Emerging Non-Thermal Technologies as Alternative to SO2 for the Production of Wine
Abstract
1. Introduction
2. Non-Thermal Cold Pasteurization Technologies for Wine Preservation
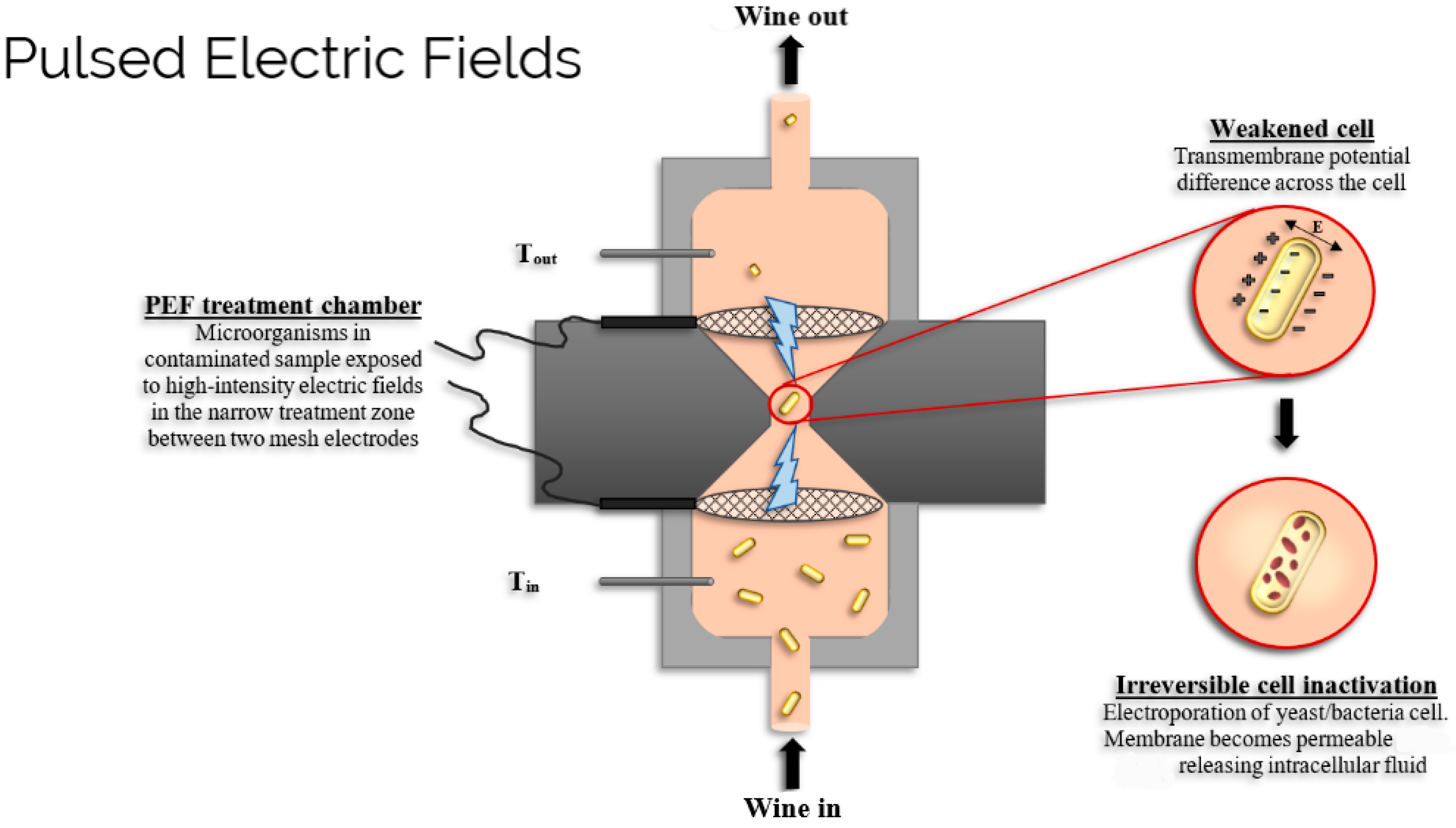
2.1. Pulsed Electric Fields (PEF)
Impact on Wine Quality
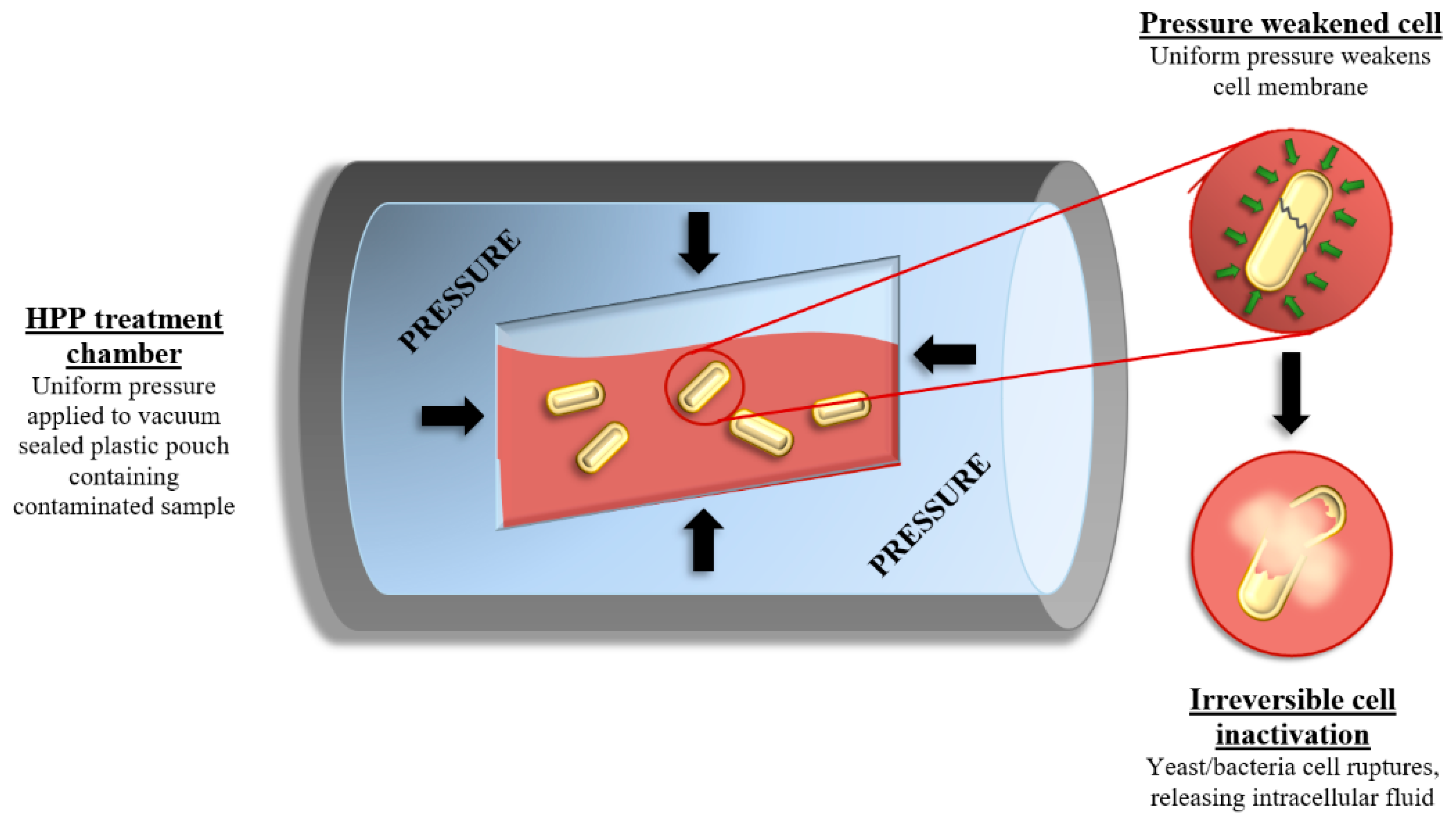
2.2. High Pressure Processing (HPP)
Impact on Wine Quality
2.3. Power Ultrasound (US)
Impact on Wine Quality
2.4. Ultraviolet (UV) Irradiation
Impact on Wine Quality
2.5. High Pressure Homogenization (HPH)
Impact on Wine Quality
2.6. Filtration
Impact on Wine Quality
2.7. Low Electric Current (LEC)
Impact on Wine Quality
3. Microbial Wine Spoilage
3.1. Brettanomyces Yeast
3.2. Other Spoilage Yeasts
3.3. Bacteria
3.4. Molds
4. Effect of PEF, HPP and Other Non-Thermal Technologies on Microbial Inactivation in Wine
4.1. Brettanomyces Bruxellensis
4.2. Yeasts Important for Wine
| Yeast Species | Pasteurization Process | Wine | Alcohol Content (% v/v) | Processing Conditions | Treatment Time | Log Reduction | Reference |
|---|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae | PEF | Red | 12.0 | 31 kV/cm, 3 µs square bipolar pulse, 40 mL/min, T ≤ 40 °C | − | 4.5 | [20] |
| Saccharomyces bayanus | PEF | Red | 13.0 | 31 kV/cm, 1 Hz, 100 pulses, batch, T < 30 °C | − | 5.4 | [17] |
| Candida lipolytica | PEF | Red | 12.0 | 31 kV/cm, 3 µs square bipolar pulse, 40 mL/min, T ≤ 40 °C | − | 4.4 | [20] |
| Hansenula anomala | PEF | Red | 12.0 | 31 kV/cm, 3 µs square bipolar pulse, 40 mL/min, T ≤ 40 °C | − | 3.2 | [20] |
| Saccharomyces cerevisiae | HPP | nr | 15.0 | 300 MPa | 360 s | >7.0 | [86] |
| Saccharomyces cerevisiae | HPP | Red & white | nr | 500 MPa | 300 s | 6.0 | [9] |
| Saccharomyces cerevisiae | HPP | White | nr | 400 MPa | 20 s | >3.5 | [87] |
| Saccharomyces ludwigii | HPP | Rosé | nr | 400 MPa | 20 s | >3.7 | [87] |
| Saccharomyces cerevisiae | US | Red | 14.0 | 24 kHz, 0.2 W/mL, T ≤ 25 °C | 20 min | 0.30 | [53] |
| Schizosaccharomyces pombe | US | Red | 14.0 | 24 kHz, 0.2 W/mL, T ≤ 25 °C | 20 min | 0.13 | [53] |
| Zygosaccharomyces bailii | US | Red | 14.0 | 24 kHz, 0.2 W/mL, T ≤ 25 °C | 20 min | No inactivation | [53] |
| Pichia membranefaciens | US | Red | 14.0 | 24 kHz, 0.2 W/mL, T ≤ 25 °C | 20 min | 0.60 | [53] |
4.3. Bacteria
| Bacterium Species | Pasteurization Process | Wine | Alcohol Content (% v/v) | Processing Conditions | Treatment Time | Log Reduction | Reference |
|---|---|---|---|---|---|---|---|
| Lactobacillus plantarum | PEF | Red | 13.0 | 31 kV/cm, 1 Hz, 100 pulses, batch, T < 30 °C | − | 4.8 | [17] |
| Lactobacillus hilgardii | PEF | Red | 13.0 | 31 kV/cm, 1 Hz, 100 pulses, batch, T < 30 °C | − | 5.2 | [17] |
| Lactobacillus delbrueckii ssp. bulgaricus | PEF | Red | 12.0 | 31 kV/cm, 3 µs square bipolar pulse, 40 mL/min, T ≤ 40 °C | − | 2.7 | [20] |
| Pediococcus parvulus | PEF | Red | nr | 20 kV/cm, 0.5 Hz, 10 µs pulse width, T ≤ 42 °C | 6000 µs | >1.0 | [19] |
| Oenococcus oeni | PEF | nr | nr | 20 kV/cm, 0.5 Hz, 10 µs pulse width, T ≤ 38 °C | 6000 µs | >5.3 | [19] |
| Lactobacillus plantarum | HPP | Red & white | nr | 500 MPa | 300 s | 8.0 | [9] |
| Pediococcus damnosus | HPP | Red | nr | 400 MPa | 20 s | >3.4 | [87] |
| Oenococcus oeni | HPP | Red & white | nr | 500 MPa | 300 s | 8.0 | [9] |
| Acetobacter aceti | HPP | Red & white | nr | 500 MPa | 300 s | 8.0 | [9] |
| Acetobacter aceti | HPP | Red | nr | 400 MPa | 20 s | >4.2 | [87] |
| Acetobacter pasteurianus | HPP | Red & white | nr | 500 MPa | 300 s | 8.0 | [9] |
| Lactobacillus plantarum | US | Red | 14.0 | 24 kHz, 0.2 W/mL, T ≤ 25 °C | 20 min | 0.13 | [53] |
| Pediococcus sp. | US | Red | 14.0 | 24 kHz, 0.2 W/mL, T ≤ 25 °C | 20 min | 0.35 | [53] |
| Oenococcus oeni | US | Red | 14.0 | 24 kHz, 0.2 W/mL, T ≤ 25 °C | 20 min | 0.22 | [53] |
| Acetobacter pasteurianus | US | Red | 14.0 | 24 kHz, 0.2 W/mL, T ≤ 25 °C | 20 min | 0.60 | [53] |
5. Comparison of Technologies and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Raposo, R.; Chinnici, F.; Ruiz-Moreno, M.J.; Puertas, B.; Cuevas, F.J.; Carbú, M.; Guerrero, R.F.; Somovilla, V.O.; Rojas, J.M.M.; Cantos-Villar, E. Sulfur free red wines through the use of grapevine shoots: Impact on the wine quality. Food Chem. 2018, 243, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, M.; Pretorius, I. Microbial Spoilage and Preservation of Wine: Using Weapons from Nature’s Own Arsenal—A Review. S. Afr. J. Enol. Vitic. 2019, 21, 74–96. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- van Wyk, S.; Silva, F.V.M. Nonthermal preservation of wine. In Preservatives and Preservation Methods for the Beverage Industry; Grumezescu, A.M., Holban, A.M., Eds.; The Science of Beverages series; Elsevier: Duxford, UK; Cambridge, MA, USA; Kidlington, UK, 2019; Chapter 7; Volume 15, pp. 203–235. [Google Scholar]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Falguera, V.; Forns, M.; Ibarz, A. UV-vis irradiation: An alternative to reduce SO2 in white wines? LWT Food Sci. Technol. 2013, 51, 59–64. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Liburdi, K.; Nanni, F.; Esti, M. Chitosan beads from microbial and animal sources as enzyme supports for wine application. Food Hydrocoll. 2016, 61, 191–200. [Google Scholar] [CrossRef]
- Silva, F.M.; Sims, C.; Balaban, M.O.; Silva, C.L.M.; O’Keefe, S. Kinetics of flavour and aroma changes in thermally processed cupuaçu (Theobroma grandiflorum) pulp. J. Sci. Food Agric. 2000, 80, 783–787. [Google Scholar] [CrossRef]
- Puig, A.; Vilavella, M.; Daoudi, L.; Guamis, B.; Mínguez, S. Microbiological and biochemical stabilization of wines by application of high pressure technique. Bull. del l’OIV 2003, 76, 596–617. [Google Scholar]
- van Wyk, S.; Silva, F.V.M. High pressure processing inactivation of Brettanomyces bruxellensis in seven different table wines. Food Control 2017, 81, 1–8. [Google Scholar] [CrossRef]
- van Wyk, S.; Silva, F.V.M. High pressure inactivation of Brettanomyces bruxellensis in red wine. Food Microbiol. 2017, 63, 199–204. [Google Scholar] [CrossRef]
- van Wyk, S.; Silva, F.V.; Farid, M.M. Pulsed electric field treatment of red wine: Inactivation of Brettanomyces and potential hazard caused by metal ion dissolution. Innov. Food Sci. Emerg. Technol. 2018, 52, 57–65. [Google Scholar] [CrossRef]
- van Wyk, S.; Farid, M.M.; Silva, F.V. SO2, high pressure processing and pulsed electric field treatments of red wine: Effect on sensory, Brettanomyces inactivation and other quality parameters during one year storage. Innov. Food Sci. Emerg. Technol. 2018, 48, 204–211. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Sulaiman, A. Polyphenoloxidase in Fruit and Vegetables: Inactivation by Thermal and Non-thermal Processes. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2018; pp. 287–301. [Google Scholar]
- Sulaiman, A.; Farid, M.; Silva, F.V.M. Quality stability and sensory attributes of apple juice processed by thermo-sonication, pulsed electric field and thermal processing. Food Sci. Technol. Int. 2017, 23, 265–276. [Google Scholar] [CrossRef]
- Milani, E.A.; Alkhafaji, S.; Silva, F.V. Pulsed Electric Field continuous pasteurization of different types of beers. Food Control 2015, 50, 223–229. [Google Scholar] [CrossRef]
- Puértolas, E.; López, N.; Condón, S.; Raso, J.; Alvarez, I. Pulsed electric fields inactivation of wine spoilage yeast and bacteria. Int. J. Food Microbiol. 2009, 130, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yu, L.; Liu, D.; Gai, L.; Wang, J. Modeling of yeast inactivation of PEF-treated Chinese rice wine: Effects of electric field intensity, treatment time and initial temperature. Food Res. Int. 2013, 54, 456–467. [Google Scholar] [CrossRef]
- Delsart, C.; Grimi, N.; Boussetta, N.; Sertier, C.M.; Ghidossi, R.; Vorobiev, E.; Peuchot, M.M. Impact of pulsed-electric field and high-voltage electrical discharges on red wine microbial stabilization and quality characteristics. J. Appl. Microbiol. 2015, 120, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Abca, E.E.; Evrendilek, G.A. Processing of Red Wine by Pulsed Electric Fields with Respect to Quality Parameters. J. Food Process. Preserv. 2014, 39, 758–767. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Marsellés-Fontanet, A.R.; Arias-Gil, M.; Ancín-Azpilicueta, C.; Martín-Belloso, O. Effect of storage conditions on the volatile composition of wines obtained from must stabilized by PEF during ageing without SO2. Innov. Food Sci. Emerg. Technol. 2008, 9, 469–476. [Google Scholar] [CrossRef]
- Puértolas, E.; Saldaña, G.; Condón, S.; Alvarez, I.; Raso, J. Evolution of polyphenolic compounds in red wine from Cabernet Sauvignon grapes processed by pulsed electric fields during aging in bottle. Food Chem. 2010, 119, 1063–1070. [Google Scholar] [CrossRef]
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving mass transfer to soften tissues by pulsed electric fields: Fundamentals and applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. [Google Scholar] [CrossRef]
- Puértolas, E.; Saldanña, G.; Alvarez, I.; Raso, J. Effect of Pulsed Electric Field Processing of Red Grapes on Wine Chromatic and Phenolic Characteristics during Aging in Oak Barrels. J. Agric. Food Chem. 2010, 58, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Application of pulsed electric fields for improving the maceration process during vinification of red wine: Influence of grape variety. Eur. Food Res. Technol. 2008, 227, 1099–1107. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Hernández-Orte, P.; Álvarez, I.; Raso, J. Effect of a pulsed electric field treatment on the anthocyanins composition and other quality parameters of Cabernet Sauvignon freshly fermented model wines obtained after different maceration times. LWT Food Sci. Technol. 2009, 42, 1225–1231. [Google Scholar] [CrossRef]
- Mok, C.; Song, K.-T.; Park, Y.-S.; Lim, S.; Ruan, R.; Chen, P. High Hydrostatic Pressure Pasteurization of Red Wine. J. Food Sci. 2006, 71, M265–M269. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V. Modeling the inactivation of psychrotrophic Bacillus cereus spores in beef slurry by 600MPa HPP combined with 38–70 °C: Comparing with thermal processing and estimating the energy requirements. Food Bioprod. Process. 2016, 99, 179–187. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V. Resistance of Byssochlamys nivea and Neosartorya fischeri mould spores of different age to high pressure thermal processing and thermosonication. J. Food Eng. 2017, 201, 9–16. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. Inactivation of pathogenic microorganisms in foods by high-pressure processing. In Food Safety and Protection; Rai, V.R., Bai, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 343–378. [Google Scholar]
- Evelyn; Silva, F.V.M. Heat assisted HPP for the inactivation of bacteria, moulds and yeasts spores in foods: Log reductions and mathematical models. Trends Food Sci. Technol. 2019, 88, 143–156. [Google Scholar] [CrossRef]
- Milani, E.A.; Silva, F. Nonthermal pasteurization of beer by high pressure processing: Modelling the inactivation of saccharomyces cerevisiae ascospores in different alcohol beers. High Press. Res. 2016, 36, 595–609. [Google Scholar] [CrossRef]
- Milani, E.A.; Ramsey, J.G.; Silva, F.V. High pressure processing and thermosonication of beer: Comparing the energy requirements and Saccharomyces cerevisiae ascospores inactivation with thermal processing and modeling. J. Food Eng. 2016, 181, 35–41. [Google Scholar] [CrossRef]
- Houška, M.; Silva, F.V.M. Introduction to high-pressure processing of fruit and vegetable products. In High Pressure Processing of Fruit and Vegetable Juices; Houška, M., Silva, F.V.M., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2018; Chapter 1; pp. 1–2. [Google Scholar]
- Silva, F.V.M.; Evelyn. High pressure processing effect on microorganisms in fruit and vegetable products. In High Pressure Processing of Fruit and Vegetable Juices; Houška, M., Silva, F.V.M., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2018; Chapter 2; pp. 3–37. [Google Scholar]
- Lee, P.Y.; Oey, I. Sensory properties of high pressure treated fruit and vegetable juices. In High Pressure Processing of Fruit and Vegetable Juices; Houška, M., Silva, F.V.M., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2018; Chapter 8; pp. 121–133. [Google Scholar]
- Sánchez-Moreno, C.; de Ancos, B. High pressure processing effect on nutrients and their stability. In High Pressure Processing of Fruit and Vegetable Juices; Houška, M., Silva, F.V.M., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2018; Chapter 6; pp. 85–103. [Google Scholar]
- Tříska, J. Health active components in fruit/vegetable juices treated by high pressure. In High Pressure Processing of Fruit and Vegetable Juices; Houška, M., Silva, F.V.M., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2018; Chapter 7; pp. 105–119. [Google Scholar]
- Norton, T.; Sun, D.-W. Recent Advances in the Use of High Pressure as an Effective Processing Technique in the Food Industry. Food Bioprocess Technol. 2007, 1, 2–34. [Google Scholar] [CrossRef]
- Rozali, S.N.; Milani, E.A.; Deed, R.C.; Silva, F.V. Bacteria, mould and yeast spore inactivation studies by scanning electron microscope observations. Int. J. Food Microbiol. 2017, 263, 17–25. [Google Scholar] [CrossRef]
- Balda, F.P.B. Current status of industrial HPP equipment for food processing. In High Pressure Processing of Fruit and Vegetable Juices; Houška, M., Silva, F.V.M., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2018; pp. 73–83. [Google Scholar]
- Uchida, R.; Silva, F.V.M. Modelling the inactivation of Alicyclobacillus acidoterrestris spores by high pressure combined with thermal processing: Study the effect of temperature and soluble solids. Food Control 2017, 73, 426–432. [Google Scholar] [CrossRef]
- Buzrul, S. High hydrostatic pressure treatment of beer and wine: A review. Innov. Food Sci. Emerg. Technol. 2012, 13, 1–12. [Google Scholar] [CrossRef]
- Houska, M.; Pravda, P. Examples of Commercial Fruit and Vegetable Juices and Smoothies Cold Pasteurized by High Pressure Chapter 10. In High Pressure Processing of Fruit and Vegetable Juices; Houška, M., Silva, F.V.M., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2018; pp. 147–154. [Google Scholar]
- Nunes, C.; Santos, M.C.; Saraiva, J.A.; Rocha, S.M.; Coimbra, M.A. Influence of high hydrostatic pressure technology on wine chemical and sensorial characteristics: Potentialities and drawbacks. Adv. Food Nutr. Res. 2017, 82, 205–235. [Google Scholar] [PubMed]
- Lonvaud-Funel, A.; Largeteau, A.; Tonello, C.; Demazeau, G. Résistance aux hautes pressions de microorganismes du vin: Applications à la stabilisation. In Oenologie 95: 5e. Symposium International d’Oenologie; Tec et Doc: Paris, France, 1996; pp. 352–356. [Google Scholar]
- Tao, Y.; Sun, D.; Górecki, A.; Blaszczak, W.; Lamparski, G.; Amarowicz, R.; Fornal, J.; Jelinski, T. Effects of high hydrostatic pressure processing on the physicochemical and sensorial properties of a red wine. Innov. Food Sci. Emerg. Technol. 2012, 16, 409–416. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Cappelle, J.; Gonçalves, F.J.; Rodrigues, A.; Saraiva, J.; Coimbra, M.A. Effect of high pressure treatments on the physicochemical properties of a sulphur dioxide-free red wine. Food Chem. 2013, 141, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.; Nunes, C.; Rocha, M.A.M.; Rodrigues, A.; Rocha, S.; Saraiva, J.; Coimbra, M.A. Impact of high pressure treatments on the physicochemical properties of a sulphur dioxide-free white wine during bottle storage: Evidence for Maillard reaction acceleration. Innov. Food Sci. Emerg. Technol. 2013, 20, 51–58. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A.; Dupont, G.; Demazeau, G.; Bignon, J. Essais de mutage de vins blancs liquoreux par traitement aux hautes pressions. OENO One 1994, 28, 57. [Google Scholar] [CrossRef]
- Takush, D.G.; Osborne, J.P. Investigating High Hydrostatic Pressure Processing as a Tool for Studying Yeast during Red Winemaking. Am. J. Enol. Vitic. 2011, 62, 536–541. [Google Scholar] [CrossRef]
- Feng, H.; Yang, W. Ultrasonic processing. In Nonthermal Processing Technologies for Food; Zhang, H.Q., Barbosa-Canovas, G.V., Balasubramaniam, V.M., Dunne, C.P., Farkas, D.F., Yuan, J.T.C., Eds.; Wiley-Blackwell: Somerset, UK, 2010; pp. 135–154. [Google Scholar]
- Luo, H.; Schmid, F.; Grbin, P.; Jiranek, V. Viability of common wine spoilage organisms after exposure to high power ultrasonics. Ultrason. Sonochem. 2012, 19, 415–420. [Google Scholar] [CrossRef]
- Gracin, L.; Jambrak, A.R.; Juretić, H.; Dobrović, S.; Barukčić, I.; Grozdanović, M.; Smoljanić, G. Influence of high power ultrasound on Brettanomyces and lactic acid bacteria in wine in continuous flow treatment. Appl. Acoust. 2016, 103, 143–147. [Google Scholar] [CrossRef]
- Kentish, S.; Feng, H. Applications of Power Ultrasound in Food Processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Sulaiman, A. Advances in thermosonication for the inactivation of endogenous enzymes in foods. In Ultrasound: Advances in Food Processing and Preservation, 1st ed.; Bermudez-Aguirre, D., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2017; pp. 101–130. [Google Scholar]
- Milani, E.A.; Silva, F.V.M. Ultrasound assisted thermal pasteurization of beers with different alcohol levels: Inactivation of Saccharomyces cerevisiae ascospores. J. Food Eng. 2017, 198, 45–53. [Google Scholar] [CrossRef]
- Sulaiman, A.; Soo, M.J.; Farid, M.; Silva, F.V. Thermosonication for polyphenoloxidase inactivation in fruits: Modeling the ultrasound and thermal kinetics in pear, apple and strawberry purees at different temperatures. J. Food Eng. 2015, 165, 133–140. [Google Scholar] [CrossRef]
- Sulaiman, A.; Farid, M.; Silva, F.V. Strawberry puree processed by thermal, high pressure, or power ultrasound: Process energy requirements and quality modeling during storage. Food Sci. Technol. Int. 2016, 23, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.G.; Sun, D.-W. Ultrasound and electric fields as novel techniques for assisting the wine ageing process: The state-of-the-art research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Evelyn; Milani, E.; Silva, F.V. Comparing high pressure thermal processing and thermosonication with thermal processing for the inactivation of bacteria, moulds, and yeasts spores in foods. J. Food Eng. 2017, 214, 90–96. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V. Ultrasound assisted thermal inactivation of spores in foods: Pathogenic and spoilage bacteria, molds and yeasts. Trends Food Sci. Technol. 2020, 105, 402–415. [Google Scholar] [CrossRef]
- Singleton, V.L.; Draper, D.E. Ultrasonic Treatment with Gas Purging as a Quick Aging Treatment for Wine. Am. J. Enol. Vitic. 1963, 14, 23–35. [Google Scholar]
- Masuzawa, N.; Ohdaira, E.; Ide, M. Effects of Ultrasonic Irradiation on Phenolic Compounds in Wine. Jpn. J. Appl. Phys. 2000, 39, 2978–2979. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Wang, T.-T. Effect of ultrasound irradiation on the evolution of color properties and major phenolic compounds in wine during storage. Food Chem. 2017, 234, 372–380. [Google Scholar] [CrossRef]
- Fredericks, I.N.; du Toit, M.; Krügel, M. Efficacy of ultraviolet radiation as an alternative technology to inactivate microorganisms in grape juices and wines. Food Microbiol. 2011, 28, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Comuzzo, P.; Calligaris, S.; Iacumin, L.; Ginaldi, F.; Palacios Paz, A.E.; Zironi, R. Potential of high pressure homogenization to induce autolysis of wine yeasts. Food Chem. 2015, 185, 340–348. [Google Scholar] [CrossRef]
- Puig, A.; Olmos, P.; Quevedo, J.; Guamis, B.; Mínguez, S. Microbiological and Sensory Effects of Musts Treated by High-pressure Homogenization. Food Sci. Technol. Int. 2008, 14, 5–11. [Google Scholar] [CrossRef]
- Zuehlke, J.M.; Petrova, B.; Edwards, C.G. Advances in the control of wine spoilage by Zygosaccharomyces and Dekkera/Brettanomyces. Annu. Rev. Food Sci. Technol. 2013, 4, 57–78. [Google Scholar] [CrossRef]
- Agnolucci, M.; Rea, F.; Sbrana, C.; Cristani, C.; Fracassetti, D.; Tirelli, A.; Nuti, M. Sulphur dioxide affects culturability and volatile phenol production by Brettanomyces/Dekkera bruxellensis. Int. J. Food Microbiol. 2010, 143, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Millet, V.; Lonvaud-Funel, A. The viable but non-culturable state of wine micro-organisms during storage. Lett. Appl. Microbiol. 2000, 30, 136–141. [Google Scholar] [CrossRef]
- Comitini, F.; Ingeniis, J.; Pepe, L.; Mannazzu, I.; Ciani, M. Corrigendum to Pichia anomala and Kluyveromyces wickerhamii killer toxins as new tools against Dekkera/Brettanomyces spoilage yeasts. FEMS Microbiol. Lett. 2004, 241, 127. [Google Scholar] [CrossRef]
- Lustrato, G.; Vigentini, I.; De Leonardis, A.; Alfano, G.; Tirelli, A.; Foschino, R.; Ranalli, G. Inactivation of wine spoilage yeasts Dekkera bruxellensis using low electric current treatment (LEC). J. Appl. Microbiol. 2010, 109, 594–604. [Google Scholar] [CrossRef]
- Arriagada-Carrazana, J.; Sáez-Navarrete, C.; Bordeu, E. Membrane filtration effects on aromatic and phenolic quality of Cabernet Sauvignon wines. J. Food Eng. 2005, 68, 363–368. [Google Scholar] [CrossRef]
- Lustrato, G.; Alfano, G.; De Leonardis, A.; Macciola, V.; Ranalli, G. Inactivation of Dekkera bruxellensis yeasts in wine storage in brand new oak barrels using low electric current technology. Ann. Microbiol. 2015, 65, 2091–2098. [Google Scholar] [CrossRef]
- Lustrato, G.; Alfano, G.; Belli, C.; Grazia, L.; Iorizzo, M.; Ranalli, G. Scaling-up in industrial winemaking using low electric current as an alternative to sulfur dioxide addition. J. Appl. Microbiol. 2006, 101, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Querol, A.; Fernaández-Espinar, M.T. Rapid Identification and Enumeration of Saccharomyces cerevisiae Cells in Wine by Real-Time PCR. Appl. Environ. Microbiol. 2005, 71, 6823–6830. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, V.; Malfeito-Ferreira, M. Dekkera/Brettanomyces spp. In Food Spoilage Microorganisms; Blackburn, C.W., Ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 354–398. [Google Scholar]
- Wedral, D.; Shewfelt, R.; Frank, J. The challenge of Brettanomyces in wine. LWT 2010, 43, 1474–1479. [Google Scholar] [CrossRef]
- Oelofse, A.; Pretorius, I.; Du Toit, M. Significance of Brettanomyces and Dekkera during Winemaking: A Synoptic Review. S. Afr. J. Enol. Vitic. 2008, 29, 124–144. [Google Scholar] [CrossRef]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Caldeira, J.; Botelheiro, R.; Pagliara, D.; Malfeito-Ferreira, M.; Loureiro, V. Survival patterns of Dekkera bruxellensis in wines and inhibitory effect of sulphur dioxide. Int. J. Food Microbiol. 2008, 121, 201–207. [Google Scholar] [CrossRef][Green Version]
- Jackson, R.S. Post-Fermentation Treatments and Related Topics. In Wine Science, 4th ed.; Jackson, R.S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 535–676. [Google Scholar]
- Silva, F.V.; Evelyn. Resistant moulds as pasteurization target for cold distributed high pressure and heat assisted high pressure processed fruit products. J. Food Eng. 2020, 282, 109998. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Sevenich, R.; Rauh, C.; López, R.; Knorr, D.; López-Alfaro, I. Inactivation of Brettanomyces bruxellensis by High Hydrostatic Pressure technology. Food Control. 2016, 59, 188–195. [Google Scholar] [CrossRef]
- Tonello, C.; Largeteau, A.; Demazeau, G.; Lonvaud-Funel, A. High pressure inactivation modelling of Saccharomyces cerevisiae in grape juice and wine. In High Pressure Biology and Medicine; Bennett, P.B., Demchenko, I., Marquis, R.F., Eds.; University of Rochester Press: New York, NY, USA, 1998; pp. 102–108. [Google Scholar]
- Tonello, C.; Largeteau, A.; Demazeau, G.; Lonvaud-Funel, A. Les applications éventuelles des hautes pressions en oenologie. Revue Française d’Oenologie 1996, 161, 13–16. [Google Scholar]
- Comuzzo, P.; Calligaris, S.; Iacumin, L.; Ginaldi, F.; Voce, S.; Zironi, R. Application of multi-pass high pressure homogenization under variable temperature regimes to induce autolysis of wine yeasts. Food Chem. 2017, 224, 105–113. [Google Scholar] [CrossRef]
- Comuzzo, P.; Calligaris, S. Potential Applications of High Pressure Homogenization in Winemaking: A Review. Beverages 2019, 5, 56. [Google Scholar] [CrossRef]
- Christofi, S.; Malliaris, D.; Katsaros, G.; Panagou, E.; Kallithraka, S. Limit SO2 content of wines by applying High Hydrostatic Pressure. Innov. Food Sci. Emerg. Technol. 2020, 62, 102342. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Portu, J.; López, N.; Santamaría, P.; Gutiérrez, A.R.; López, R.; López-Alfaro, I. Pulsed Electric Field treatment after malolactic fermentation of Tempranillo Rioja wines: Influence on microbial, physicochemical and sensorial quality. Innov. Food Sci. Emerg. Technol. 2019, 51, 57–63. [Google Scholar] [CrossRef]
| Pasteurization Technology | Wine | Alcohol Content (% v/v) | Processing Conditions | Treatment Time | Log Reduction | Reference |
|---|---|---|---|---|---|---|
| PEF | Red | 13.0 | 31 kV/cm, 1 Hz, 100 pulses, batch, T < 30 °C | − | 5.2 | [17] |
| PEF | Red | nr | 20 kV/cm, 0.5 Hz, 10 µs pulse width, T ≤ 37 °C | 6000 µs | >4.8 | [19] |
| PEF | Red | 13.5 | 50 kV/cm, 100 Hz, 1.7 µs pulse width, T < 40 °C | 39 µs | 3.0 | [12] |
| HPP | Red Cabernet Sauvignon | 13.4 | 400 MPa | 5 s | >7.0 | [11] |
| HPP | White Chardonnay | 13.0 | 200 MPa | 15 s | >7.0 | [10] |
| HPP | Rosé | 12.5 | 200 MPa | 120 s | >6.0 | [10] |
| HPP | Red Pinot Noir | 13.0 | 200 MPa | 180 s | 6.0 | [10] |
| HPP | Red & white | nr | 500 MPa | 300 s | 6.0 | [9] |
| HPP | Red Cabernet Sauvignon | 13.5 | 200 MPa | 180 s | 5.8 | [10] |
| HPP | Red Syrah | 12.5 | 200 MPa | 180 s | 5.0 | [10] |
| HPP | Red SO2-free Cabernet Merlot | 13.7 | 200 MPa | 180 s | 3.8 | [10] |
| HPP | Red Dolcetto Syrah | 10.5 14.0 | 200 MPa | 180 s | 3.0 4.2 | [10] |
| US | Red | 14.0 | 24 kHz, 0.2 W/mL, T ≤ 25 °C | 20 min | 0.24 | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.V.M.; van Wyk, S. Emerging Non-Thermal Technologies as Alternative to SO2 for the Production of Wine. Foods 2021, 10, 2175. https://doi.org/10.3390/foods10092175
Silva FVM, van Wyk S. Emerging Non-Thermal Technologies as Alternative to SO2 for the Production of Wine. Foods. 2021; 10(9):2175. https://doi.org/10.3390/foods10092175
Chicago/Turabian StyleSilva, Filipa V. M., and Sanelle van Wyk. 2021. "Emerging Non-Thermal Technologies as Alternative to SO2 for the Production of Wine" Foods 10, no. 9: 2175. https://doi.org/10.3390/foods10092175
APA StyleSilva, F. V. M., & van Wyk, S. (2021). Emerging Non-Thermal Technologies as Alternative to SO2 for the Production of Wine. Foods, 10(9), 2175. https://doi.org/10.3390/foods10092175







