Comparison of Nutritional and Nutraceutical Properties of Burdock Roots Cultivated in Fengxian and Peixian of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Preparation of Burdock Root Powder and Extract Samples
2.3. Chemical Composition Analysis
2.4. Determination of Amino Acids
2.5. In Vitro Antioxidant Capacity
2.6. Ultra Performance Liquid Chromatography Tandem Mass Spectrometry (UPLC-MS/MS) for Identification of Phenolic Composition
2.7. Determination of Volatile Compounds
2.8. Statistical Analysis
3. Results and Discussion
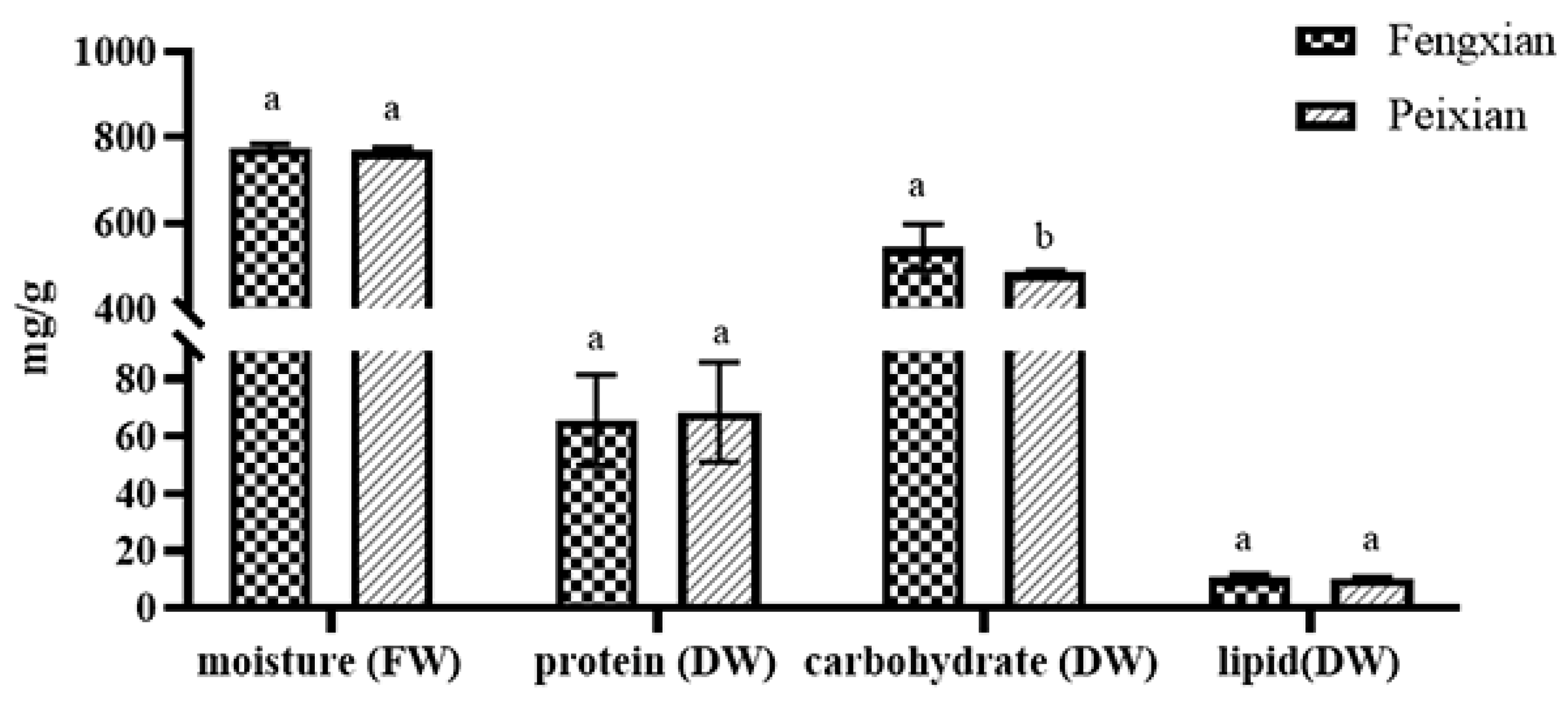
3.1. Nutritional Analysis of Burdock Root Powder Samples
3.2. Determination of Amino Acids in Burdock Root Powder Samples
3.3. Chemical Composition and In Vitro Antioxidant Activity of BRP Samples
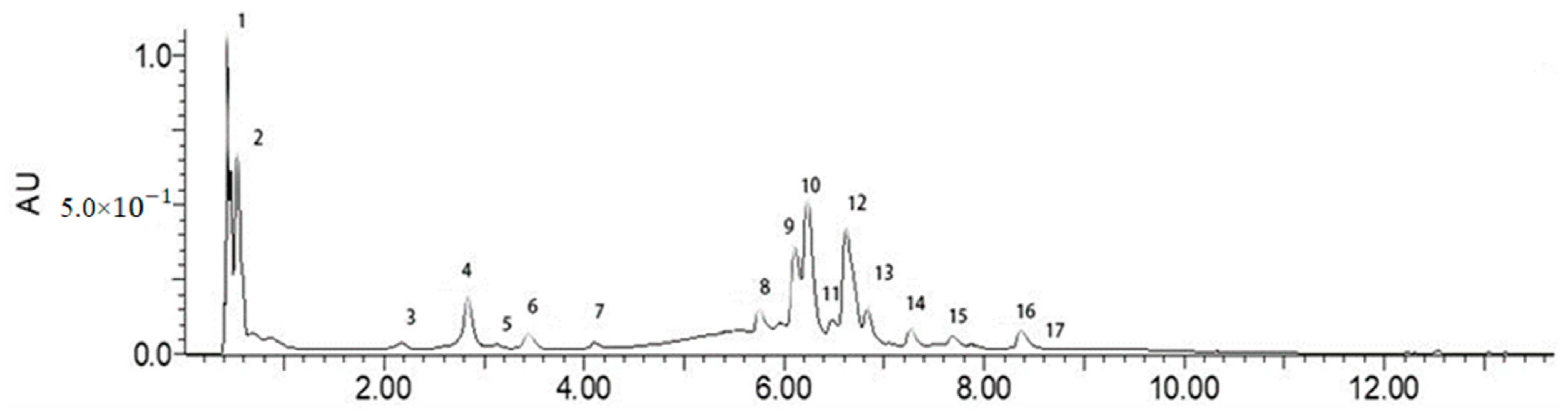
3.4. Phenolic Composition of F-BRP
3.5. Element Content
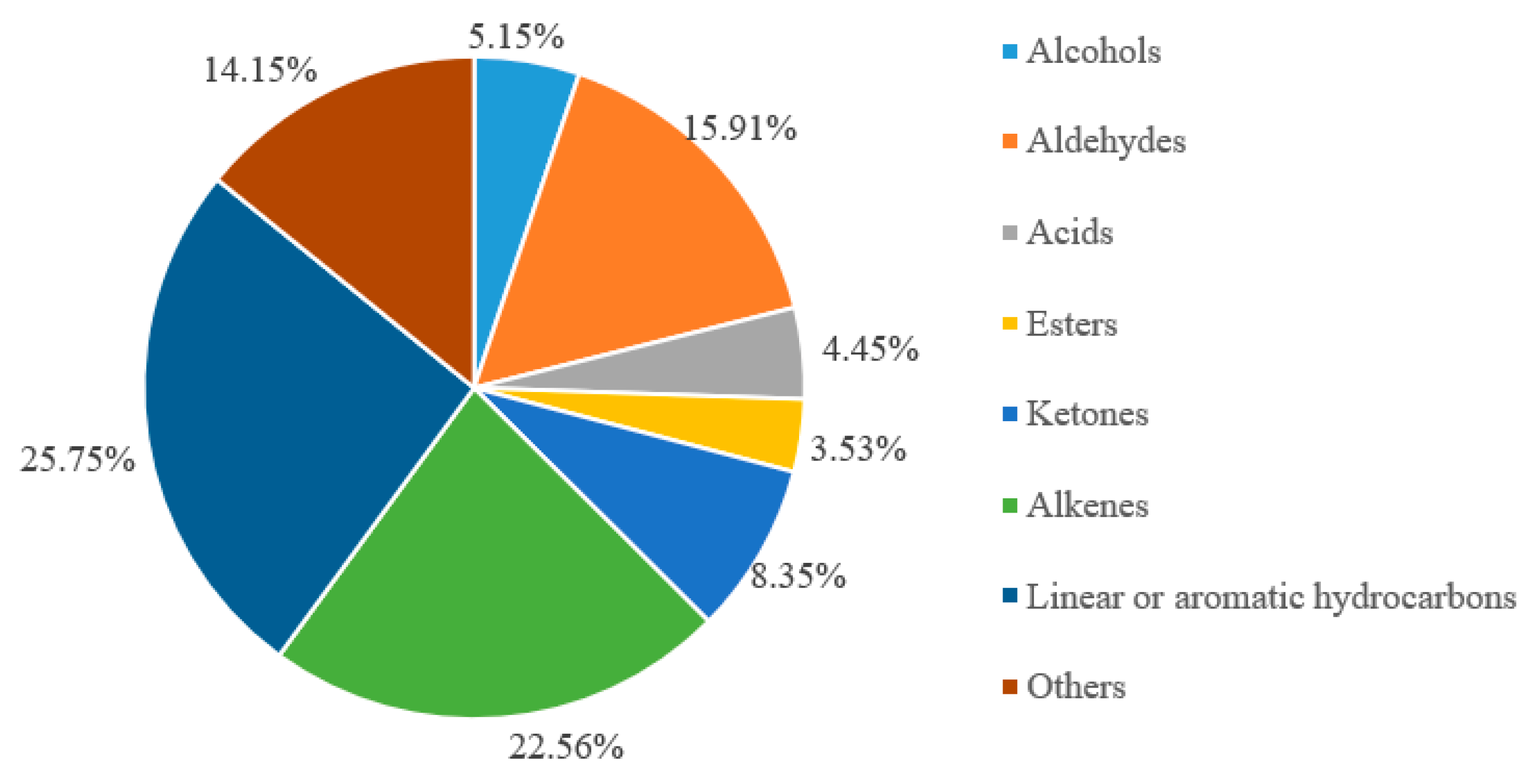
3.6. Volatile Composition of F-BRP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.D.; Bădărau, A.S.; Swamy, M.K.; Shaw, S.; Maggi, F.; da Silva, L.E.; López, V.; Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Arctium species secondary metabolites chemodiversity and bioactivities. Front. Plant. Sci. 2019, 10, 834. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Beta, T.; Chai, Z.; Zhang, X.X.; Li, Y.; Huang, W.Y. Effect of in vitro gastro-intestinal digestion on the phenolic composition and antioxidant capacity of Burdock roots at different harvest time. Food Chem. 2021, 358, 129897. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, M.Y.; Leung, P.H.; Yu, H.F.; Chan, S.W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharmaceut. Biomed. 2010, 51, 399–404. [Google Scholar] [CrossRef]
- Liu, J.Y.; Cai, Y.Z.; Wong, R.N.; Lee, C.K.; Tang, S.C.; Sze, S.C.; Tong, Y.; Zhang, Y.B. Comparative analysis of caffeoylquinic acids and lignans in roots and seeds among various burdock (Arctium lappa) genotypes with high antioxidant activity. J. Agric. Food Chem. 2012, 60, 4067–4075. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.A.; Wu, A.B.; Chen, C.Y. The influence of different treatments on the free radical scavenging activity of burdock and variations of its active components. Food Chem. 2004, 86, 479–484. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, J.M.; Yang, J.J.; Chuang, S.C.; Ujiie, T. Anti-inflammatory and radical scavenge effects of Arctium lappa. Am. J. Chin. Med. 1996, 24, 127–137. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Allemand, A.; Mendes, D.A.G.B.; Dos Santos, A.C.; Andr´e, E.; De Souza, L.M.; Werner, M.F. Ethanolic extract of roots from Arctium lappa L. accelerates the healing of acetic acid-induced gastric ulcer in rats: Involvement of the antioxidant system. Food Chem. Toxicol. 2013, 51, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Wang, W.; Gao, H.; Cai, S.; Wang, C. Effects of aqueous extract of Arctium lappa L. roots on serum lipid metabolism. J. Int. Med. Res. 2018, 46, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.L.; Gu, L.W.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Uni. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.M.; Huang, W.Y.; Chen, W.X.; Han, L.; Zhang, H.D. Optimization of extraction conditions of areca seed polyphenols and evaluation of their antioxidant activities. Molecules 2014, 19, 16416–16427. [Google Scholar] [CrossRef] [PubMed]
- Kriukova, A.I.; Vladymyrova, I.M.; Levashova, O.L.; Tishakova, T.S. Determination of amino acid composition in the harpagophytum procumbens root. Dhaka Uni. J. Pharm. Sci. 2019, 18, 85–91. [Google Scholar] [CrossRef]
- Wu, H.; Chai, Z.; Hutabarat, R.P.; Zeng, Q.L.; Niu, L.Y.; Li, D.J.; Yu, H.; Huang, W.Y. Blueberry leaves from 73 different cultivars in southeastern China as nutraceutical supplements rich in antioxidants. Food Res. Int. 2019, 122, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.A.; Nur, A.R.; Anisah, J.; Shaiful, A.S.; Kamariah, L. Enhancement of phenolic acid content and antioxidant activity of rice bran fermented with Rhizopus oligosporus and Monascus purpureus. Biocatal. Agric. Biotechnol. 2015, 4, 33–38. [Google Scholar]
- Lin, L.Z.; Harnly, J.M. Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. J. Agric. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef]
- Chai, Z.; Tian, L.L.; Yu, H.; Zhang, L.C.; Zeng, Q.L.; Wu, H.; Yan, Z.; Li, D.J.; Hutabarat, R.P.; Huang, W.Y. Comparison on chemical compositions and antioxidant capacities of the green, oolong, and red tea from blueberry leaves. Food Sci. Nutr. 2020, 8, 1688–1699. [Google Scholar] [CrossRef]
- Feng, F.J.; Li, M.J.; Ma, F.W.; Cheng, L.L. Effects of location within the tree canopy on carbohydrates, organic acids, amino acids and phenolic compounds in the fruit peel and flesh from three apple (Malus × domestica) cultivars. Hortic. Res. 2014, 1, 14019. [Google Scholar] [CrossRef] [Green Version]
- Oloumi, H. Study the correlation between some climate parameters and the content of phenolic compounds in roots of Glycyrrhiza glabra. J. Med. Plant. Res. 2011, 5, 6011–6016. [Google Scholar]
- Song, W.W.; Yang, R.P.; Wu, T.T.; Wu, C.X.; Sun, S.; Zhang, S.W.; Jiang, B.J.; Tian, S.Y.; Liu, X.B.; Han, T.F. Analyzing the effects of climate factors on soybean protein, oil contents, and composition by extensive and high-density sampling in China. J. Agric. Food Chem. 2016, 64, 4121–4130. [Google Scholar] [CrossRef]
- Wu, G.Y.; Jaeger, L.A.; Bazer, F.W.; Rhoads, J.M. Arginine deficiency in preterm infants: Biochemical mechanisms and nutritional implications. J. Nutr. Biochem. 2004, 15, 442–451. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A truly functional amino acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef]
- Ritterhoff, J.; Young, S.; Villet, O.; Shao, D.; Neto, C.F.; Bettcher, F.L.; Hsu, A.Y.; Kolwicz, C.S.; Raftery, D.; Tian, R. Metabolic remodeling promotes cardiac hypertrophy by directing glucose to aspartate biosynthesis. Circ. Res. 2020, 126, 182–196. [Google Scholar] [CrossRef]
- Xavier, A.A.; Pérez-Gálvez, A. Carotenoids as a source of antioxidants in the diet. Subcell. Biochem. 2016, 79, 359–375. [Google Scholar]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Woznicki, T.L.; Heide, O.M.; Sønsteby, A.; Wold, A.B.; Remberg, S.F. Effects of controlled post-flowering temperature and daylength on chemical composition of four black currant (Ribes nigrum L.) cultivars of contrasting origin. Sci. Hortic.-Amst. 2015, 197, 627–636. [Google Scholar] [CrossRef]
- Prior, R.L.; Gao, G. In vivo total antioxidant capacity: Composition of different analytical methods. Free Radic. Bio. Med. 1999, 27, 1173–1181. [Google Scholar] [CrossRef]
- Lee, J.; Seo, W.; Lim, W.; Cho, K. Phenolic contents and antioxidant activities from different tissues of Baekseohyang (Daphne kiusiana). Food Sci. Biotechnol. 2011, 20, 695–702. [Google Scholar] [CrossRef]
- Lou, Z.X.; Wang, H.X.; Li, J.; Chen, S.W.; Zhu, S.; Ma, C.Y.; Wang, Z.P. Antioxidant activity and chemical composition of the fractions from burdock leaves. J. Food Sci. 2010, 75, C413–C419. [Google Scholar] [CrossRef] [PubMed]
- Zacarías-García, J.; Rey, F.; Gil, J.-V.; Rodrigo, M.J.; Zacarías, L. Antioxidant capacity in fruit of Citrus cultivars with marked differences in pulp coloration: Contribution of carotenoids and vitamin C. Food Sci. Technol. Int. 2020, 27, 108201322094401. [Google Scholar]
- Maadane, A.; Merghoub, N.; Ainane, T.; Arroussi, H.E.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant activity of some Moroccan marine microalgae: Pufaprofiles, carotenoids and phenolic content. J. Biotechnol. 2015, 215, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Carlotto, J.; da Silva, L.M.; Dartora, N.; Maria-Ferreira, D.; Sabry, D.; Filho, A.P.; Werner, M.F.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M.; et al. Identification of a dicaffeoylquinic acid isomer from Arctium lappa with a potent anti-ulcer activity. Talanta 2015, 135, 50–57. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kuhnert, N. Identification and characterization of five new classes of chlorogenic acids in burdock (Arctium lappa L.) roots by liquid chromatography/tandem mass spectrometry. Food Funct. 2011, 2, 63–71. [Google Scholar] [CrossRef]
- Jiang, X.W.; Bai, J.P.; Zhang, Q.; Hu, X.L.; Tian, X.; Zhu, J.; Liu, J.; Meng, W.H.; Zhao, Q.C. Caffeoylquinic acid derivatives from the roots of Arctium lappa L. (burdock) and their structure–activity relationships (SARs) of free radical scavenging activities. Phytochem. Lett. 2016, 15, 159–163. [Google Scholar] [CrossRef]
- Reid, I.R.; Bolland, M.J. Controversies in medicine: The role of calcium and vitamin D supplements in adults. Med. J. Aust. 2019, 211, 468–473. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Erikson, K.M.; Aschner, M. Manganese: Its role in disease and health. Metal. Ions Life Sci. 2019, 19, 253–266. [Google Scholar]
- Saghiri, M.A.; Asatourian, A.; Orangi, J.; Sorenson, C.M.; Sheibani, N. Functional role of inorganic trace elements in angiogenesis—Part II: Cr, Si, Zn, Cu, and S. Crit. Rev. Oncol. Hematol. 2015, 96, 143–155. [Google Scholar] [CrossRef]
- Maret, W. Chromium supplementation in human health, metabolic syndrome, and diabetes. Met. Ions Life Sci. 2019, 19, 231–251. [Google Scholar]
- Choi, S.Y.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.Y.; Gan, X.Q.; Lu, L.Y.; Wei, B.H. Nutritional quality analysis of different burdock varieties roots. China Veg. 2015, 6, 73–75. [Google Scholar]
- Robin, J.; Ashu, G. Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. Food Chem. 2015, 167, 290–298. [Google Scholar]
- Peng, X.Y.; Li, X.; Shi, X.D.; Guo, S.T. Evaluation of the aroma quality of Chinese traditional soy paste during storage based on principal component analysis. Food Chem. 2014, 151, 532–538. [Google Scholar] [CrossRef]
- Li, Z.W.; Wang, J.H. Identification and similarity analysis of aroma substances in main types of Fenghuang Dancong tea. PLoS ONE 2020, 15, e0244224. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.Y.; Liu, L.L.; Zheng, J.; Chen, T.; Chen, F.; Li, P.P.; Zhang, M.; Shen, X.J. Correlation analysis between aroma components and microbial communities in Wuliangye-flavor raw liquor based on HS-SPME/LLME-GC-MS and PLFA. Food Res. Int. 2021, 140, 109995. [Google Scholar] [CrossRef]
| Amino Acid 1 | F-BRP | P-BRP |
|---|---|---|
| Aspartic acid (Asp) | 11.062 | 13.014 |
| Glutamic acid (Glu) | 18.289 | 17.656 |
| Serine (Ser) | 3.382 | 3.165 |
| Histidine (His) | 0.806 | 0.848 |
| Glycine (Gly) | 2.659 | 2.366 |
| Arginine (Arg) | 19.696 | 16.526 |
| Alanine (Ala) | 1.978 | 1.971 |
| Tyrosine (Tyr) | 2.151 | 1.735 |
| Cysteine (Cys) | 1.035 | 1.459 |
| Proline (Pro) | 6.420 | 5.650 |
| Valine (Val) | 2.279 | 2.368 |
| Phenylalanine (Phe) | 4.371 | 4.512 |
| Isoleucine (Ile) | 2.000 | 2.148 |
| Leucine (Leu) | 2.784 | 2.884 |
| Lysine (Lys) | 1.849 | 1.460 |
| Threonine (Thr) | 2.868 | 2.690 |
| EAA | 16.151 | 16.062 |
| NAA | 67.478 | 64.390 |
| PAA | 62.710 | 60.566 |
| SAA | 14.439 | 13.152 |
| UAA | 29.351 | 30.670 |
| BAA | 31.936 | 29.286 |
| TAA | 80.761 | 77.762 |
| Sample 1 | Carotenoid | Vitamin C | TPC | TFC | DPPH | ABTS | FRAP | ORAC |
|---|---|---|---|---|---|---|---|---|
| (mg/g DW) | (mg/g DW) | (mg GAE/g DW) | (mg RTE/g DW) | (mg TEAC/g DW) | (mg TEAC/g DW) | (mg FEAC/g DW) | (mg TEAC/g DW) | |
| Fengxian | ||||||||
| BRP | 10.08 ± 0.66 a | 1.76 ± 0.18 c | 186.20 ± 0.76 c | 8.98 ± 0.10 b | 40.20 ± 0.66 c | 10.88 ± 0.21 c | 4.52 ± 0.21 c | 66.78 ± 1.96 c |
| PBRP | 6.03 ± 0.18 b | 1.77 ± 0.43 c | 156.33 ± 1.04 d | 6.23 ± 0.11 c | 29.29 ± 0.24 d | 8.21 ± 0.49 d | 3.21 ± 0.12 d | 28.46 ± 1.19 e |
| BRPP | 1.95 ± 0.23 d | 13.02 ± 1.61 a | 776.46 ± 2.86 a | 43.65 ± 2.68 a | 109.76 ± 10.14 a | 41.24 ± 0.75 b | 26.51 ± 0.24 a | 144.38 ± 5.76 a |
| Peixian | ||||||||
| BRP | 10.24 ± 0.65 a | 1.65 ± 0.18 c | 151.31 ± 1.04 e | 8.62 ± 0.07 b | 38.76 ± 1.42 c | 8.70 ± 0.18 d | 2.89 ± 0.12 d | 47.76 ± 1.22 d |
| PBRP | 4.89 ± 0.45 c | 1.49 ± 0.16 c | 127.53 ± 0.58 f | 6.60 ± 0.37 c | 29.69 ± 1.12 d | 7.26 ± 0.33 e | 2.09 ± 0.09 e | 19.83 ± 2.40 f |
| BRPP | 2.04 ± 0.31 d | 12.31 ± 1.53 b | 722.14 ± 3.64 b | 41.75 ± 1.53 a | 94.71 ± 1.42 b | 49.86 ± 0.32 a | 22.87 ± 0.76 b | 94.63 ± 2.53 b |
| Peak | Rt (min) 1 | Mass Parent ion m/z | Calc. MW | Tentative Compound |
|---|---|---|---|---|
| 1 | 0.43 | 191 | 192 | Quinic acid |
| 2 | 0.53 | 191 | 192 | Citric acid |
| 3 | 2.175 | 163 | 164 | p-Coumaric acid |
| 4 | 2.836 | 353 | 354 | 3-caffeoylquinic acid (Chlorogenic acid) |
| 5 | 3.131 | 353 | 354 | 4-caffeoylquinic acid (Cryptochlorogenic acid) |
| 6 | 3.445 | 397 | 398 | 5-sinapoylquinic acid |
| 7 | 4.104 | 515 | 516 | 3,4-di-O-caffeoylquinic acid |
| 8 | 5.754 | 631 | 632 | caffeoylquinic acid glycoside |
| 9 | 6.112 | 615 | 616 | 1,5-di-O-caffeoyl-3-O-succinoylquinic acid |
| 10 | 6.235 | 515 | 516 | 1,5-di-O-caffeoylquinic acid |
| 11 | 6.486 | 367 | 368 | O-Feruloylquinic acid |
| 12 | 6.622 | 615 | 616 | 1,5-di-O-caffeoyl-4-O-succinoylquinic acid |
| 13 | 6.826 | 301 | 302 | Quercetin |
| 14 | 7.266 | 631 | 632 | 3,4-di-O-caffeoyl-5-O-maloylquinic acid |
| 15 | 7.687 | 515 | 516 | 3,5-di-O-caffeoylquinic acid |
| 16 | 8.376 | 631 | 632 | 1,5-di-O-caffeoyl-4-O-maloylquinic acid |
| 17 | 8.597 | 179 | 180 | Caffeic acid |
| Element | Content (µg/g) |
|---|---|
| aluminum (Al) | 192.48 |
| barium (Ba) | 2.90 |
| beryllium (Be) | <0.50 |
| calcium (Ca) | 4205.58 |
| cadmium (Cd) | <0.50 |
| cobalt (Co) | <0.50 |
| chromium (Cr) | 20.45 |
| copper (Cu) | 13.23 |
| iron (Fe) | 58.49 |
| potassium (K) | 10,897.38 |
| magnesium (Mg) | 2424.32 |
| manganese (Mn) | 4.82 |
| sodium (Na) | 751.57 |
| nickel (Ni) | <0.50 |
| phosphorus (P) | 3176.83 |
| lead (Pb) | <0.50 |
| antimony (Sb) | <0.50 |
| titanium (Ti) | 4.59 |
| vanadium (V) | <0.50 |
| zinc (Zn) | 16.73 |
| Compounds | RT (min) 1 | Area (%) |
|---|---|---|
| Alcohols | ||
| hexyl alcohol | 9.440 | 0.82 |
| benzyl alcohol | 14.863 | 0.66 |
| phenethyl alcohol | 17.957 | 1.00 |
| 1-penten-3-ol, 2-methyl- | 20.939 | 0.41 |
| elemol | 27.986 | 0.33 |
| 1-hexadecanol | 31.103 | 0.79 |
| Aldehydes | ||
| hexanal | 7.828 | 4.64 |
| furfural | 8.664 | 0.42 |
| (E)-2-hexenal | 9.128 | 0.44 |
| (E)-2-heptenal | 12.034 | 0.56 |
| benzaldehyde | 12.340 | 1.11 |
| (E,E)-2,4-heptadienal | 13.963 | 0.56 |
| phenylethanal | 15.310 | 0.87 |
| (E)-2-octenal | 15.734 | 1.52 |
| n-nonanal | 17.486 | 2.38 |
| (E)-2-nonenal | 19.622 | 0.70 |
| n-decanal | 21.339 | 1.16 |
| (E,E)-2,4-nonadienal | 21.698 | 0.45 |
| Acids | ||
| hexanoic acid | 12.828 | 3.05 |
| 2-ethyl-hexanoic acid | 17.781 | 0.31 |
| 2-((2-chloroethoxy) carbonyl) benzoic acid | 43.844 | 0.46 |
| Alkenes | ||
| 3-(2-methylpropyl)-cyclohexene | 13.328 | 3.53 |
| 1-dodecene | 27.874 | 0.39 |
| β-elemene | 28.221 | 2.48 |
| cadinene | 28.668 | 0.40 |
| α-cedrene | 30.968 | 0.45 |
| α-curcumene | 31.062 | 1.01 |
| (-)-alloaromadendrene | 31.433 | 0.67 |
| (Z,Z)-1,8,11-heptadecatriene | 36.756 | 1.28 |
| (Z,Z,Z)-1,8,11,14-heptadecatetraene | 36.985 | 11.35 |
| Esters | ||
| sulfurous acid, hexyl octyl ester | 25.039 | 0.40 |
| oxalic acid, 2-ethylhexyl isohexyl ester | 26.986 | 0.35 |
| 2,2,4-trimethyl-1,3-pentanediol diisobutyrate | 34.585 | 1.05 |
| Ketones | ||
| octa-3-ene-2-one | 14.987 | 1.03 |
| (Z,E) and (E,E)-3,5-octadien-2-one | 16.210 | 2.28 |
| (E,E)-3,5-octadien-2-one | 17.086 | 2.20 |
| 2,6-bis(1,1-dimethylethyl)-4-hydroxy-4-methyl-2,5-Cyclohexadien-1-one | 30.615 | 1.18 |
| Linear or aromatic hydrocarbons | ||
| n-undecane | 17.268 | 0.38 |
| decamethyl-cyclopentasiloxane | 19.239 | 2.07 |
| naphthalene | 20.851 | 0.76 |
| dodecane | 21.074 | 1.95 |
| n-tridecane | 24.715 | 1.89 |
| 1-methyl-naphthalene | 24.892 | 0.43 |
| 5-(2-methylpropyl)-nonane | 25.968 | 0.36 |
| 5-Ethyldecane | 26.092 | 0.32 |
| 4,5-diethyl-octane | 26.368 | 0.60 |
| 3,5-dimethyldodecane | 27.156 | 1.31 |
| 2,6,10,14-tetramethyl-hexadecane | 27.368 | 0.32 |
| 3-methyl-6-methylene-octane | 27.674 | 0.43 |
| tetradecane | 28.145 | 2.98 |
| nonyl-cyclopentane | 29.827 | 0.56 |
| 2,6,10-trimethyltridecane | 30.150 | 0.47 |
| tetradecamethyl-cycloheptasiloxane | 31.291 | 0.76 |
| tetradecane | 31.333 | 0.50 |
| 1,2,3,4,4a,5,6,8a-octahydro-4a,8-dimethyl-2-(1-methylethenyl)-naphthalene | 31.691 | 0.62 |
| n-tetradecane | 33.503 | 0.49 |
| hexadecane | 34.427 | 0.47 |
| Others | ||
| dimethyl sulfide | 4.440 | 1.28 |
| 3-methyl-5-hydroxy-isoxazole | 10.169 | 0.32 |
| 2-sec-butyl-3-methoxypyrazine | 20.157 | 0.31 |
| 2-methoxy-3-isobutyl pyrazine | 20.475 | 0.33 |
| 2-(1,1-dimethylethyl)-6-methyl-phenol | 26.915 | 0.39 |
| 2,4-di-tert-butylphenol | 31.797 | 0.98 |
| 4-methyl-2,6-di-tert-butyl | 31.991 | 2.19 |
| 2-(3,5-dimethyl-1h-pyrazol-1-yl) pyridine | 36.150 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Herrera-Balandrano, D.D.; Huang, W.; Chai, Z.; Beta, T.; Wang, J.; Feng, J.; Li, Y. Comparison of Nutritional and Nutraceutical Properties of Burdock Roots Cultivated in Fengxian and Peixian of China. Foods 2021, 10, 2095. https://doi.org/10.3390/foods10092095
Zhang X, Herrera-Balandrano DD, Huang W, Chai Z, Beta T, Wang J, Feng J, Li Y. Comparison of Nutritional and Nutraceutical Properties of Burdock Roots Cultivated in Fengxian and Peixian of China. Foods. 2021; 10(9):2095. https://doi.org/10.3390/foods10092095
Chicago/Turabian StyleZhang, Xiaoxiao, Daniela D. Herrera-Balandrano, Wuyang Huang, Zhi Chai, Trust Beta, Jing Wang, Jin Feng, and Ying Li. 2021. "Comparison of Nutritional and Nutraceutical Properties of Burdock Roots Cultivated in Fengxian and Peixian of China" Foods 10, no. 9: 2095. https://doi.org/10.3390/foods10092095
APA StyleZhang, X., Herrera-Balandrano, D. D., Huang, W., Chai, Z., Beta, T., Wang, J., Feng, J., & Li, Y. (2021). Comparison of Nutritional and Nutraceutical Properties of Burdock Roots Cultivated in Fengxian and Peixian of China. Foods, 10(9), 2095. https://doi.org/10.3390/foods10092095








