Antioxidants Bioaccessibility and Lactobacillus salivarius (CECT 4063) Survival Following the In Vitro Digestion of Vacuum Impregnated Apple Slices: Effect of the Drying Technique, the Addition of Trehalose, and High-Pressure Homogenization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strain and Raw Materials
2.2. Sample Preparation
2.3. In Vitro Simulation of Gastrointestinal Digestion
2.4. Analytical Determinations
2.4.1. Moisture Content and Water Activity
2.4.2. Microbial Counts
2.4.3. Antioxidant Properties
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of the Food Matrix and the Vacuum Impregnation Solution on Probiotic Survival during In Vitro Digestion
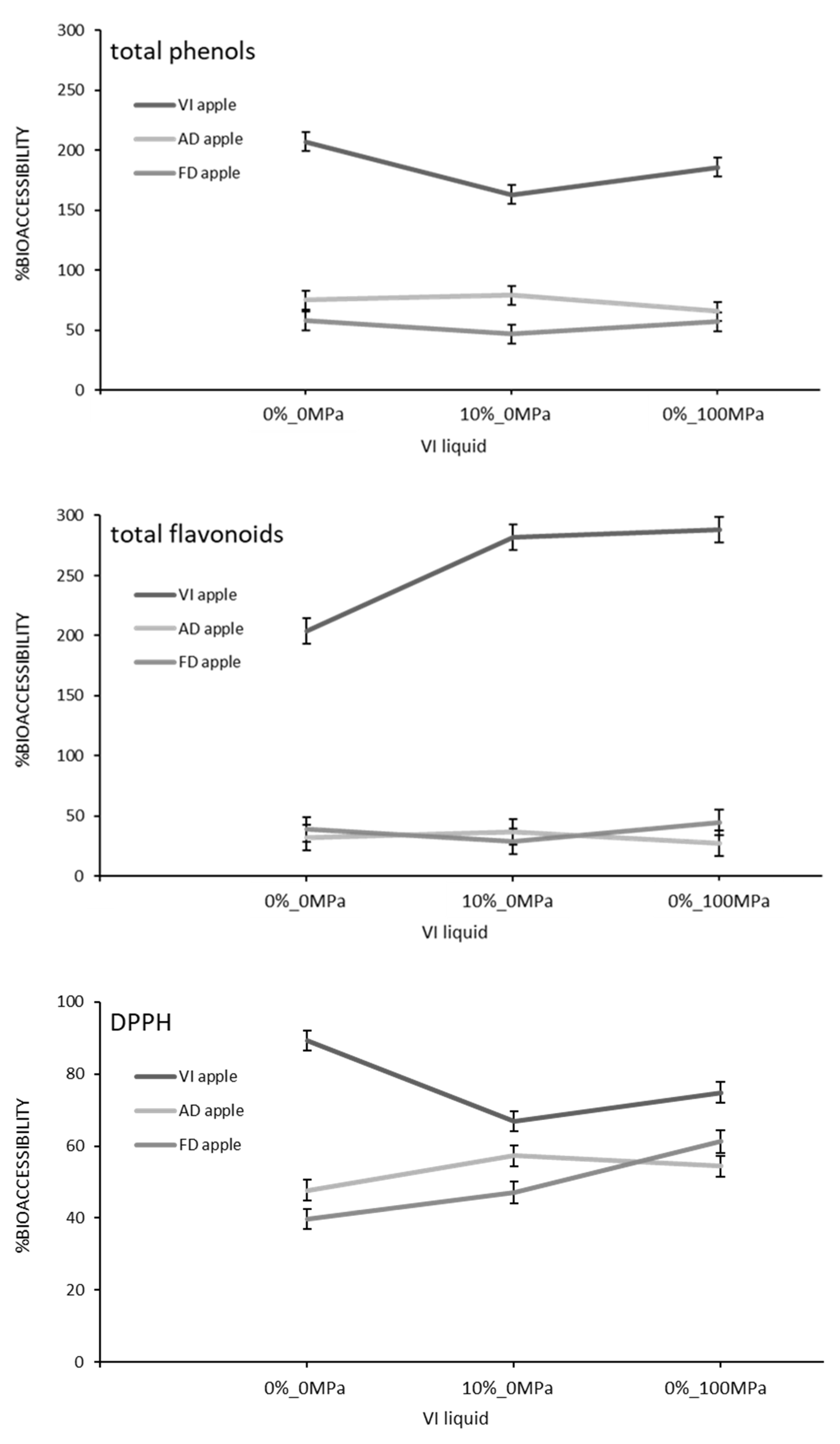
3.2. Effect of the Food Matrix and the Vacuum Impregnation Solution on the Bioaccesibility of Antioxidants during In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janssen, M.; Chang, B.P.I.; Hristov, H.; Pravst, I.; Profeta, A.; Millard, J. Changes in Food Consumption During the COVID-19 Pandemic: Analysis of Consumer Survey Data from the First Lockdown Period in Denmark, Germany, and Slovenia. Front. Nutr. 2021, 8, 635859. [Google Scholar] [CrossRef] [PubMed]
- Fortune Business Insights. Functional Food and Beverage Market Size, Share & COVID-19 Impact Analysis by Type (Functional Cereals & Grains, Functional Dairy Products, Functional Bakery Products, Functional Fats & Oils, an Other Functional/Fortidfied Foods), Distribution Channel (Supermarkets/Hypermarkets, Convenience Stores, Online Retail, an Others) and Regional Forecast 2021–2028. In Market Research Report; Fortune Business Insights: Pune, India, 2021; Available online: https://www.fortunebusinessinsights.com/functional-foods-market-102269 (accessed on 1 June 2021).
- Aponte, M.; Murru, N.; Shoukat, M. Therapeutic, Prophylactic, and Functional Use of Probiotics: A Current Perspective. Front. Microbiol. 2020, 11, 2120–2136. [Google Scholar] [CrossRef]
- Sharma, A. Importance of Probiotics in Cancer Prevention and Treatment. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA, 2019; Chapter 4; pp. 33–45. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed. Pharmacother. 2020, 130, 110625. [Google Scholar] [CrossRef]
- Hajavi, J.; Esmaeili, S.-A.; Varasteh, A.; Vazini, H.; Atabati, H.; Mardani, F.; Momtazi-Borojeni, A.A.; Hashemi, M.; Sankian, M.; Sahebkar, A. The immunomodulatory role of probiotics in allergy therapy. J. Cell. Physiol. 2019, 234, 2386–2398. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [Green Version]
- Boricha, A.A.; Shekh, S.L.; Pithva, S.P.; Ambalam, P.S.; Vyas, B.R.M. In vitro evaluation of probiotic properties of Lactobacillus species of food and human origin. LWT 2019, 106, 201–208. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kenallaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT 2019, 105, 242–249. [Google Scholar] [CrossRef]
- Gomand, F.; Borges, F.; Burgain, J.; Guerin, J.; Revol-Junelles, A.-M.; Gaiani, C. Food Matrix Design for Effective Lactic Acid Bacteria Delivery. Annu. Rev. Food Sci. Technol. 2019, 10, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Betoret, N.; Puente, L.; Díaz, M.; Pagán, M.; García, M.; Gras, M.; Martínez-Monzó, J.; Fito, P. Development of probiotic-enriched dried fruits by vacuum impregnation. J. Food Eng. 2003, 56, 273–277. [Google Scholar] [CrossRef]
- Akman, P.K.; Uysal, E.; Ozkaya, G.U.; Tornuk, F.; Durak, M.Z. Development of probiotic carrier dried apples for consumption as snack food with the impregnation of Lactobacillus paracasei. LWT 2019, 103, 60–68. [Google Scholar] [CrossRef]
- Koutsos, A.; Lovegrove, J.A. An Apple a Day Keeps the Doctor Away—Inter-Relationship Between Apple Consumption, the Gut Microbiota and Cardiometabolic Disease Risk Reduction. In Diet-Microbe Interactions in the Gut: Effects on Human Health and Disease; Tuohy, K., Del Rio, D., Eds.; Academic Press: Cambridge, MA, USA, 2015; Chapter 12; pp. 173–194. [Google Scholar] [CrossRef]
- Valerio, F.; Volpe, M.G.; Santagata, G.; Boscaino, F.; Barbarisi, C.; Di Biase, M.; Bavaro, A.R.; Lonigro, S.L.; Lavermicocca, P. The viability of probiotic Lactobacillus paracasei IMPC2.1 coating on apple slices during dehydration and simulated gastro-intestinal digestion. Food Biosci. 2020, 34, 100533. [Google Scholar] [CrossRef]
- Serra, A.T.; Rocha, J.; Sepodes, B.; Matias, A.A.; Feliciano, R.P.; De Carvalho, A.; Bronze, M.; Duarte, C.; Figueira, M.E. Evaluation of cardiovascular protective effect of different apple varieties—Correlation of response with composition. Food Chem. 2012, 135, 2378–2386. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Balderas, C.; Sánchez-Moreno, C.; De Ancos, B. Impact of an in vitro dynamic gastrointestinal digestion on phenolic compounds and antioxidant capacity of apple treated by high-pressure processing. Innov. Food Sci. Emerg. Technol. 2020, 66, 102486. [Google Scholar] [CrossRef]
- García-Hernández, J.; Hernández-Pérez, M.; Peinado, I.; Andrés, A.; Heredia, A. Tomato-antioxidants enhance viability of L. reuteri under gastrointestinal conditions while the probiotic negatively affects bioaccessibility of lycopene and phenols. J. Funct. Foods 2018, 43, 1–7. [Google Scholar] [CrossRef]
- Patrignani, F.; Vannini, L.; Kamdem, S.L.S.; Lanciotti, R.; Guerzoni, M.E. Effect of high pressure homogenization on Saccharomyces cerevisiae inactivation and physico-chemical features in apricot and carrot juices. Int. J. Food Microbiol. 2009, 136, 26–31. [Google Scholar] [CrossRef]
- Burns, P.G.; Patrignani, F.; Tabanelli, G.; Vinderola, G.C.; Siroli, L.; Reinheimer, J.A.; Gardini, F.; Lanciotti, R. Potential of high pressure homogenisation on probiotic Caciotta cheese quality and functionality. J. Funct. Foods 2015, 13, 126–136. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Betoret, E.; Taccari, A.; Dalla Rosa, M.; Bordoni, A. Impact of processing on the nutritional and functional value of mandarin juice. J. Sci. Food Agric. 2020, 100, 4558–4564. [Google Scholar] [CrossRef]
- Oikonomopoulou, V.P.; Krokida, M.K. Novel Aspects of Formation of Food Structure during Drying. Dry. Technol. 2013, 31, 990–1007. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Arilla, A.; Bennár, M.; Barrera, C.; Codoñer, P.; Fito, P. No invasive methodology to produce a probiotic low humid apple snack with potential effect against Helicobacter pylori. J. Food Eng. 2012, 110, 289–293. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Calabuig-Jiménez, L.; Patrignani, F.; Barrera, C.; Lanciotti, R.; Dalla Rosa, M. Probiotic survival and in vitro digestion of L. salivarius spp. salivarius encapsulated by high homogenization pressures and incorporated into a fruit matrix. LWT 2019, 111, 883–888. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Calabuig-Jiménez, L.; Barrera, C.; Dalla Rosa, M. Effect of Drying Process, Encapsulation, and Storage on the Survival Rates and Gastrointestinal Resistance of L. salivarius spp. salivarius Included into a Fruit Matrix. Microorganisms 2020, 8, 654. [Google Scholar] [CrossRef]
- Burca-Busaga, C.G.; Betoret, N.; Seguí, L.; Betoret, E.; Barrera, C. Survival of Lactobacillus salivarius CECT 4063 and Stability of Antioxidant Compounds in Dried Apple Snacks as Affected by the Water Activity, the Addition of Trehalose and High Pressure Homogenization. Microorganisms 2020, 8, 1095. [Google Scholar] [CrossRef]
- Fito, P.; Chiralt, A.; Betoret, N.; Gras, M.; Cháfer, M.; Martínez-Monzó, J.; Andrés, A.; Vidal, D. Vacuum impregnation and osmotic dehydration in matrix engineering: Application in functional fresh food development. J. Food Eng. 2001, 49, 175–183. [Google Scholar] [CrossRef]
- Barrera, C.; Burca, C.; Betoret, E.; García-Hernandez, J.; Hernández, M.; Betoret, N. Improving antioxidant properties and probiotic effect of clementine juice inoculated with Lactobacillus salivarius spp. salivarius (CECT 4063) by trehalose addition and/or sublethal homogenisation. Int. J. Food Sci. Technol. 2019, 54, 2109–2122. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Wiczkowska, J. Rehydration of Apple Dried by Different Methods. Int. J. Food Prop. 2007, 9, 217–226. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Castagnini, J.M.; Rocculi, P.; Rosa, M.D.; Fito, P. Analysis by non-linear irreversible thermodynamics of compositional and structural changes occurred during air drying of vacuum impregnated apple (cv. Granny smith): Calcium and trehalose effects. J. Food Eng. 2015, 147, 95–101. [Google Scholar] [CrossRef]
- Zhu, W.; Lyu, F.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional Efficacy of Probiotic Lactobacillus sanfranciscensis in Apple, Orange and Tomato Juices with Special Reference to Storage Stability and In Vitro Gastrointestinal Survival. Beverages 2020, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Panpetch, W.; Spinler, J.K.; Versalovic, J.; Tumwasorn, S. Characterization of Lactobacillus salivarius strains B37 and B60 capable of inhibiting IL-8 production in Helicobacter pylori-stimulated gastric epithelial cells. BMC Microbiol. 2016, 16, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, P.; Lin, K.; Zhang, J.; Han, X.; Lyu, L.; Yi, H.; Sun, J.; Zhang, L. Enhancing spray drying tolerance of Lactobacillus bulgaricus by intracellular trehalose delivery via electroporation. Food Res. Int. 2020, 127, 108725. [Google Scholar] [CrossRef]
- Nayak, P.K.; Chandrasekar, C.M.; Sundarsingh, A.; Kesavan, R.K. Effect of in-vitro digestion on the bio active compounds and biological activities of fruit pomaces. J. Food Sci. Technol. 2020, 57, 4707–4715. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martinez, P.; Martín-Belloso, O. Changes in Vitamin C, Phenolic, and Carotenoid Profiles Throughout In Vitro Gastrointestinal Digestion of a Blended Fruit Juice. J. Agric. Food Chem. 2013, 61, 1859–1867. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Impact of in vitro digestion phases on the stability and bioaccessibility of carotenoids and their esters in mandarin pulps. Food Funct. 2017, 8, 3951–3963. [Google Scholar] [CrossRef]
- Liu, G.; Ying, D.; Guo, B.; Cheng, L.J.; May, B.; Bird, T.; Sanguansri, L.; Cao, Y.; Augustin, M. Extrusion of apple pomace increases antioxidant activity upon in vitro digestion. Food Funct. 2019, 10, 951–963. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Zhao, Y.-Y.; Luo, C.-X.; Li, J.; Gao, Y.-Q. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crop. Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.-A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Pavez-Guajardo, C.; Ferreira, S.R.S.; Mazzutti, S.; Guerra-Valle, M.E.; Sáez-Trautmann, G.; Moreno, J. Influence of In Vitro Digestion on Antioxidant Activity of Enriched Apple Snacks with Grape Juice. Foods 2020, 9, 1681. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Barber, X.; Fernández-López, J.; Pérez-Alvarez, J.A.; Viuda-Martos, M. Bioaccessibility, changes in the antioxidant potential and colonic fermentation of date pits and apple bagasse flours obtained from co-products during simulated in vitro gastrointestinal digestion. Food Res. Int. 2015, 78, 169–176. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Jayabalan, R. Effect of probiotification with Lactobacillus plantarum MCC 2974 on quality of Sohiong juice. LWT Food Sci. Technol. 2019, 108, 55–60. [Google Scholar] [CrossRef]
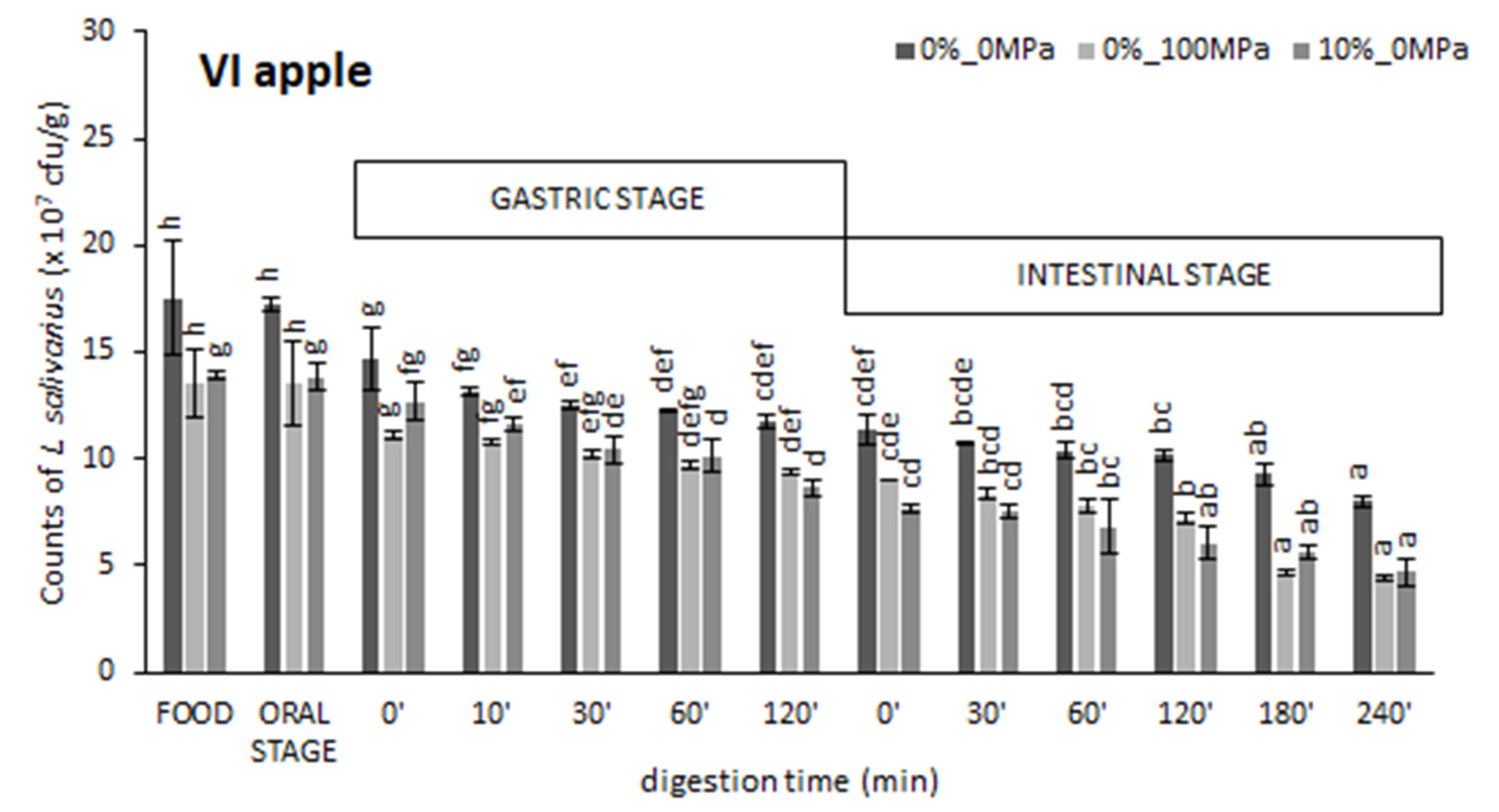
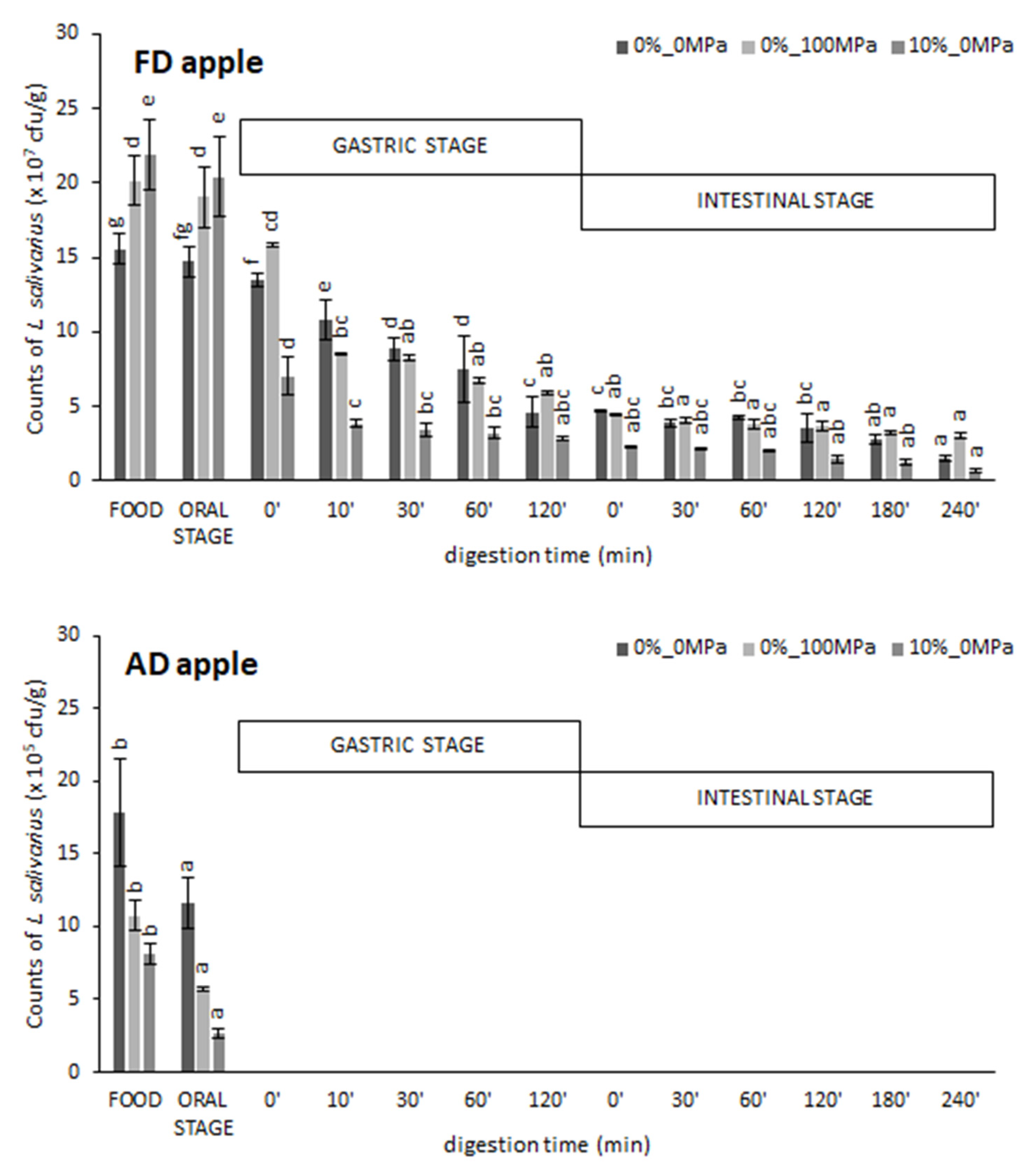
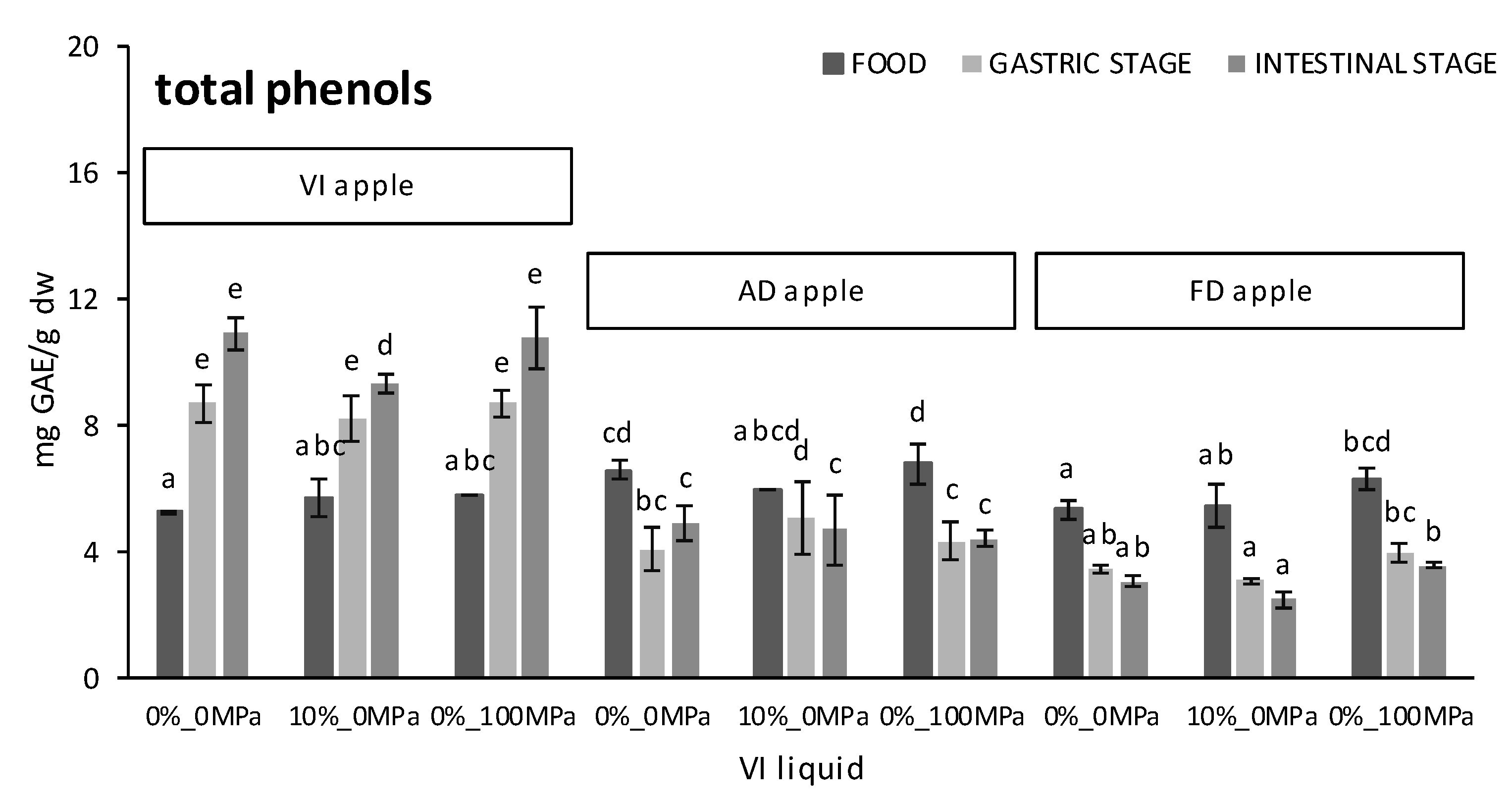
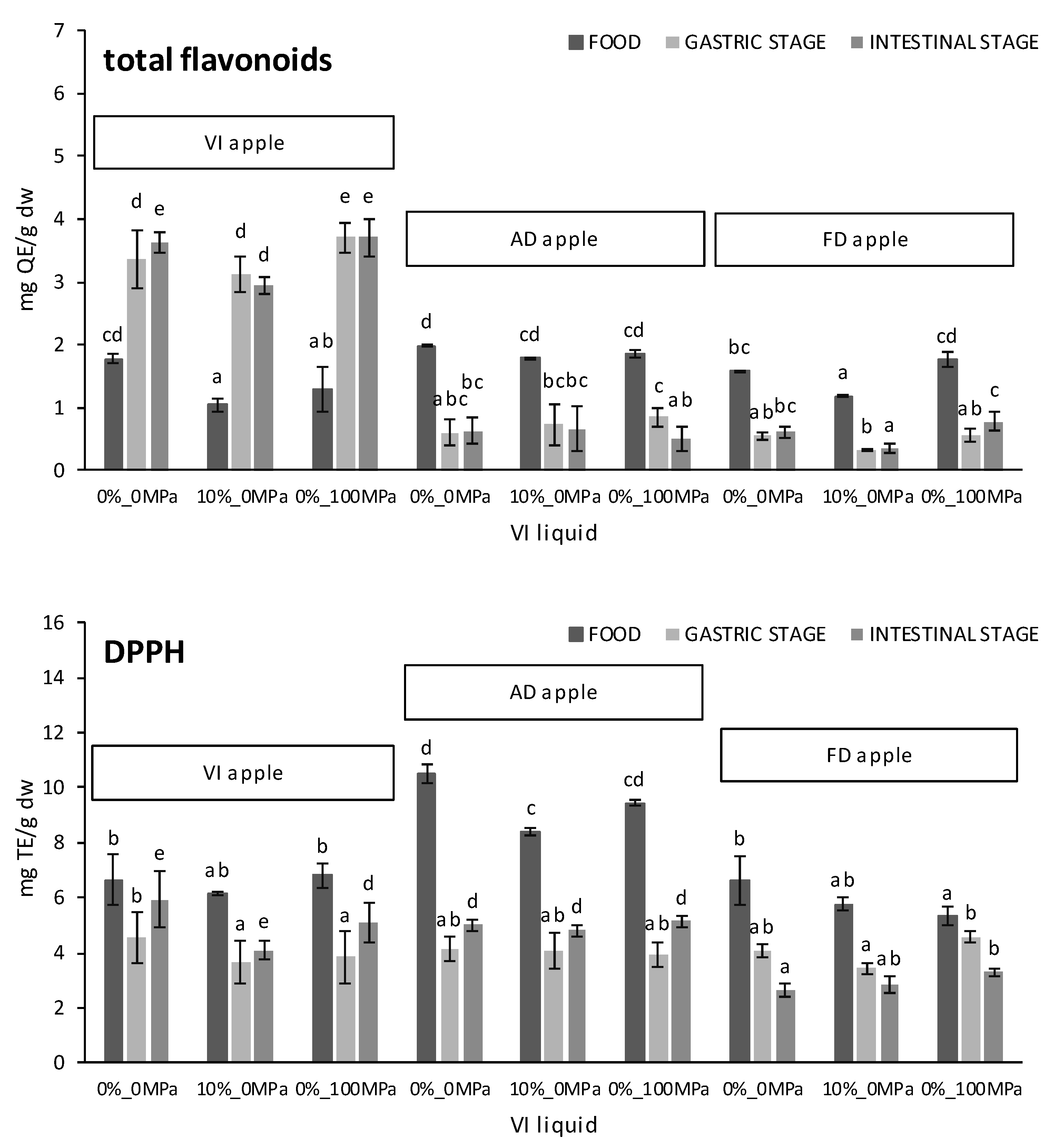
| Treatment | Oral Stage | Gastric Stage | Intestinal Stage | Total | Final Counts (Log cfu/g) | |
|---|---|---|---|---|---|---|
| MRS Broth | - | 33 ± 2 b | 48.50 ± 0.13 bc | 15.8 ± 1.4 b | 8.566 ± 0.004 f | |
| VI apple | 0%_0 MPa 0%_100 MPa 10%_0 MPa | 97 ± 4 de 100 ± 2 e 93 ± 7 d | 68 ± 3 d 65 ± 7 d 55 ± 9 c | 68 ± 8 d 48 ± 2 b 54 ± 6 bc | 47 ± 5 d 31 ± 4 c 28 ± 5 c | 7.904 ± 0.012 e 7.644 ± 0.015 d 7.67 ± 0.06 d |
| FD apple | 0%_0 MPa 0%_100 MPa 10%_0 MPa | 99 ± 2 de 94 ± 4 de 93 ± 2 de | 17 ± 5 a 28 ± 5 b 14 ± 3 a | 53 ± 9 bc 58 ± 5 c 24 ± 3 a | 9 ± 2 a 16 ± 3 b 3.1 ± 0.2 a | 7.19 ± 0.05 c 7.438 ± 0.012 b 6.82 ± 0.07 a |
| AD apple | 0%_0 MPa 0%_100 MPa 10%_0 MPa | 66 ± 4 c 53 ± 6 b 31.9 ± 1.2 a | n d n d n d | n d n d n d | n d n d n d | n d n d n d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burca-Busaga, C.G.; Betoret, N.; Seguí, L.; García-Hernández, J.; Hernández, M.; Barrera, C. Antioxidants Bioaccessibility and Lactobacillus salivarius (CECT 4063) Survival Following the In Vitro Digestion of Vacuum Impregnated Apple Slices: Effect of the Drying Technique, the Addition of Trehalose, and High-Pressure Homogenization. Foods 2021, 10, 2155. https://doi.org/10.3390/foods10092155
Burca-Busaga CG, Betoret N, Seguí L, García-Hernández J, Hernández M, Barrera C. Antioxidants Bioaccessibility and Lactobacillus salivarius (CECT 4063) Survival Following the In Vitro Digestion of Vacuum Impregnated Apple Slices: Effect of the Drying Technique, the Addition of Trehalose, and High-Pressure Homogenization. Foods. 2021; 10(9):2155. https://doi.org/10.3390/foods10092155
Chicago/Turabian StyleBurca-Busaga, Cristina Gabriela, Noelia Betoret, Lucía Seguí, Jorge García-Hernández, Manuel Hernández, and Cristina Barrera. 2021. "Antioxidants Bioaccessibility and Lactobacillus salivarius (CECT 4063) Survival Following the In Vitro Digestion of Vacuum Impregnated Apple Slices: Effect of the Drying Technique, the Addition of Trehalose, and High-Pressure Homogenization" Foods 10, no. 9: 2155. https://doi.org/10.3390/foods10092155
APA StyleBurca-Busaga, C. G., Betoret, N., Seguí, L., García-Hernández, J., Hernández, M., & Barrera, C. (2021). Antioxidants Bioaccessibility and Lactobacillus salivarius (CECT 4063) Survival Following the In Vitro Digestion of Vacuum Impregnated Apple Slices: Effect of the Drying Technique, the Addition of Trehalose, and High-Pressure Homogenization. Foods, 10(9), 2155. https://doi.org/10.3390/foods10092155










