Impact of Pressurized Liquid Extraction and pH on Protein Yield, Changes in Molecular Size Distribution and Antioxidant Compounds Recovery from Spirulina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Samples
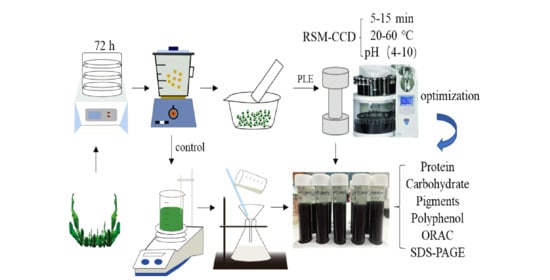
2.3. PLE Extraction Process
2.4. Total Protein Determination
2.5. Total Carbohydrate Content Determination
2.6. Pigments Determination
2.7. Polyphenols Determination
2.8. Antioxidant Capacity
2.9. Protein Molecular Size Distribution (SDS-PAGE Gels)
2.10. Experimental Design and Statistical Analysis
3. Results and Discussion
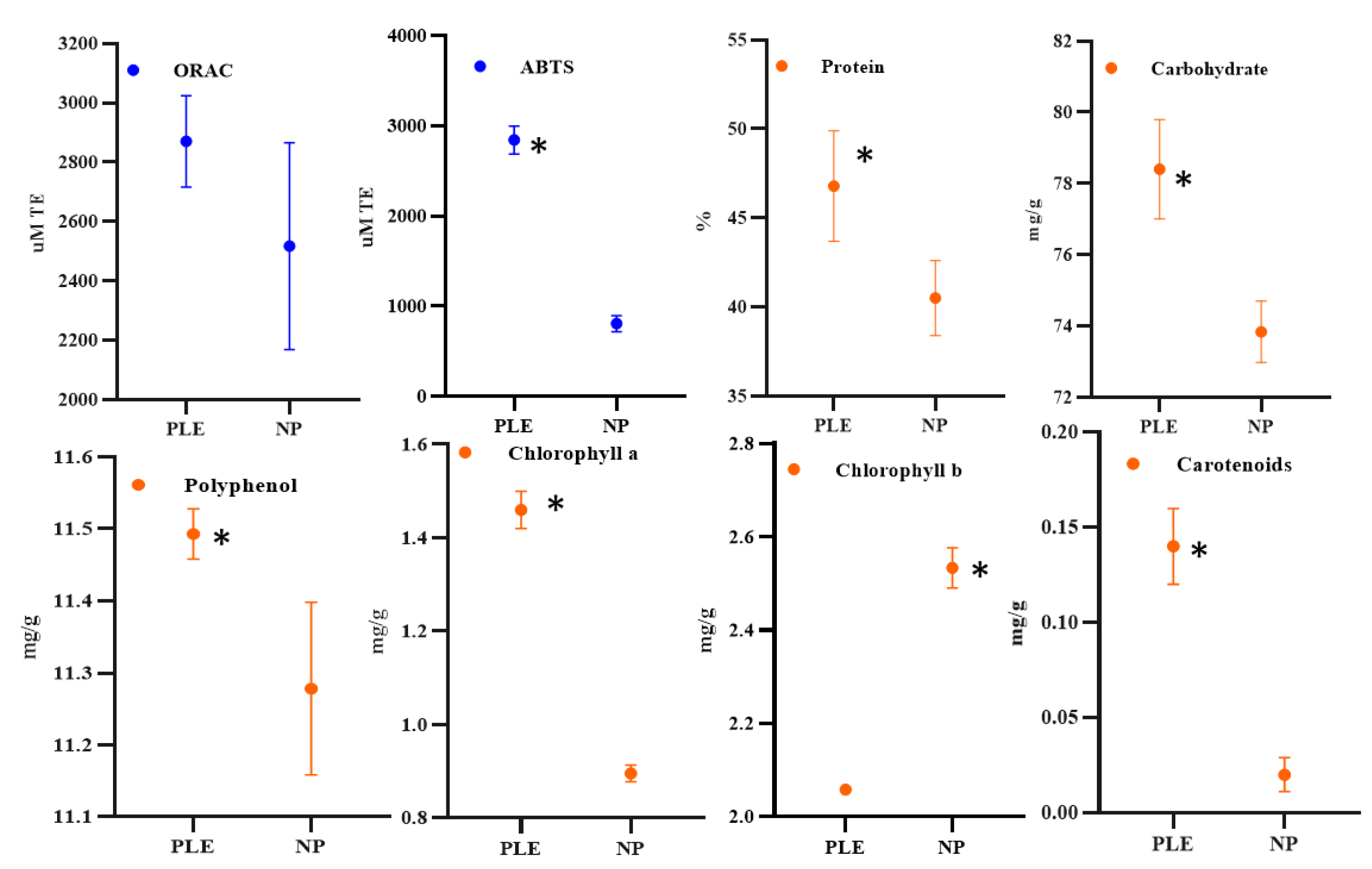
3.1. Effect of PLE on High-Added-Value Compounds
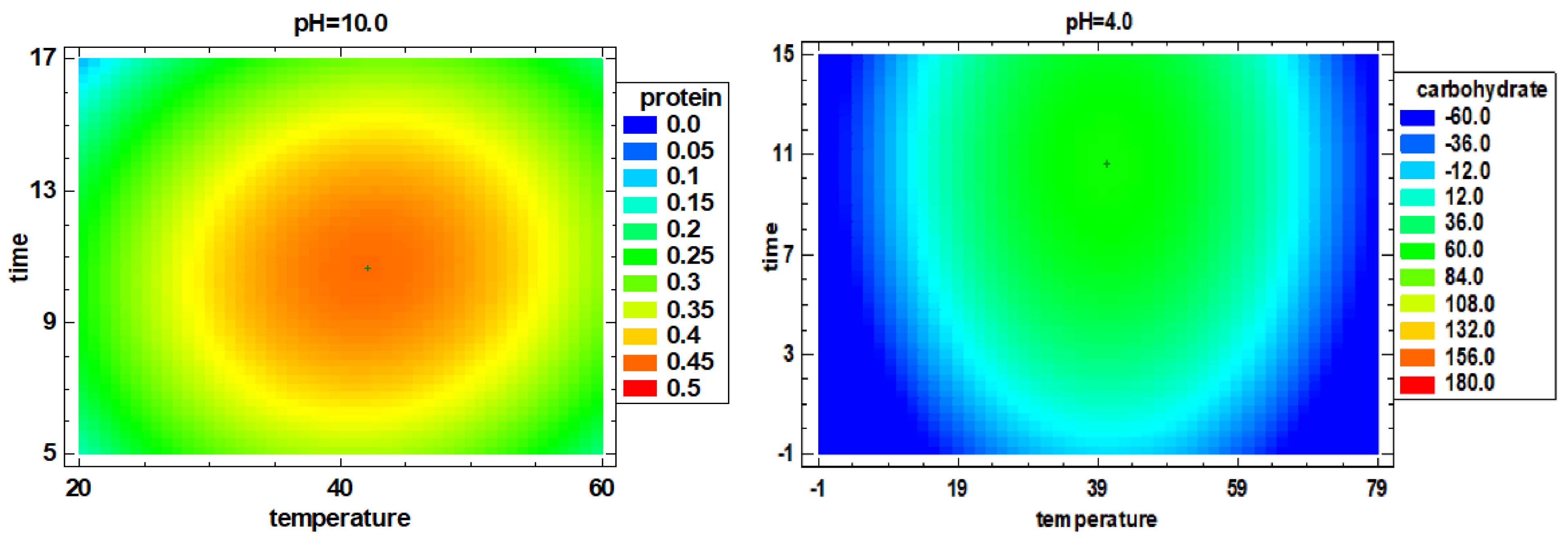
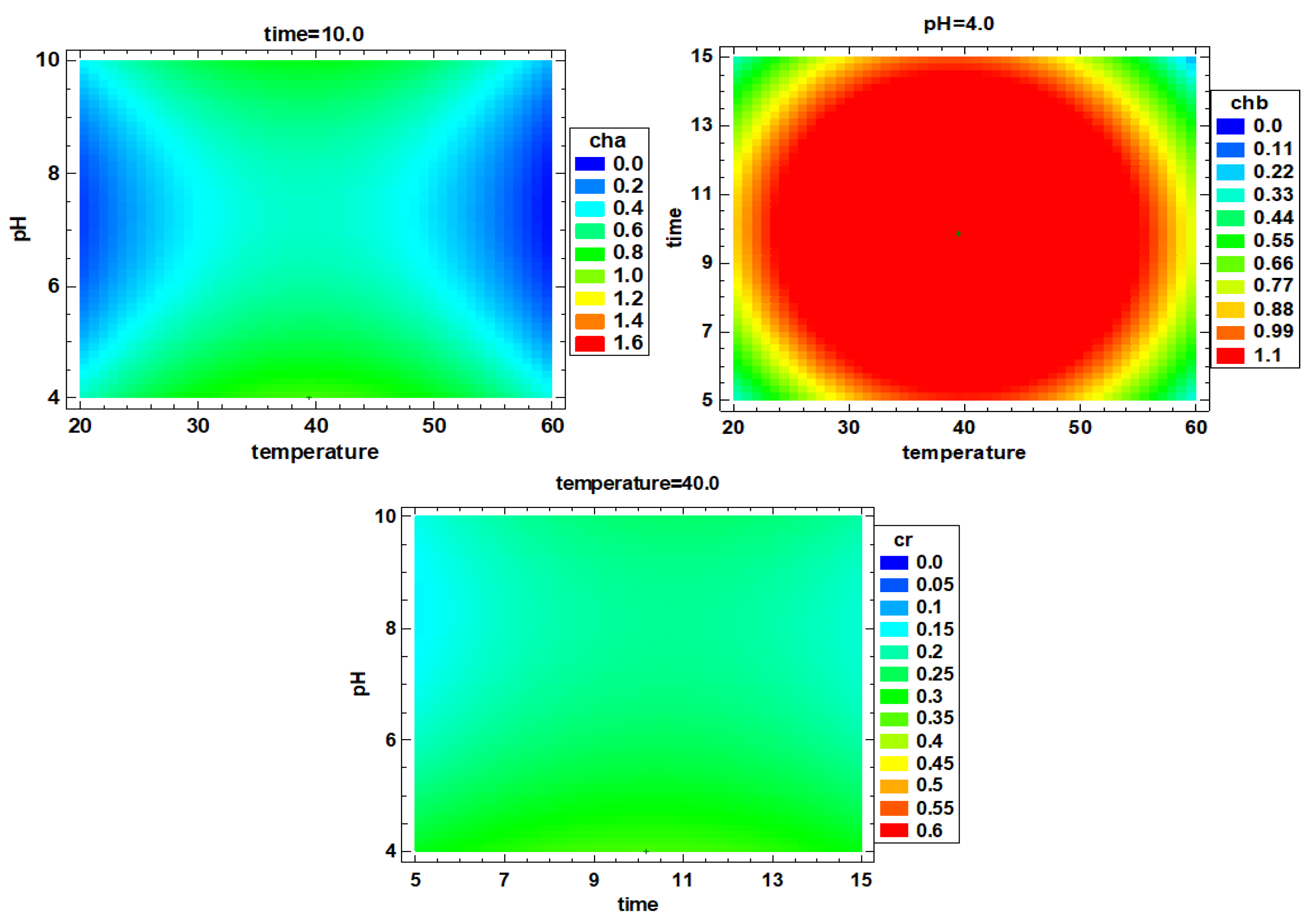
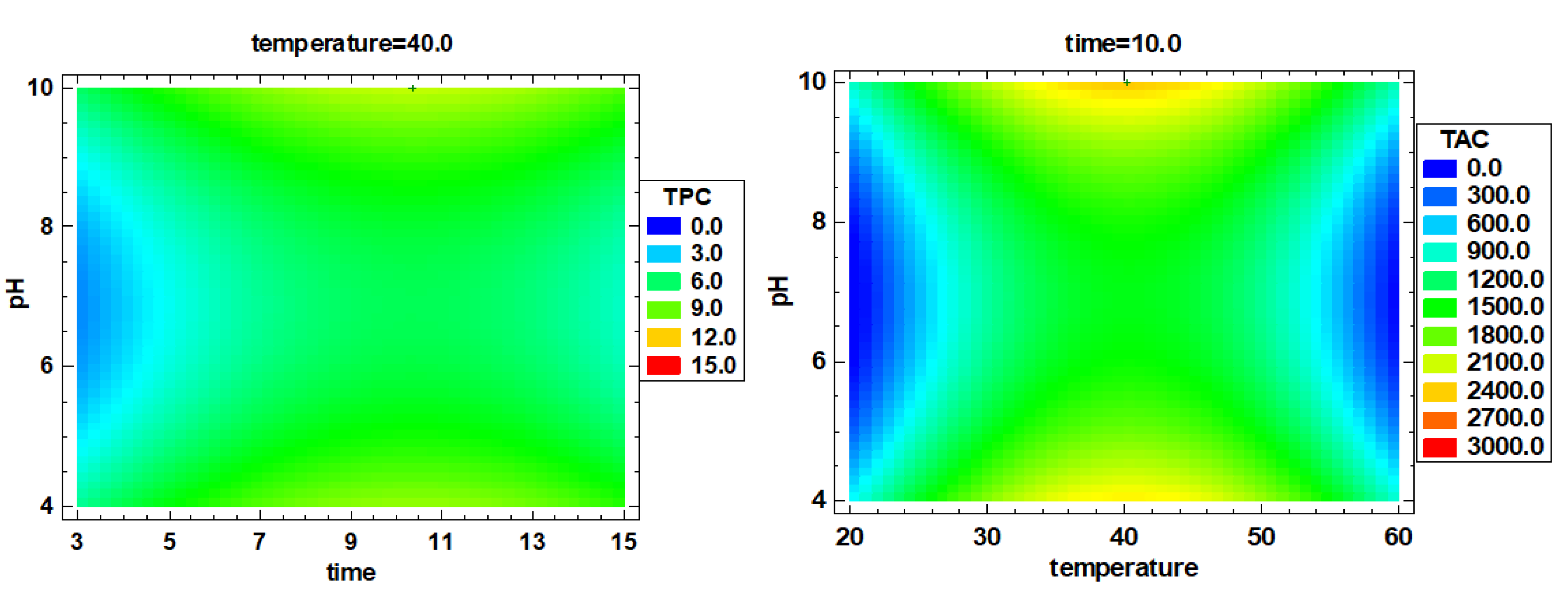
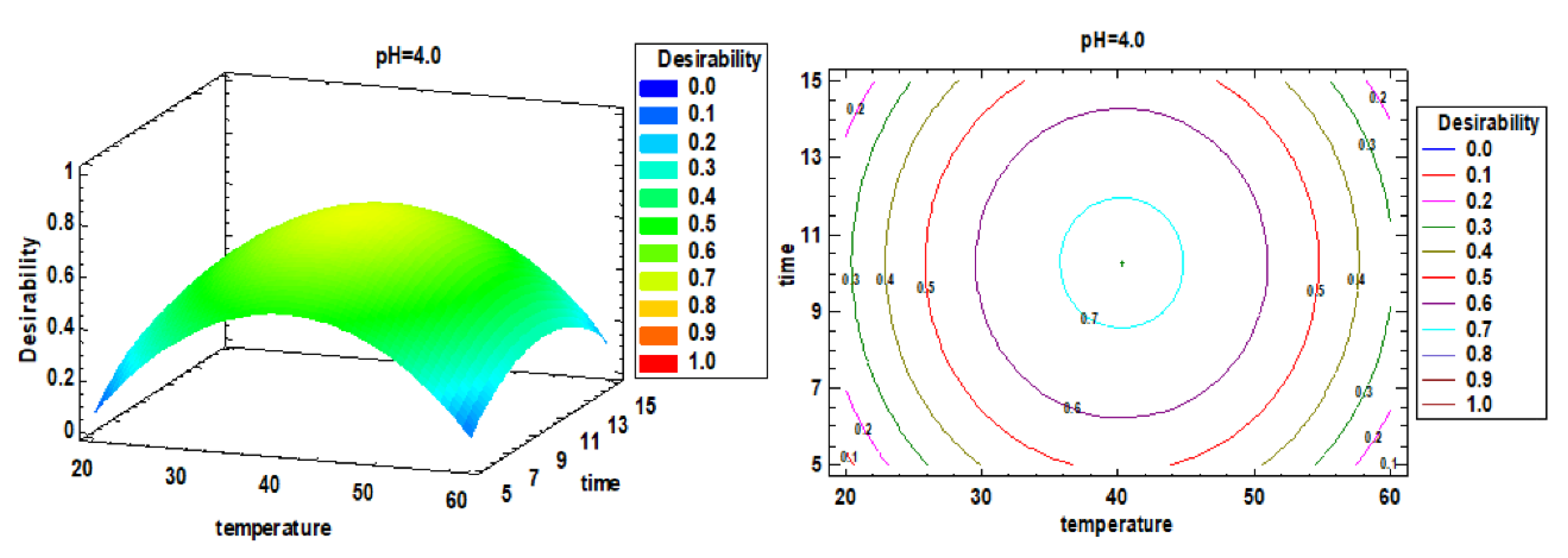
3.2. Multiple Factor Response Results and Verification Experiments
3.3. Effect of Pressure on Compounds Extraction and Antioxidant Capacity
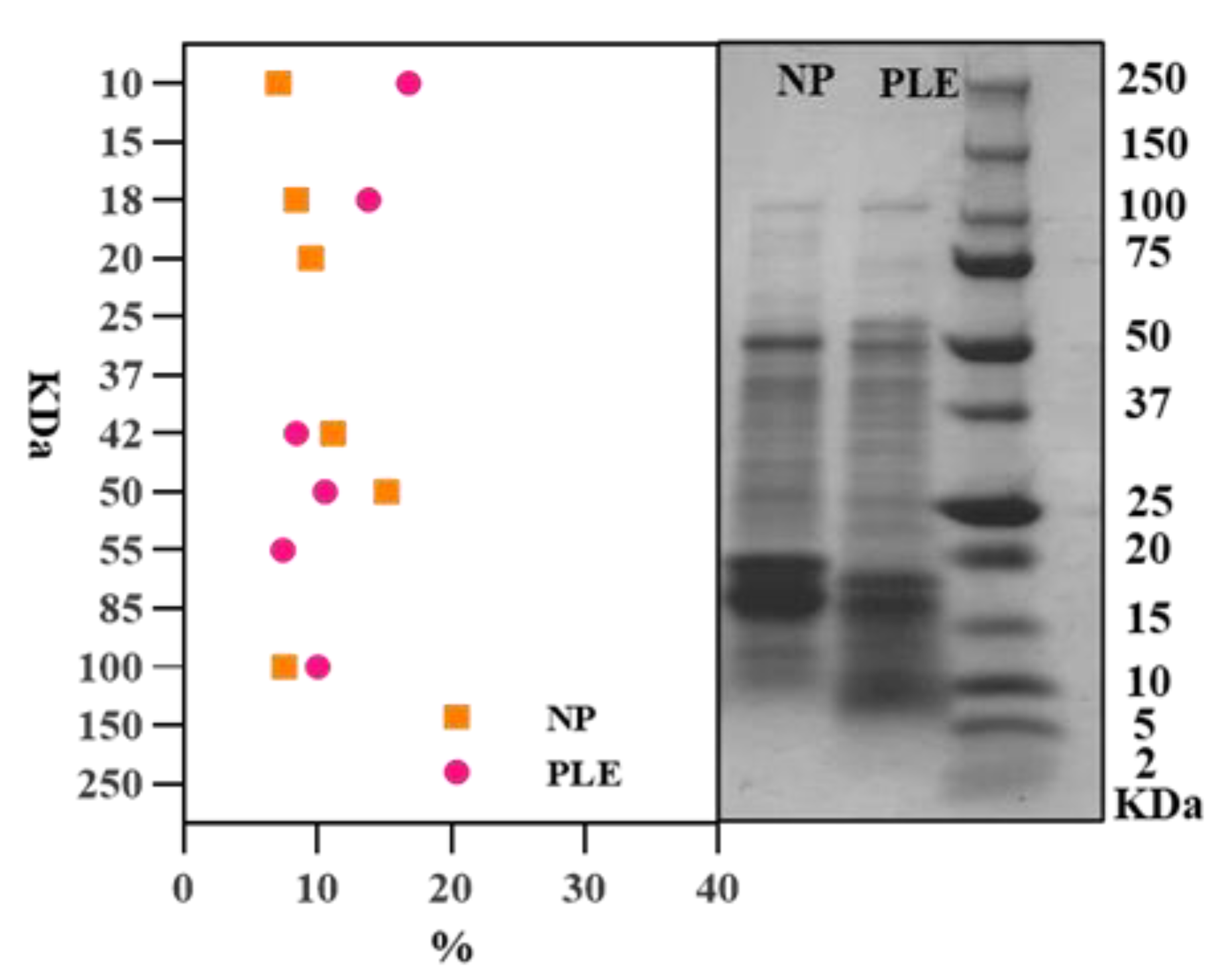
3.4. SDS-PAGE Analysis of Protein Molecules in Spirulina Extracts
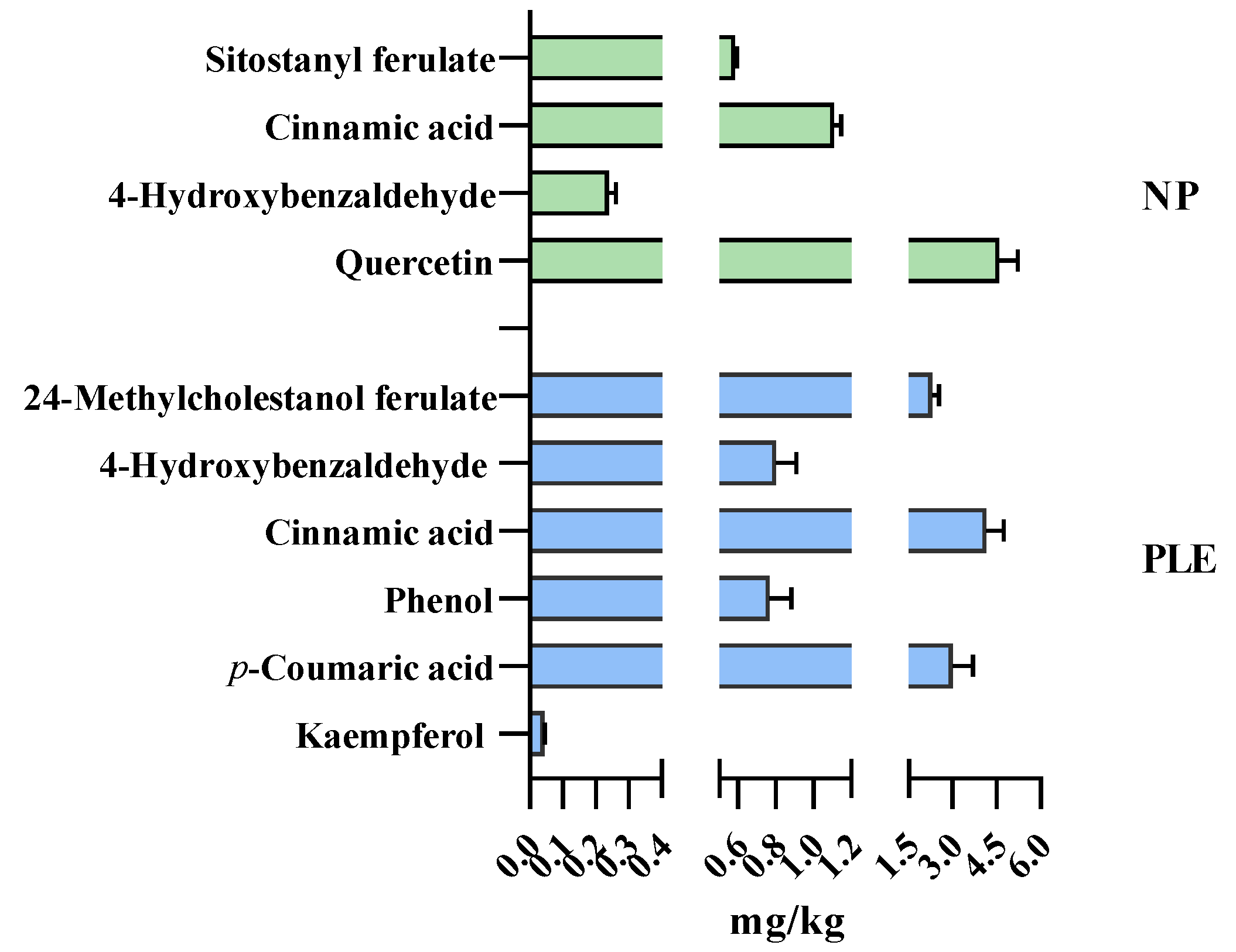
3.5. Polyphenol Profile of the Extracts: Triple TOF–LC–MS–MS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rangel-Yagui, C.D.O.; Danesi, E.D.G.; De Carvalho, J.C.M.; Sato, S. Chlorophyll production from Spirulina platensis: Cultivation with urea addition by fed-batch process. Bioresour. Technol. 2004, 92, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic diversity in the macromolecular composition of microalgae. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Gutierrez, C.M.; Guerra-Rivas, G.; Soria-Mercado, I.E.; Ayala-Sánchez, N.E. Marine edible algae as disease preventers. Adv. Food Nutr. Res. 2011, 64, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, M.; Fang, Z. Recent development in efficient processing technology for edible algae: A review. Trends Food Sci. Technol. 2019, 88, 251–259. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhang, Y.M.; Chen, F. Application of mathematical models to the determination optimal glucose concentration and light intensity for mixotrophic culture of Spirulina platensis. Process Biochem. 1999, 34, 477–481. [Google Scholar] [CrossRef]
- Danesi, E.D.G.; de O. Rangel-Yagui, C.; de Carvalho, J.C.M.; Sato, S. An investigation of effect of replacing nitrate by urea in the growth and production of chlorophyll by Spirulina platensis. Biomass Bioenergy 2002, 23, 261–269. [Google Scholar] [CrossRef]
- Morist, A.; Montesinos, J.L.; Cusidó, J.A.; Gò Dia, F. Recovery and treatment of Spirulina platensis cells cultured in a continuous photobioreactor to be used as food. Process Biochem. 2001, 37, 535–547. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef]
- Hosikian, A.; Lim, S.; Halim, R.; Bio, M.K.D. Chlorophyll extraction from microalgae: A review on the process engineering aspects. Int. J. Chem. Eng. 2010, 2010, 391632. [Google Scholar] [CrossRef] [Green Version]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field and pH assisted selective extraction of intracellular components from microalgae Nannochloropsis. Algal Res. 2015, 8, 128–134. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New Approaches for the Use of Non-conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae. Food Eng. Rev. 2014, 7, 45–62. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Ji, Y.; Li, X.; Wang, Z.; Xiao, W.; He, Z.; Xiong, Z.; Zhao, L. Extraction optimization of accelerated solvent extraction for eight active compounds from Yaobitong capsule using response surface methodology: Comparison with ultrasonic and reflux extraction. J. Chromatogr. A 2020, 1620, 460984. [Google Scholar] [CrossRef]
- Pearson, C.H.; Cornish, K.; McMahan, C.M.; Rath, D.J.; Whalen, M. Natural rubber quantification in sunflower using an automated solvent extractor. Ind. Crops Prod. 2010, 31, 469–475. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, S.; Moon, B. Optimization by response surface methodology of lutein recovery from paprika leaves using accelerated solvent extraction. Food Chem. 2016, 205, 140–145. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kundu, A.; Sharma, K.; Paul, R.K. Optimal extraction and fingerprinting of carotenoids by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry. Food Chem. 2015, 177, 369–375. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Hayes, M.; Kerry, J.P.; O’Brien, N.M. The effect of solvents on the antio xidant acti vity in Caco-2 cells of Ir ish br own sea weed extr acts pr epar ed using acceler ated solv ent extr action (ASE â). J. Funct. Foods 2013, 5, 940–948. [Google Scholar] [CrossRef]
- Denery, J.R.; Dragull, K.; Tang, C.S.; Li, Q.X. Pressurized fluid extraction of carotenoids from Haematococcus pluvialis and Dunaliella salina and kavalactones from Piper methysticum. Anal. Chim. Acta 2004, 501, 175–181. [Google Scholar] [CrossRef] [Green Version]
- de Sousa e Silva, A.; Moreira, L.M.; De Magalhães, W.T.; Farias, W.R.L.; Rocha, M.V.P.; Bastos, A.K.P. Extraction of biomolecules from Spirulina platensis using non-conventional processes and harmless solvents. J. Environ. Chem. Eng. 2017, 5, 2101–2106. [Google Scholar] [CrossRef]
- Herrero, M.; Martín-Álvarez, P.J.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef]
- de Lourdes MendesFinete, M.; Gouvêa, M.M.; de CarvalhoMarques, F.F.; Netto, A.D.P. Is it possible to screen for milk or whey protein adulteration with melamine, urea and ammonium sulphate, combining Kjeldahl and classical spectrophotometric methods? Food Chem. 2013, 141, 3649–3655. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, L.; Ebert, S.; Hinrichs, J.; Weiss, J. Production of protein-rich extracts from disrupted microalgae cells: Impact of solvent treatment and lyophilization. Algal Res. 2018, 36, 67–76. [Google Scholar] [CrossRef]
- Zhang, W.H.; Wu, J.; Weng, L.; Zhang, H.; Zhang, J.; Wu, A. An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr. Polym. 2020, 227. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Du, B.; Xu, B. Oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) of β-glucans from different sources with various molecular weight. Bioact. Carbohydrates Diet. Fibre 2014, 3, 11–16. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate composition, phenolic content and in vitro antioxidant activity of aqueous extracts of the seaweeds Ascophyllum nodosum, Bifurcaria bifurcata and Fucus vesiculosus. Effect of addition of the extracts on the oxidative stability of canola oil unde. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Jong, L.W.; Thien, V.Y.; Yong, Y.S.; Rodrigues, K.F.; Yong, W.T.L. Micropropagation and protein profile analysis by SDS-PAGE of Gracilaria changii (Rhodophyta, Solieriaceae). Aquac. Reports 2015, 1, 10–14. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Collado, M.C.; Barba, F.J. Accelerated Solvent Extraction and Pulsed Electric Fields for Valorization of Rainbow Trout (Oncorhynchus mykiss) and Sole (Dover sole) By-Products: Protein Content, Molecular Weight Distribution and Antioxidant Potential of the Extracts. Mar. Drugs 2021, 19, 207. [Google Scholar] [CrossRef]
- Parimi, N.S.; Singh, M.; Kastner, J.R.; Das, K.C.; Forsberg, L.S.; Azadi, P. Optimization of protein extraction from Spirulina platensis to generate a potential co-product and a biofuel feedstock with reduced nitrogen content. Front. Energy Res. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, W.; McKague, B.; Reeve, D.; Carey, J. A comparative evaluation of accelerated solvent extraction and Polytron extraction for quantification of lipids and extractable organochlorine in fish. Chemosphere 2004, 54, 467–480. [Google Scholar] [CrossRef]
- Vernès, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, G.; Wang, L.; Yuan, W. Optimization of antioxidant extraction from edible brown algae Ascophyllum nodosum using response surface methodology. Food Bioprod. Process. 2019, 114, 205–215. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Perez-Won, M.; Zamarca, M.; Aguilera-Radic, J.M.; Tabilo-Munizaga, G. Effects of high hydrostatic pressure on microstructure, texture, colour and biochemical changes of red abalone (Haliotis rufecens) during cold storage time. Innov. Food Sci. Emerg. Technol. 2012, 13, 42–50. [Google Scholar] [CrossRef]
- Escobedo-Avellaneda, Z.; Yildiz, S.; Lavilla, M.; Welti-Chanes, J. Strategies for development of new ingredients and food products based on HPP-induced changes in rheology. In Present and Future of High Pressure Processing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 353–380. [Google Scholar]
- Ahmad, R.; Ahmad, N.; Al-Anaki, W.S.; Ismail, F.A.; Al-Jishi, F. Solvent and temperature effect of accelerated solvent extraction (ASE) coupled with ultra-high-pressure liquid chromatography (UHPLC-PDA) for the determination of methyl xanthines in commercial tea and coffee. Food Chem. 2020, 311, 126021. [Google Scholar] [CrossRef] [PubMed]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
| Experiment | Temperature (°C) | Time (min) | pH |
|---|---|---|---|
| 1 | 20 | 10 | 7 |
| 2 | 40 | 10 | 7 |
| 3 | 40 | 5 | 7 |
| 4 | 40 | 15 | 7 |
| 5 | 40 | 10 | 7 |
| 6 | 60 | 10 | 7 |
| 7 | 20 | 5 | 4 |
| 8 | 20 | 15 | 4 |
| 9 | 40 | 10 | 4 |
| 10 | 60 | 15 | 4 |
| 11 | 60 | 5 | 4 |
| 12 | 20 | 15 | 10 |
| 13 | 20 | 5 | 10 |
| 14 | 40 | 10 | 10 |
| 15 | 60 | 15 | 10 |
| 16 | 60 | 5 | 10 |
| Run | Protein % | Carbohydrate mg/g | Chlorophyll a mg/g | Chlorophyll b mg/g | Carotenoids mg/g | Total Polyphenol mg/g | ORAC µM TE |
|---|---|---|---|---|---|---|---|
| 1 | 18.32 ± 1.10 abc | 12.98 ± 0.62 bcde | 0.267 ± 0.004 e | 0.524 ± 0.003 d | 0.092 ± 0.003 f | 1.59 ± 0.02 cd | 480.1 ± 5.5 c |
| 2 | 25.64 ± 0.00 de | 23.51 ± 0.75 f | 0.195 ± 0.003 e | 0.301 ± 0.001 c | 0.133 ± 0.004 g | 3.63 ± 0.08 fg | 365.9 ± 14.9 abc |
| 3 | 22.13 ± 0.00 cd | 26.15 ± 0.18 f | 0.283 ± 0.003 e | 0.527 ± 0.010 d | 0.137 ± 0.002 g | 4.64 ± 0.01 cde | 1491.0 ± 96.4 d |
| 4 | 29.82 ± 0.76 e | 39.83 ± 0.64 g | 0.436 ± 0.002 g | 0.600 ± 0.012 e | 0.265 ± 0.003 i | 6.23 ± 0.00 f | 1595.4 ± 96.4 d |
| 5 | 25.89 ± 0.72 de | 21.21 ± 0.65 f | 0.204 ± 0.010 e | 0.344 ± 0.002 c | 0.145 ± 0.000 g | 3.71 + ±0.08 f | 419.9 ± 58.9 bc |
| 6 | 19.48 ± 0.00 bc | 12.58 ± 0.57 bcd | 0.089 ± 0.001 b | 0.194 ± 0.007 b | 0.036 ± 0.002 d | 1.93 ± 0.01 b | 359.1 ± 47.8 abc |
| 7 | 15.21 ± 0.49 ab | 6.90 ± 0.25 a | 0.110 ± 0.000 c | 0.216 ± 0.002 b | 0.031 ± 0.000 cd | 0.97 ± 0.01 a | 148.1 ± 7.6 ab |
| 8 | 14.02 ± 1.08 a | 10.80 ± 0.37 abc | 0.057 ± 0.001 a | 0.120 ± 0.002 a | 0.004 ± 0.000 a | 0.63 ± 0.04 a | 129.2 ± 16.1 a |
| 9 | 47.87 ± 1.20 f | 80.95 ± 2.90 i | 1.406 ± 0.007 i | 2.017 ± 0.017 g | 0.648 ± 0.002 j | 11.61 ± 0.39 h | 2915.5 ± 371.4 e |
| 10 | 24.07 ± 1.60 d | 14.06 ± 1.06 cde | 0.061 ± 0.001 a | 0.106 ± 0.002 a | 0.013 ± 0.000 b | 2.79 ± 0.17 cde | 414.2 ± 48.3 bc |
| 11 | 15.15 ± 0.68 ab | 8.81 ± 0.14 ab | 0.058 ± 0.001 a | 0.129 ± 0.002 a | 0.015 ± 0.001 b | 1.61 ± 0.06 b | 247.0 ± 19.2 abc |
| 12 | 15.66 ± 0.51 ab | 15.55 ± 0.15 de | 0.225 ± 0.003 d | 0.487 ± 0.002 d | 0.085 ± 0.001 ef | 2.78 ± 0.07 de | 427.8 ± 30.6 c |
| 13 | 14.43 ± 0.00 a | 10.55 ± 0.38 abc | 0.090 ± 0.011 bc | 0.140 ± 0.003 a | 0.001 ± 0.000 a | 2.41 ± 0.02 g | 280.6 ± 34.7 abc |
| 14 | 45.81 ± 0.58 f | 58.90 ± 3.02 h | 0.516 ± 0.008 h | 1.626 ± 0.023 f | 0.002 ± 0.000 a | 10.85 ± 0.19 h | 2831.1 ± 295.5 e |
| 15 | 25.37 ± 1.21 de | 14.41 ± 0.40 cde | 0.058 ± 0.000 a | 0.095 ± 0.001 a | 0.029 ± 0.000 c | 2.75 ± 0.08 e | 468.5 ± 53.5 c |
| 16 | 19.33 ± 0.00 bc | 13.04 ± 0.36 bcde | 0.114 ± 0.001 c | 0.118 ± 0.001 a | 0.081 ± 0.001 e | 2.35 ± 0.06 c | 485.4 ± 61.6 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wang, M.; Carrillo, C.; Zhu, Z.; Brncic, M.; Berrada, H.; Barba, F.J. Impact of Pressurized Liquid Extraction and pH on Protein Yield, Changes in Molecular Size Distribution and Antioxidant Compounds Recovery from Spirulina. Foods 2021, 10, 2153. https://doi.org/10.3390/foods10092153
Zhou J, Wang M, Carrillo C, Zhu Z, Brncic M, Berrada H, Barba FJ. Impact of Pressurized Liquid Extraction and pH on Protein Yield, Changes in Molecular Size Distribution and Antioxidant Compounds Recovery from Spirulina. Foods. 2021; 10(9):2153. https://doi.org/10.3390/foods10092153
Chicago/Turabian StyleZhou, Jianjun, Min Wang, Celia Carrillo, Zhenzhou Zhu, Mladen Brncic, Houda Berrada, and Francisco J. Barba. 2021. "Impact of Pressurized Liquid Extraction and pH on Protein Yield, Changes in Molecular Size Distribution and Antioxidant Compounds Recovery from Spirulina" Foods 10, no. 9: 2153. https://doi.org/10.3390/foods10092153
APA StyleZhou, J., Wang, M., Carrillo, C., Zhu, Z., Brncic, M., Berrada, H., & Barba, F. J. (2021). Impact of Pressurized Liquid Extraction and pH on Protein Yield, Changes in Molecular Size Distribution and Antioxidant Compounds Recovery from Spirulina. Foods, 10(9), 2153. https://doi.org/10.3390/foods10092153