.07 | 1.74 ± 0.12 | 1.40 ± 0.25 | |
| 24 | 1.64 ± 0.00 | 1.85 ± 0.00 | 1.66 ± 0.01 | ||
| 48 | 2.02 ± 0.01 | 1.80 ± 0.03 | 1.73 ± 0.01 | ||
| Citric acid | 0 | 0.77 ± 0.06 | 1.04 ± 0.04 | 0.87 ± 0.00 | |
| 24 | 0.73 ± 0.01 | 0.98 ± 0.01 | 0.75 ± 0.00 | ||
| 48 | 0.74 ± 0.00 | 0.95 ± 0.01 | 0.68 ± 0.00 | ||
| Formic acid | 0 | 0.21 ± 0.01 | 0.03 ± 0.00 | 0.10 ± 0.00 | |
| 24 | 0.24 ± 0.02 | 0.16 ± 0.03 | 0.24 ± 0.01 | ||
| 48 | 0.20 ± 0.03 | 0.27 ± 0.06 | 0.12 ± 0.03 | ||
| Lactobacillus rhamnosus R11 | pH value | 0 | 5.98 ± 0.01 | 6.82 ± 0.02 | 6.59 ± 0.00 |
| 24 | 6.04 ± 0.00 | 6.11 ± 0.01 | 5.53 ± 0.01 | ||
| 48 | 4.41 ± 0.01 | 5.68 ± 0.01 | 5.44 ± 0.01 | ||
| Glucose | 0 | 1.15 ± 0.07 | 0.07 ± 0.00 | 1.15 ± 0.08 | |
| 24 | 3.36 ± 0.01 | 0.05 ± 0.00 | 1.01 ± 0.00 | ||
| 48 | 2.12 ± 0.04 | 0.03 ± 0.00 | 0.48 ± 0.02 | ||
| Fructose | 0 | 1.51 ± 0.16 | 0.01 ± 0.00 | 1.51 ± 0.16 | |
| 24 | 2.67 ± 0.04 | 0.25 ± 0.00 | 0.00 ± 0.00 | ||
| 48 | 2.73 ±0.09 | 0.24 ± 0.00 | 0.00 ± 0.00 | ||
| Lactic acid | 0 | 1.02 ± 0.01 | 0.10 ± 0.01 | 1.02 ± 0.01 | |
| 24 | 0.47 ± 0.01 | 0.75 ± 0.01 | 1.24 ± 0.01 | ||
| 48 | 5.41 ± 0.02 | 0.72 ± 0.04 | 1.19 ± 0.01 | ||
| Acetic acid | 0 | 1.96 ± 0.02 | 1.40 ± 0.02 | 1.96 ± 0.02 | |
| 24 | 1.66 ± 0.01 | 2.13 ± 0.01 | 2.04 ± 0.00 | ||
| 48 | 1.73 ± 0.01 | 2.14 ± 0.01 | 1.91 ± 0.03 | ||
| Citric acid | 0 | 0.79 ± 0.02 | 0.90 ± 0.01 | 2.95 ± 0.96 | |
| 24 | 0.75 ± 0.02 | 0.17 ± 0.01 | 0.15 ± 0.02 | ||
| 48 | 0.68 ± 0.00 | 0.16 ± 0.00 | 0.12 ± 0.00 | ||
| Formic acid | 0 | 0.47 ± 0.09 | 0.15 ± 0.01 | 0.47 ± 0.09 | |
| 24 | 0.24 ± 0.01 | 0.19 ± 0.03 | 0.24 ± 0.01 | ||
| 48 | 0.15 ± 0.03 | 0.28 ± 0.04 | 0.24 ± 0.02 | ||
| Pineapple By-Product | GIT Stage | ABTS Assay (mg AAE/100 g) | ORAC Assay (mg TE/100 g) | Folin–Ciocalteu (mg GAE/100 g) |
|---|---|---|---|---|
| Peel liquid fraction | T0 | 430.9 ± 9.2 | 21.7 ± 0.6 | 476.1 ± 10.3 |
| After stomach | 352.8 ± 12.9 a | 14.7 ± 0.5 a | 783.9 ± 21.3 a | |
| After intestine | 883.5 ± 24.0 a | 16.2 ± 0.3 a | 680.3 ± 27.7 a | |
| Stem liquid fraction | T0 | 369.6 ± 8.9 | 19.5 ± 1.4 | 529.7 ± 16.6 |
| After stomach | 452.1 ± 27.7 a | 25.9 ± 1.8 a | 708.8 ± 7.3 a | |
| After intestine | 826.3 ± 15.0 a | 29.3 ± 0.7 a | 590.7 ± 39.7 a |
| Pineapple By-Product | GIT Stage | Chlorogenic Acid (mg/100 g DB) | Caffeic Acid (mg/100 g DB) | Coumaric Acid (mg/100 g DB) | Ferulic Acid (mg/100 g DB) |
|---|---|---|---|---|---|
| Peel liquid fraction | T0 | 16.96 ± 1.42 | 34.54 ± 4.46 | 0.00 ± 0.00 | 26.68 ± 3.80 |
| After stomach | 21.28 ± 0.68 | 12.60 ± 1.4 | 0.00 ± 0.00 | 2.68 ± 0.12 | |
| After intestine | 133.58 ± 12.44 | 11.46 ± 0.76 | 0.00 ± 0.00 | 0.50 ± 0.44 | |
| Stem liquid fraction | T0 | 106.96 ± 1.9 | 14.62 ± 0.58 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| After stomach | 26.16 ± 0.72 | 47.24 ± 1.84 | 0.00 ± 0.00 | 13.58 ± 0.34 | |
| After intestine | 141.58 ± 0.84 | 82.16 ± 1.64 | 43.74 ± 0.72 | 19.14 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-García, R.; Vilas-Boas, A.A.; Oliveira, A.; Amorim, M.; Teixeira, J.A.; Pastrana, L.; Pintado, M.M.; Campos, D.A. Impact of Simulated Human Gastrointestinal Digestion on the Bioactive Fraction of Upcycled Pineapple By-Products. Foods 2022, 11, 126. https://doi.org/10.3390/foods11010126
Gómez-García R, Vilas-Boas AA, Oliveira A, Amorim M, Teixeira JA, Pastrana L, Pintado MM, Campos DA. Impact of Simulated Human Gastrointestinal Digestion on the Bioactive Fraction of Upcycled Pineapple By-Products. Foods. 2022; 11(1):126. https://doi.org/10.3390/foods11010126
Chicago/Turabian StyleGómez-García, Ricardo, Ana A. Vilas-Boas, Ana Oliveira, Manuela Amorim, José A. Teixeira, Lorenzo Pastrana, Maria Manuela Pintado, and Débora A. Campos. 2022. "Impact of Simulated Human Gastrointestinal Digestion on the Bioactive Fraction of Upcycled Pineapple By-Products" Foods 11, no. 1: 126. https://doi.org/10.3390/foods11010126
APA StyleGómez-García, R., Vilas-Boas, A. A., Oliveira, A., Amorim, M., Teixeira, J. A., Pastrana, L., Pintado, M. M., & Campos, D. A. (2022). Impact of Simulated Human Gastrointestinal Digestion on the Bioactive Fraction of Upcycled Pineapple By-Products. Foods, 11(1), 126. https://doi.org/10.3390/foods11010126