Avian Eggshell Membrane as a Novel Biomaterial: A Review
Abstract
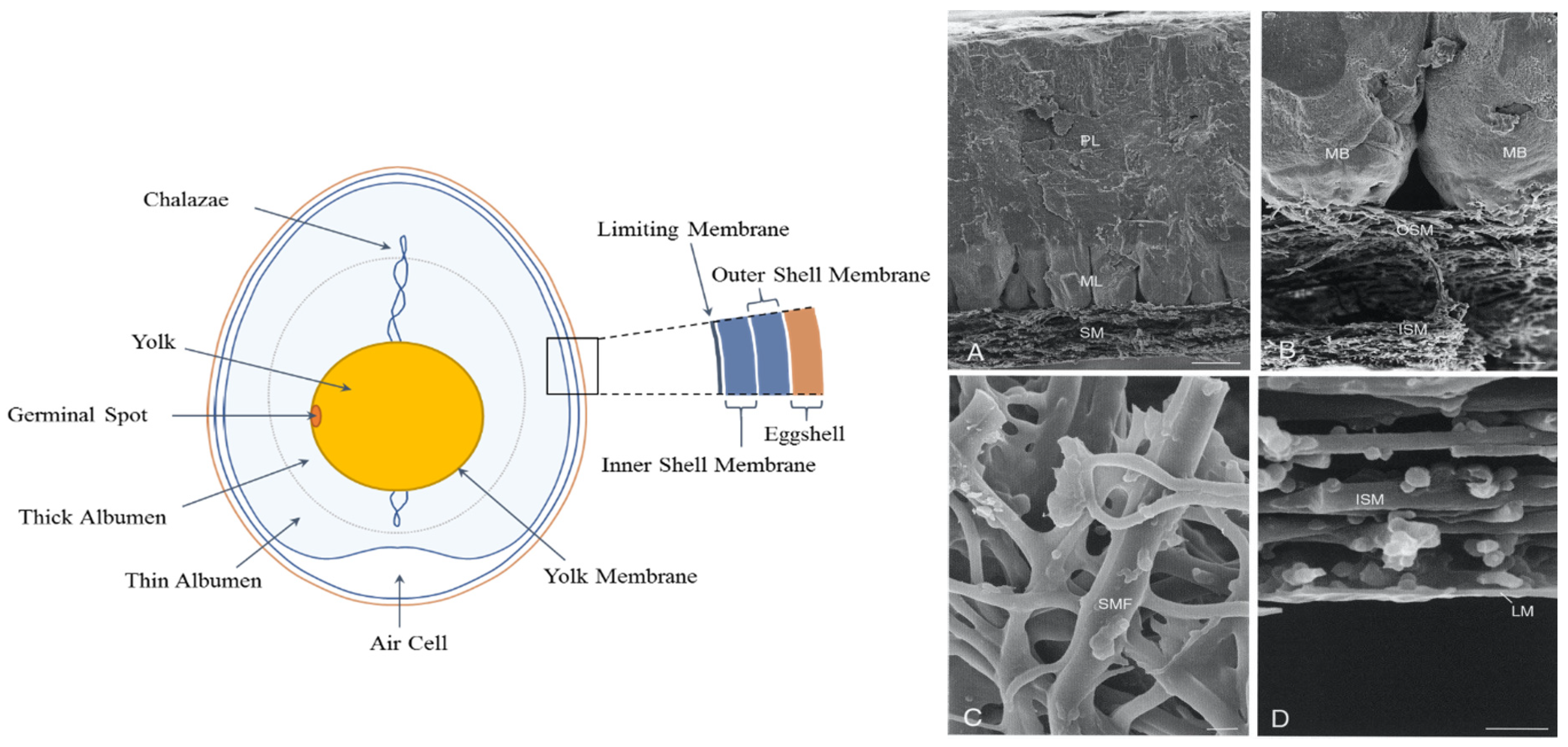
:1. Structure and Chemical Composition of the Eggshell and Eggshell Membrane
2. Isolation and Solubilization of the Eggshell Membrane
3. Application of Eggshell Membrane as a Novel Biomaterial
3.1. ESM for Joint Health
3.2. ESM for Wound Healing
3.3. ESM for Gut Health
3.4. ESM for Anti-Inflammatory and Antioxidant Activity
3.5. ESM for the Control of Bacteria
3.6. ESM for Biomineralization
3.7. ESM for Immobilisation
3.8. ESM for Tissue Engineering
3.9. ESM for Food Packaging
3.10. ESM for Biosorbent Activities
4. Safety Evaluation of Eggshell Membrane
5. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hincke, M.T.; Nys, Y.; Gautron, J. The role of matrix proteins in eggshell formation. J. Poult. Sci. 2010, 47, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Mann, K.; Maek, B.; Olsen, J.V. Proteomic analysis of the acidsoluble organic matrix of the chicken calcified eggshell layer. Proteomics 2006, 6, 3801–3810. [Google Scholar] [CrossRef]
- Nakano, T.; Ikawa, N.I.; Ozimek, L. Chemical composition of chicken eggshell and shell membranes. Poult. Sci. 2003, 82, 510–514. [Google Scholar] [CrossRef]
- Gautron, J.; Hincke, M.T.; Panheleux, M.; Garcia-Ruiz, J.M.; Boldicke, T.; Nys, Y. Ovotransferrin is a matrix protein of the hen eggshell membranes and basal calcified layer. Connect. Tissue Res. 2001, 42, 255–267. [Google Scholar] [CrossRef]
- Hincke, M.T.; Gautron, J.; Panheleux, M.; Garcia-Ruiz, J.; McKee, M.D.; Nys, Y. Identification and localization of lysozyme as a component of eggshell membranes and eggshell matrix. Matrix Biol. 2000, 19, 443–453. [Google Scholar] [CrossRef]
- Nys, Y.; Gautron, J.; Garcia-Ruiz, J.M.; Hincke, M.T. Avian eggshell mineralization: Biochemical and functional characterization of matrix proteins. Comptes Rendus Palevol 2004, 3, 549–562. [Google Scholar] [CrossRef]
- Arias, J.L.; Fink, D.J.; Xiao, S.-Q.; Heuer, A.H.; Caplan, A.I. Biomineralization and Eggshells: Cell-Mediated Acellular Compartments of Mineralized Extracellular Matrix. In International Review of Cytology; Jeon, K.W., Jarvik, J., Eds.; Academic Press: Cambridge, MA, USA, 1993; Volume 145, pp. 217–250. [Google Scholar]
- Li, Y.; Li, Y.; Liu, S.; Tang, Y.; Mo, B.; Liao, H. New zonal structure and transition of the membrane to mammillae in the eggshell of chicken Gallus domesticus. J. Struct. Biol. 2018, 203, 162–169. [Google Scholar] [CrossRef]
- Lee, S.-M.; Grass, G.; Kim, G.-M.; Dresbach, C.; Zhang, L.; Gösele, U.; Knez, M. Low-temperature ZnO atomic layer deposition on biotemplates: Flexible photocatalytic ZnO structures from eggshell membranes. Phys. Chem. Chem. Phys. 2009, 11, 3608–3614. [Google Scholar] [CrossRef] [PubMed]
- Bellairs, R.; Boyde, A. Scanning electron microscopy of the shell membranes of the hen’s egg. Z. Für Zellforsch. Mikrosk. Anat. 1969, 96, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Liong, J.; Frank, J.F.; Bailey, S. Visualization of Eggshell Membranes and Their Interaction with Salmonella enteritidis Using Confocal Scanning Laser Microscopy. J. Food Prot. 1997, 60, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, S.; Nie, F.; Feng, L.; Zhu, G.; Jiang, L. Elaborate architecture of the hierarchical hen’s eggshell. Nano Res. 2011, 4, 171–179. [Google Scholar] [CrossRef]
- Baláž, M. Eggshell membrane biomaterial as a platform for applications in materials science. Acta Biomater. 2014, 10, 3827–3843. [Google Scholar] [CrossRef]
- Chowdhury, S.D. Shell membrane protein system in relation to lathyrogen toxicity and copper deficiency. World’s Poult. Sci. J. 1990, 46, 153–169. [Google Scholar] [CrossRef]
- D’Ambrosio, C.; Arena, S.; Scaloni, A.; Guerrier, L.; Boschetti, E.; Mendieta, M.E.; Citterio, A.; Righetti, P.G. Exploring the Chicken Egg White Proteome with Combinatorial Peptide Ligand Libraries. J. Proteome Res. 2008, 7, 3461–3474. [Google Scholar] [CrossRef]
- Leach, R.M. Biochemistry of the Organic Matrix of the Eggshell. Poult. Sci. 1982, 61, 2040–2047. [Google Scholar] [CrossRef]
- Wong, M.; Hendrix, M.J.C.; von der Mark, K.; Little, C.; Stern, R. Collagen in the egg shell membranes of the hen. Dev. Biol. 1984, 104, 28–36. [Google Scholar] [CrossRef]
- Carrino, D.A.; Dennis, J.E.; Wu, T.M.; Arias, J.L.; Fernandez, M.S.; Rodriguez, J.P.; Fink, D.J.; Heuer, A.H.; Caplan, A.I. The avian eggshell extracellular matrix as a model for biomineralization. Connect. Tissue Res. 1996, 35, 325–329. [Google Scholar] [CrossRef]
- Arias, J.L.; Nakamura, O.; Fernández, M.S.; Wu, J.J.; Knigge, P.; Eyre, D.R.; Caplan, A.I. Role of type X collagen on experimental mineralization of eggshell membranes. Connect. Tissue Res. 1997, 36, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Schwarzbauer, J.E. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005, 24, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.F.; Wolinetz, C.D.; Killian, G.J. Influence of arginine-glycine-aspartic acid (RGD), integrins (αV and α5) and osteopontin on bovine sperm-egg binding, and fertilization in vitro. Theriogenology 2007, 67, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Nys, Y.; Gautron, J.; Mckee, M.D. Biochemical and functional characterisation of eggshell matrix proteins in hens. Worlds Poult. Sci. J. 2001, 57, 401–413. [Google Scholar] [CrossRef]
- Rønning, S.B.; Berg, R.S.; Høst, V.; Veiseth-Kent, E.; Wilhelmsen, C.R.; Haugen, E.; Suso, H.P.; Barham, P.; Schmidt, R.; Pedersen, M.E. Processed Eggshell Membrane Powder Is a Promising Biomaterial for Use in Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 8130. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Suso, H.P.; Maqbool, A.; Hincke, M.T. Processed eggshell membrane powder: Bioinspiration for an innovative wound healing product. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Padrão, T.; Coelho, C.C.; Costa, P.; Alegrete, N.; Monteiro, F.J.; Sousa, S.R. Combining local antibiotic delivery with heparinized nanohydroxyapatite/collagen bone substitute: A novel strategy for osteomyelitis treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111329. [Google Scholar] [CrossRef] [PubMed]
- Rose-Martel, M.; Smiley, S.; Hincke, M.T. Novel identification of matrix proteins involved in calcitic biomineralization. J. Proteom. 2015, 116, 81–96. [Google Scholar] [CrossRef]
- Jensen, T.; Dolatshahi-Pirouz, A.; Foss, M.; Baas, J.; Lovmand, J.; Duch, M.; Pedersen, F.S.; Kassem, M.; Bünger, C.; Søballe, K.; et al. Interaction of human mesenchymal stem cells with osteopontin coated hydroxyapatite surfaces. Colloids Surf. B Biointerfaces 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Gu, Y.; Jiang, C.; Chen, L. Osteonectin regulates the extracellular matrix mineralization of osteoblasts through P38 signaling pathway. J. Cell. Physiol. 2020, 235, 2220–2231. [Google Scholar] [CrossRef]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e919159. [Google Scholar] [CrossRef]
- Scatena, M.; Liaw, L.; Giachelli, C.M. Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2302–2309. [Google Scholar] [CrossRef] [Green Version]
- Hunter, G.K. Role of osteopontin in modulation of hydroxyapatite formation. Calcif. Tissue Int. 2013, 93, 348–354. [Google Scholar] [CrossRef]
- De Fusco, C.; Messina, A.; Monda, V.; Viggiano, E.; Moscatelli, F.; Valenzano, A.; Esposito, T.; Sergio, C.; Cibelli, G.; Monda, M.; et al. Osteopontin: Relation between Adipose Tissue and Bone Homeostasis. Stem Cells Int. 2017, 2017, 4045238. [Google Scholar] [CrossRef] [Green Version]
- Valenick, L.V.; Hsia, H.C.; Schwarzbauer, J.E. Fibronectin fragmentation promotes alpha4beta1 integrin-mediated contraction of a fibrin-fibronectin provisional matrix. Exp. Cell Res. 2005, 309, 48–55. [Google Scholar] [CrossRef]
- Lenselink, E.A. Role of fibronectin in normal wound healing. Int. Wound J. 2015, 12, 313–316. [Google Scholar] [CrossRef]
- Esparza, Y.; Ullah, A.; Wu, J. Molecular mechanism and characterization of self-assembly of feather keratin gelation. Int. J. Biol. Macromol. 2018, 107, 290–296. [Google Scholar] [CrossRef]
- Wang, S.; Taraballi, F.; Tan, L.P.; Ng, K.W. Human keratin hydrogels support fibroblast attachment and proliferation in vitro. Cell Tissue Res. 2012, 347, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Barman, H.K.; Nakayama, M.; Takami, Y.; Nakayama, T. Participation of histones, histone modifying enzymes and histone chaperones in vertebrate cell functions. Sub-Cell. Biochem. 2006, 40, 225–243. [Google Scholar]
- Doolin, T.; Amir, H.M.; Duong, L.; Rosenzweig, R.; Urban, L.A.; Bosch, M.; Pol, A.; Gross, S.P.; Siryaporn, A. Mammalian histones facilitate antimicrobial synergy by disrupting the bacterial proton gradient and chromosome organization. Nat. Commun. 2020, 3888, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y. Avian β-defensins expression for the innate immune system in hen reproductive organs. Poult. Sci. 2015, 94, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, H.; Yu, P.L. Avian antimicrobial peptides: The defense role of beta-defensins. Biochem. Biophys. Res. Commun. 2004, 323, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.M.; Esmaili, H.; Ansah, G.; Hincke, M.T. Ovocalyxin-36 is a pattern recognition protein in chicken eggshell membranes. PLoS ONE 2013, 8, e84112. [Google Scholar]
- Kovacsnolan, J.; Cordeiro, C.; Young, D.; Mine, Y.; Hincke, M. Ovocalyxin-36 is an effector protein modulating the production of proinflammatory mediators. Vet. Immunol. Immunopathol. 2014, 160, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, C.M.; Hincke, M.T. Quantitative proteomics analysis of eggshell membrane proteins during chick embryonic development. J. Proteom. 2015, 130, 11–25. [Google Scholar] [CrossRef]
- Makkar, S.K.; Liyanage, R.; Kannan, L.; Packialakshmi, B.; Lay, J.; Rath, N.C. Chicken egg shell membrane associated proteins and peptides. J. Agric. Food Chem. 2015, 63, 9888–9898. [Google Scholar] [CrossRef] [PubMed]
- Concha, M.; Vidal, A.; Giacaman, A.; Ojeda, J.; Pavicic, F.; Oyarzun-Ampuero, F.A.; Torres, C.; Cabrera, M.; Moreno-Villoslada, I.; Orellana, S.L. Aerogels made of chitosan and chondroitin sulfate at high degree of neutralization: Biological properties toward wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, X.; Cai, D.; Li, J.; Mu, Q.; Zhang, W.; Zhu, S.; Jiang, Y.; Shen, W.; Zhang, S.; et al. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhang, C.; Mo, H.; Zhang, H.; Qin, X.; Li, J.; Zhang, Z.; Richel, A. Fabrication of chondroitin sulfate calcium complex and its chondrocyte proliferation in vitro. Carbohydr. Polym. 2021, 254, 117282. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.L.; Mishra, M.K.; Moussienko, D.; Laflamme, N.; Rivest, S.; Ling, C.C.; Yong, V.W. Chondroitin sulfate proteoglycans as novel drivers of leucocyte infiltration in multiple sclerosis. Brain J. Neurol. 2018, 141, 1094–1110. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Khoshfetrat, A.B.; Eskandarnezhad, S.; Sani, N.F.; Ebrahimi, S. Sequential optimization strategy for hyaluronic acid extraction from eggshell and its partial characterization. J. Ind. Eng. Chem. 2014, 20, 4371–4376. [Google Scholar] [CrossRef]
- Vulganová, K.; Ürgeová, E. Extraction of hyaluronic acid from eggshell membranes. Curr. Opin. Biotechnol. 2013, 24, S106. [Google Scholar] [CrossRef]
- Sri Devi Kumari, T.; Prem Kumar, T. Synthesis of macroporous LiMn2O4 with avian egg membrane as a template. Ionics 2010, 16, 61–66. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, F.; Peng, Q.; Xu, S.; Lei, X.; Evans, D.G.; Duan, X. Layered double hydroxide/eggshell membrane: An inorganic biocomposite membrane as an efficient adsorbent for Cr(VI) removal. Chem. Eng. J. 2011, 166, 81–87. [Google Scholar] [CrossRef]
- Devi, P.S.; Banerjee, S.; Chowdhury, S.R.; Kumar, G.S. Eggshell membrane: A natural biotemplate to synthesize fluorescent gold nanoparticles. RSC Adv. 2012, 2, 11578–11585. [Google Scholar] [CrossRef]
- Ishikawa, S.-I.; Suyama, K.; Arihara, K.; Itoh, M. Uptake and recovery of gold ions from electroplating wastes using eggshell membrane. Bioresour. Technol. 2002, 81, 201–206. [Google Scholar] [CrossRef]
- Su, H.; Wang, N.; Dong, Q.; Zhang, D. Incubating lead selenide nanoclusters and nanocubes on the eggshell membrane at room temperature. J. Membr. Sci. 2006, 283, 7–12. [Google Scholar] [CrossRef]
- Marcet, I.; Salvadores, M.; Rendueles, M.; Díaz, M. The effect of ultrasound on the alkali extraction of proteins from eggshell membranes. J. Sci. Food Agric. 2018, 98, 1765–1772. [Google Scholar] [CrossRef]
- Lunge, S.; Thakre, D.; Kamble, S.; Labhsetwar, N.; Rayalu, S. Alumina supported carbon composite material with exceptionally high defluoridation property from eggshell waste. J. Hazard. Mater. 2012, 237–238, 161–169. [Google Scholar] [CrossRef]
- Su, H.; Han, J.; Wang, N.; Dong, Q.; Zhang, D.; Zhang, C. In situsynthesis of lead sulfide nanoclusters on eggshell membrane fibers by an ambient bio-inspired technique. Smart Mater. Struct. 2008, 17, 015045. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, N.; Xu, Q.; Harlina, P.W.; Ma, M. An Efficient Method for Co-purification of Eggshell Matrix Proteins OC-17, OC-116, and OCX-36. Korean J. Food Sci. Anim. Resour. 2016, 36, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Kodali, V.K.; Gannon, S.A.; Paramasivam, S.; Raje, S.; Polenova, T.; Thorpe, C. A Novel Disulfide-Rich Protein Motif from Avian Eggshell Membranes. PLoS ONE 2011, 6, e18187. [Google Scholar] [CrossRef]
- Baker, J.R.; Balch, D.A. A study of the organic material of hen’s-egg shell. Biochem. J. 1962, 82, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Crombie, G.; Snider, R.; Faris, B.; Franzblau, C. Lysine-derived cross-links in the egg shell membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 1981, 640, 365–367. [Google Scholar] [CrossRef]
- Sah, M.K.; Pramanik, K. Soluble-eggshell-membrane-protein-modified porous silk fibroin scaffolds with enhanced cell adhesion and proliferation properties. J. Appl. Polym. Sci. 2014, 131, 40138. [Google Scholar] [CrossRef]
- Liddington, R.C.; Ginsberg, M.H. Integrin activation takes shape. J. Cell Biol. 2002, 158, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Yu, J.; Guo, Z.X.; Zhang, L.X.; Li, Q. Natural Bioactive Material: A Preparation of Soluble Eggshell Membrane Protein. Macromol. Biosci. 2003, 3, 234–237. [Google Scholar] [CrossRef]
- Jia, J.; Liu, G.; Guo, Z.-X.; Yu, J.; Duan, Y. Preparation and Characterization of Soluble Eggshell Membrane Protein/PLGA Electrospun Nanofibers for Guided Tissue Regeneration Membrane. J. Nanomater. 2012, 2012, 282736. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.; Mohammadifar, M.A.; Mortazavian, A.M.; Rouhi, M.; Ghasemi, J.B.; Delshadian, Z. Extraction optimization of pepsin-soluble collagen from eggshell membrane by response surface methodology (RSM). Food Chem. 2016, 190, 186. [Google Scholar] [CrossRef]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Rong, T.; Mine, Y. Antioxidant activity of enzymatic hydrolysates from eggshell membrane proteins and its protective capacity in human intestinal epithelial Caco-2 cells. J. Funct. Foods 2014, 10, 35–45. [Google Scholar] [CrossRef]
- Ruff, K.J.; Morrison, D.; Duncan, S.A.; Back, M.; Aydogan, C.; Theodosakis, J. Beneficial effects of natural eggshell membrane versus placebo in exercise-induced joint pain, stiffness, and cartilage turnover in healthy, postmenopausal women. Clin. Interv. Aging 2018, 13, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Hewlings, S.; Kalman, D.; Schneider, L.V. A Randomized, Double-Blind, Placebo-Controlled, Prospective Clinical Trial Evaluating Water-Soluble Chicken Eggshell Membrane for Improvement in Joint Health in Adults with Knee Osteoarthritis. J. Med. Food 2019, 22, 875–884. [Google Scholar] [CrossRef] [Green Version]
- Kiers, J.L.; Bult, J.H.F. Mildly Processed Natural Eggshell Membrane Alleviates Joint Pain Associated with Osteoarthritis of the Knee: A Randomized Double-Blind Placebo-Controlled Study. J. Med. Food 2020, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.M.; Gopinathan, J.; Senthil Kumar, R.; Sathish Kumar, G.; Shanthakumari, S.; Sahanand, K.S.; Bhattacharyya, A.; Selvakumar, R. Tissue engineering of human knee meniscus using functionalized and reinforced silk-polyvinyl alcohol composite three-dimensional scaffolds: Understanding the in vitro and in vivo behavior. J. Biomed. Mater. Res. Part A 2018, 106, 1722–1731. [Google Scholar] [CrossRef]
- Adali, T.; Kalkan, R.; Karimizarandi, L. The chondrocyte cell proliferation of a chitosan/silk fibroin/egg shell membrane hydrogels. Int. J. Biol. Macromol. 2019, 124, 541–547. [Google Scholar] [CrossRef]
- Ohto-Fujita, E.; Shimizu, M.; Sano, S.; Kurimoto, M.; Yamazawa, K.; Atomi, T.; Sakurai, T.; Murakami, Y.; Takami, T.; Murakami, T.; et al. Solubilized eggshell membrane supplies a type III collagen-rich elastic dermal papilla. Cell Tissue Res. 2019, 376, 123–135. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Suso, H.P.; Hincke, M.T. Experimental datasets on processed eggshell membrane powder for wound healing. Data Brief 2019, 26, 104457. [Google Scholar] [CrossRef]
- Vuong, T.T.; Rønning, S.B.; Ahmed, T.A.E.; Brathagen, K.; Høst, V.; Hincke, M.T.; Suso, H.P.; Pedersen, M.E. Processed eggshell membrane powder regulates cellular functions and increase MMP-activity important in early wound healing processes. PLoS ONE 2018, 13, e0201975. [Google Scholar]
- Jung, J.Y.; Yun, H.C.; Kim, T.M.; Joo, J.W.; Song, I.S.; Rah, Y.C.; Chang, J.; Im, G.J.; Choi, J. Analysis of Effect of Eggshell Membrane Patching for Moderate-to-Large Traumatic Tympanic Membrane Perforation. J. Audiol. Otol. 2017, 21, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Jun, H.J.; Oh, K.H.; Yoo, J.; Han, W.G.; Chang, J.; Jung, H.H.; Choi, J. A new patch material for tympanic membrane perforation by trauma: The membrane of a hen egg shell. Acta Oto-Laryngol. 2014, 134, 250–254. [Google Scholar] [CrossRef]
- Li, J.; Zhai, D.; Lv, F.; Yu, Q.; Ma, H.; Yin, J.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016, 36, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Luo, G.; Wang, Y.; Xu, R.; Wang, Y.; He, W.; Tan, J.; Xing, M.; Wu, J. Nano-silver-decorated microfibrous eggshell membrane: Processing, cytotoxicity assessment and optimization, antibacterial activity and wound healing. Sci. Rep. 2017, 7, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Hanate, M.; Aw, W.; Itoh, H.; Saito, K.; Kobayashi, S.; Hachimura, S.; Fukuda, S.; Tomita, M.; Hasebe, Y.; et al. Eggshell membrane powder ameliorates intestinal inflammation by facilitating the restitution of epithelial injury and alleviating microbial dysbiosis. Sci. Rep. 2017, 7, 43993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramli, N.S.; Jia, H.; Sekine, A.; Lyu, W.; Furukawa, K.; Saito, K.; Hasebe, Y.; Kato, H. Eggshell membrane powder lowers plasma triglyceride and liver total cholesterol by modulating gut microbiota and accelerating lipid metabolism in high-fat diet-fed mice. Food Sci. Nutr. 2020, 8, 2512–2523. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Rupa, P.; Jiang, B.; Mine, Y. Hydrolysate from eggshell membrane ameliorates intestinal inflammation in mice. Int. J. Mol. Sci. 2014, 15, 22728–22742. [Google Scholar] [CrossRef] [Green Version]
- Kulshreshtha, G.; Ahmed, T.A.E.; Wu, L.; Diep, T.; Hincke, M.T. A novel eco-friendly green approach to produce particalized eggshell membrane (PEM) for skin health applications. Biomater. Sci. 2020, 8, 5346–5361. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.F.; Ruff, K.J.; Jensen, G.S. Effects of natural eggshell membrane (NEM) on cytokine production in cultures of peripheral blood mononuclear cells: Increased suppression of tumor necrosis factor-α levels after in vitro digestion. J. Med. Food 2012, 15, 360–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuong, T.T.; Rønning, S.B.; Suso, H.-P.; Schmidt, R.; Prydz, K.; Lundström, M.; Moen, A.; Pedersen, M.E. The extracellular matrix of eggshell displays anti-inflammatory activities through NF-κB in LPS-triggered human immune cells. J. Inflamm. Res. 2017, 10, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Jinhee, Y.; Kimoon, P.; Youngji, Y.; Jongkeun, K.; Yang, H.; Youngjae, S. Effects of Egg Shell Membrane Hydrolysates on Anti-Inflammatory, Anti-Wrinkle, Anti-Microbial Activity and Moisture-Protection. Hangug Chugsan Sigpum Haghoeji Korean J. Food Sci. Anim. Resour. 2014, 34, 26–32. [Google Scholar]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Rong, T.; Mine, Y. Peptides derived from eggshell membrane improve antioxidant enzyme activity and glutathione synthesis against oxidative damage in Caco-2 cells. J. Funct. Foods 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Production and characterization of functional properties of protein hydrolysates from egg shell membranes by lactic acid bacteria fermentation. J. Food Sci. Technol. 2017, 54, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- Nagamalli, H.; Sitaraman, M.; Kandalai, K.K.; Mudhole, G.R. Chicken egg shell as a potential substrate for production of alkaline protease by Bacillus altitudinis GVC11 and its applications. 3 Biotech 2017, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.C.; Zhao, J.Y.; Ahn, D.U.; Jin, Y.G.; Huang, X. Separation and Identification of Highly Efficient Antioxidant Peptides from Eggshell Membrane. Antioxidants 2019, 8, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Cai, Z.; Ahn, D.U.; Huang, X. Development of an antibacterial nanobiomaterial for wound-care based on the absorption of AgNPs on the eggshell membrane. Colloids Surf. B Biointerfaces 2019, 183, 110449. [Google Scholar] [CrossRef] [PubMed]
- Preda, N.; Costas, A.; Beregoi, M.; Apostol, N.; Kuncser, A.; Curutiu, C.; Iordache, F.; Enculescu, I. Functionalization of eggshell membranes with CuO-ZnO based p-n junctions for visible light induced antibacterial activity against Escherichia coli. Sci. Rep. 2020, 10, 20960. [Google Scholar] [CrossRef]
- Li, X.; Ma, M.; Ahn, D.U.; Huang, X. Preparation and characterization of novel eggshell membrane-chitosan blend films for potential wound-care dressing: From waste to medicinal products. Int. J. Biol. Macromol. 2019, 123, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Armitage, O.E.; Strange, D.; Oyen, M.L. Biomimetic calcium carbonate-gelatin composites as a model system for eggshell mineralization. J. Mater. Res. 2012, 27, 3157–3164. [Google Scholar] [CrossRef]
- Kirsch, T.; Swoboda, B.; von der Mark, K. Ascorbate independent differentiation of human chondrocytes in vitro: Simultaneous expression of types I and × collagen and matrix mineralization. Differentiation 1992, 52, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Ji, X.; Banks, C.E.; Song, J. Flower-like agglomerates of hydroxyapatite crystals formed on an egg-shell membrane. Colloids Surf. B Biointerfaces 2011, 82, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.S.; Valenzuela, F.; Arias, J.I.; Neira-Carrillo, A.; Arias, J.L. Is the snail shell repair process really influenced by eggshell membrane as a template of foreign scaffold? J. Struct. Biol. 2016, 196, 187–196. [Google Scholar] [CrossRef]
- Xu, Z.; Neoh, K.G.; Kishen, A. A biomimetic strategy to form calcium phosphate crystals on type I collagen substrate. Mater. Sci. Eng. C 2010, 30, 822–826. [Google Scholar] [CrossRef]
- Arias, J.L.; Silva, K.; Neira-Carrillo, A.; Ortiz, L.; Fernández, M. Polycarboxylated Eggshell Membrane Scaffold as Template for Calcium Carbonate Mineralization. Crystals 2020, 10, 797. [Google Scholar] [CrossRef]
- D’Souza, S.F.; Kumar, J.; Jha, S.K.; Kubal, B.S. Immobilization of the urease on eggshell membrane and its application in biosensor. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 850–854. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, H.; Fan, Z.; Li, M.; Du, P.; Liu, C.; Li, Y.; Li, H.; Cao, H. A Highly Sensitive and Selective Hydrogen Peroxide Biosensor Based on Gold Nanoparticles and Three-Dimensional Porous Carbonized Chicken Eggshell Membrane. PLoS ONE 2015, 10, e0130156. [Google Scholar]
- Li, Y.; Wang, A.; Bai, Y.; Wang, S. Acriflavine-immobilized eggshell membrane as a new solid-state biosensor for Sudan I-IV detection based on fluorescence resonance energy transfer. Food Chem. 2017, 237, 966–973. [Google Scholar] [CrossRef]
- Golafshan, N.; Gharibi, H.; Kharaziha, M.; Fathi, M. A facile one-step strategy for development of a double network fibrous scaffold for nerve tissue engineering. Biofabrication 2017, 9, 025008. [Google Scholar] [CrossRef]
- Golafshan, N.; Kharaziha, M.; Alehosseini, M. A three-layered hollow tubular scaffold as an enhancement of nerve regeneration potential. Biomed. Mater. 2018, 13, 065005. [Google Scholar] [CrossRef]
- Li, Q.; Bai, Y.; Jin, T.; Wang, S.; Cui, W.; Stanciulescu, I.; Yang, R.; Nie, H.; Wang, L.; Zhang, X. Bioinspired Engineering of Poly(ethylene glycol) Hydrogels and Natural Protein Fibers for Layered Heart Valve Constructs. ACS Appl. Mater. Interfaces 2017, 9, 16524–16535. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Napiwocki, B.; Xu, Y.; Zhang, J.; Zhang, X.; Wang, X.; Crone, W.C.; Li, Q.; Turng, L.S. Wavy small-diameter vascular graft made of eggshell membrane and thermoplastic polyurethane. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110311. [Google Scholar] [CrossRef] [PubMed]
- Umaraw, P.; Munekata, P.E.; Verma, A.K.; Barba, F.J.; Singh, V.; Kumar, P.; Lorenzo, P. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Reza, M.; Mohammad, A.M.; Milad, R.; Mohaddeseh, K.; Amir, M.M.; Ehsan, S.; Sara, H. Physico-mechanical and structural properties of eggshell membrane gelatin-chitosan blend edible films. Int. J. Biol. Macromol. 2018, 107, 406–412. [Google Scholar]
- Li, L.; Xia, N.; Zhang, H.; Li, T.; Zhang, H.; Chi, Y.; Zhang, Y.; Liu, X.; Li, H. Structure and properties of edible packaging film prepared by soy protein isolate-eggshell membrane conjugates loaded with Eugenol. Int. J. Food Eng. 2020, 16, 20200099. [Google Scholar] [CrossRef]
- Xin, Y.; Li, C.; Liu, J.; Liu, J.; Liu, Y.; He, W.; Gao, Y. Adsorption of heavy metal with modified eggshell membrane and the in situ synthesis of Cu-Ag/modified eggshell membrane composites. R. Soc. Open Sci. 2018, 5, 180532. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghouti, M.A.; Khan, M. Eggshell membrane as a novel bio sorbent for remediation of boron from desalinated water. J. Environ. Manag. 2018, 207, 405–416. [Google Scholar] [CrossRef]
- Mirzaei, S.; Javanbakht, V. Dye removal from aqueous solution by a novel dual cross-linked biocomposite obtained from mucilage of Plantago Psyllium and eggshell membrane. Int. J. Biol. Macromol. 2019, 134, 1187–1204. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, X.; Xu, H.; Weng, Y.; Chen, Y. Fabrication of recyclable magnetic biosorbent from eggshell membrane for efficient adsorption of dye. Environ. Technol. 2020, 41, 1–13. [Google Scholar] [CrossRef]
- Li, Y.; Wang, A.; Bai, Y.; Wang, S. Evaluation of a mixed anionic-nonionic surfactant modified eggshell membrane as an advantageous adsorbent for the solid-phase extraction of Sudan I-IV as model analytes. J. Sep. Sci. 2017, 40, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Bessashia, W.; Berredjem, Y.; Hattab, Z.; Bououdina, M. Removal of Basic Fuchsin from water by using mussel powdered eggshell membrane as novel bioadsorbent: Equilibrium, kinetics, and thermodynamic studies. Environ. Res. 2020, 186, 109484. [Google Scholar] [CrossRef] [PubMed]
- Parvin, S.; Biswas, B.K.; Rahman, M.A.; Rahman, M.H.; Anik, M.S.; Uddin, M.R. Study on adsorption of Congo red onto chemically modified egg shell membrane. Chemosphere 2019, 236, 124326. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, K.J.; Ruff, K.J.; Atwell, C.A.; Evans, J.L.; Bendele, A.M. Beneficial effects of natural eggshell membrane (NEM) on multiple indices of arthritis in collagen-induced arthritic rats. Mod. Rheumatol. 2017, 27, 838–848. [Google Scholar] [CrossRef] [Green Version]
- Guarderas, F.; Leavell, Y.; Sengupta, T.; Zhukova, M.; Megraw, T.L. Assessment of Chicken-Egg Membrane as a Dressing for Wound Healing. Adv. Ski. Wound Care 2016, 29, 131–134. [Google Scholar] [CrossRef]
- Kessi, E.; Arias, J.L. Using Natural Waste Material as a Matrix for the Immobilization of Enzymes: Chicken Eggshell Membrane Powder for β-Galactosidase Immobilization. Appl. Biochem. Biotechnol. 2019, 187, 101–115. [Google Scholar] [CrossRef]
- Datta, S.; Kanjilal, B.; Sarkar, P. Silver nanoparticles decorated eggshell membrane as an effective platform for interference free sensing of dopamine. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2018, 53, 1048–1055. [Google Scholar] [CrossRef]
- Girelli, A.M.; Scuto, F.R. Eggshell membrane as feedstock in enzyme immobilization. J. Biotechnol. 2020, 325, 241–249. [Google Scholar] [CrossRef]
- Xue, G.; Yue, Z.; Bing, Z.; Yiwei, T.; Xiuying, L.; Jianrong, L. Highly-sensitive organophosphorus pesticide biosensors based on CdTe quantum dots and bi-enzyme immobilized eggshell membranes. Analyst 2016, 141, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, T.; Zhang, X.; Jian, Z.; Xia, H.; Yang, J.; Hu, X.; Xing, M.; Luo, G.; Wu, J. Fabrication of KR-12 peptide-containing hyaluronic acid immobilized fibrous eggshell membrane effectively kills multi-drug-resistant bacteria, promotes angiogenesis and accelerates re-epithelialization. Int. J. Nanomed. 2019, 14, 3345–3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Li, Y.; Ma, M.; Lin, Q.; Sun, S.; Zhang, B.; Feng, X.; Liu, J. Protective effect of soluble eggshell membrane protein hydrolysate on cardiac ischemia/reperfusion injury. Food Nutr. Res. 2015, 59, 28870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farjah, G.H.; Mohammdzadeh, S.; Zirak Javanmard, M. The effect of lycopene in egg shell membrane guidance channel on sciatic nerve regeneration in rats. Iran. J. Basic Med. Sci. 2020, 23, 527–533. [Google Scholar] [PubMed]
- Asgari, G.; Dayari, A. Experimental dataset on acid treated eggshell for removing cyanide ions from synthetic and industrial wastewaters. Data Brief 2018, 16, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J. Use of methyl esterified eggshell membrane for treatment of aqueous solutions contaminated with anionic sulfur dye. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2017, 76, 2638–2646. [Google Scholar] [CrossRef]
- Ruff, K.J.; Endres, J.R.; Clewell, A.E.; Szabo, J.R.; Schauss, A.G. Safety evaluation of a natural eggshell membrane-derived product. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 604–611. [Google Scholar] [CrossRef]
- Park, S.; Choi, K.S.; Lee, D.; Kim, D.; Lim, K.T.; Lee, K.H.; Seonwoo, H.; Kom, J. Eggshell membrane: Review and impact on engineering. Biosyst. Eng. 2016, 151, 446–463. [Google Scholar] [CrossRef]
| Main Components | Major Biochemical Functions |
|---|---|
| Collagens | |
| Osteopontin | |
| Fibronectin | |
| Keratin | |
| Cysteine-rich eggshell membrane proteins (CREMPs) |
|
| Histones | |
| Avian beta defensins | |
| Ovocalyxin-36 | |
| Apolipoproteins |
|
| Protocadherin |
|
| Chondroitin sulfate | |
| Hyaluronic acid |
| Applications | Methods | Functions |
|---|---|---|
| Joint Health |
|
|
|
| |
|
| |
|
| |
| Wound healing |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Immobilization |
|
|
|
| |
|
| |
|
| |
| Antimicrobial activity |
|
|
|
| |
| Gut health |
|
|
|
| |
|
| |
| Anti-inflammatory and antioxidant activity |
|
|
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Tissue engineering |
|
|
|
| |
|
| |
|
| |
|
| |
| Food packaging |
|
|
|
| |
| Biosorbent |
|
|
|
| |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhou, K.; Li, D.; Guyonnet, V.; Hincke, M.T.; Mine, Y. Avian Eggshell Membrane as a Novel Biomaterial: A Review. Foods 2021, 10, 2178. https://doi.org/10.3390/foods10092178
Shi Y, Zhou K, Li D, Guyonnet V, Hincke MT, Mine Y. Avian Eggshell Membrane as a Novel Biomaterial: A Review. Foods. 2021; 10(9):2178. https://doi.org/10.3390/foods10092178
Chicago/Turabian StyleShi, Yaning, Kai Zhou, Dandan Li, Vincent Guyonnet, Maxwell T. Hincke, and Yoshinori Mine. 2021. "Avian Eggshell Membrane as a Novel Biomaterial: A Review" Foods 10, no. 9: 2178. https://doi.org/10.3390/foods10092178
APA StyleShi, Y., Zhou, K., Li, D., Guyonnet, V., Hincke, M. T., & Mine, Y. (2021). Avian Eggshell Membrane as a Novel Biomaterial: A Review. Foods, 10(9), 2178. https://doi.org/10.3390/foods10092178







