The Impact of Different Closures on the Flavor Composition of Wines during Bottle Aging
Abstract
:1. Introduction
2. Types of Wine Bottle Closures
2.1. Cork Stoppers
2.2. Synthetic Closures
2.3. Screw Caps
3. Contribution of Different Closures to Wine Flavor Composition during Aging
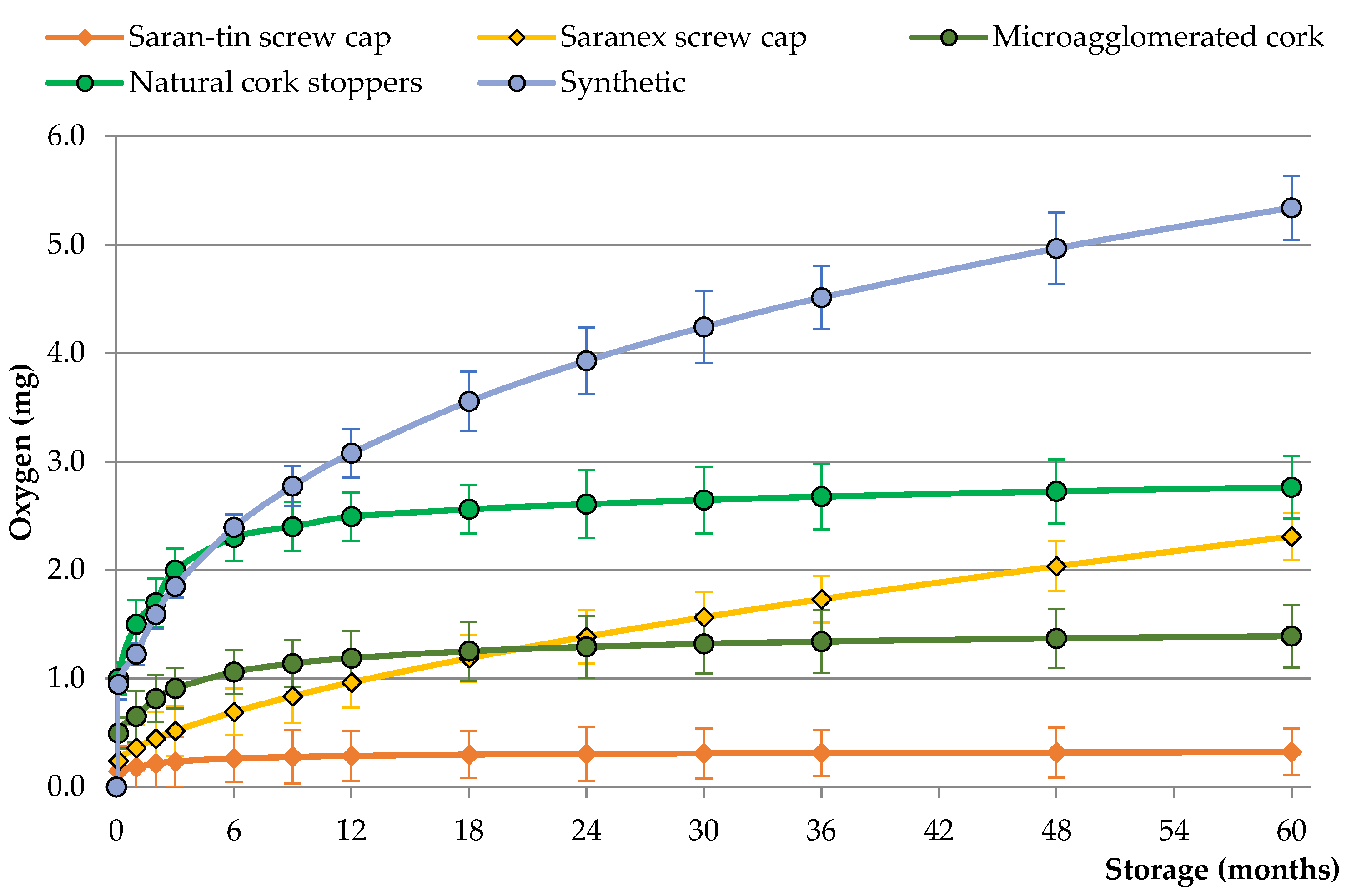
3.1. Oxygen Transmission Rate (OTR) of Closures
3.2. Desorption of Volatile Compounds from Closures into Wine
3.3. Scalping of Volatile Compounds Present in Wine by Closures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145. [Google Scholar] [CrossRef]
- Clarke, R.J.; Bakker, J. Factors influencing sensory perception. In Wine Flavour Chemistry; Blackwell Publishing: Oxford, UK, 2004; pp. 202–204. [Google Scholar]
- Silva, M.A.; Julien, M.; Jourdes, M.; Teissedre, P.-L. Impact of closures on wine post-bottling development: A review. Eur. Food Res. Technol. 2011, 233, 905–914. [Google Scholar] [CrossRef]
- Hopfer, H.; Buffon, P.A.; Ebeler, S.E.; Heymann, H. The combined effects of storage temperature and packaging on the sensory, chemical, and physical properties of a Cabernet Sauvignon wine. J. Agric. Food Chem. 2013, 61, 3320–3334. [Google Scholar] [CrossRef]
- Hopfer, H.; Ebeler, S.E.; Heymann, H. The combined effects of storage temperature and packaging type on the sensory and chemical properties of chardonnay. J. Agric. Food Chem. 2012, 60, 10743–10754. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Valiente, J.; Valladares, F.; Gil, L.; Aranda, I. Population differences in juvenile survival under increasing drought are mediated by seed size in cork oak (Quercus suber L.). For. Ecol. Manag. 2009, 257, 1676–1683. [Google Scholar] [CrossRef]
- Anjos, O.; Pereira, H.; Rosa, M.E. Tensile properties of cork in the tangential direction: Variation with quality, porosity, density and radial position in the cork plank. Mater. Des. 2010, 31, 2085–2090. [Google Scholar] [CrossRef]
- Anjos, O.; Pereira, H.; Rosa, M.E. Effect of quality, porosity and density on the compression properties of cork. Holz Als Roh-Und Werkst. 2008, 66, 295. [Google Scholar] [CrossRef]
- Pereira, H. Cork: Biology, Production and Uses, 1st ed.; Elsevier: Amsterdam, The Netherlands; London, UK, 2007. [Google Scholar]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef] [Green Version]
- Gil, L. Cork composites: A review. Materials 2009, 2, 776–789. [Google Scholar] [CrossRef] [Green Version]
- Shinde, A.M.; Tate, G.J.; Shinde, R.R.; Kadam, S.P.; Patil, P.M. An overview of properties of cork—A bottling approach. Int. Res. J. Eng. Technol. 2016, 3, 2762–2765. [Google Scholar]
- Gardner, D. Innovative Packaging for the Wine Industry: A Look at Wine Closures; Virginia Tech, Food Science and Technology: Blacksburg, VA, USA, 2008. [Google Scholar]
- Liu, N.; Song, Y.-Y.; Dang, G.-F.; Ye, D.-Q.; Gong, X.; Liu, Y.-L. Effect of wine closures on the aroma properties of Chardonnay wines after four years of storage. S. Afr. J. Enol. Vitic. 2015, 36, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Vidal, J.-C.; Guillemat, B.; Chayvialle, C. Oxygen transmission rate of screwcaps by chemoluminescence and air/capsule/headspace/acidified water system. Bull. L’OIV 2011, 84, 189–198. [Google Scholar]
- Hopfer, H.; Nelson, J.; Mitchell, A.E.; Heymann, H.; Ebeler, S.E. Profiling the trace metal composition of wine as a function of storage temperature and packaging type. J. Anal. At. Spectrom. 2013, 28, 1288–1291. [Google Scholar] [CrossRef]
- Lopes, P.; Saucier, C.; Teissedre, P.-L.; Glories, Y. Main routes of oxygen ingress through different closures into wine bottles. J. Agric. Food Chem. 2007, 55, 5167–5170. [Google Scholar] [CrossRef]
- ASTM D1434-82(2015)e1. Standard Test Method for Determining Gas Permeability Characteristics of Plastic Film and Sheeting; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Lopes, P.; Saucier, C.; Glories, Y. Nondestructive colorimetric method to determine the oxygen diffusion rate through closures used in winemaking. J. Agric. Food Chem. 2005, 53, 6967–6973. [Google Scholar] [CrossRef]
- Lopes, P.; Saucier, C.; Teissedre, P.-L.; Glories, Y. Impact of storage position on oxygen ingress through different closures into wine bottles. J. Agric. Food Chem. 2006, 54, 6741–6746. [Google Scholar] [CrossRef]
- Brotto, L.; Battistutta, F.; Tat, L.; Comuzzo, P.; Zironi, R. Modified nondestructive colorimetric method to evaluate the variability of oxygen diffusion rate through wine bottle closures. J. Agric. Food Chem. 2010, 58, 3567–3572. [Google Scholar] [CrossRef] [PubMed]
- Diéval, J.B.; Vidal, S.; Aagaard, O. Measurement of the oxygen transmission rate of co-extruded wine bottle closures using a luminescence-based technique. Packag. Technol. Sci. 2011, 24, 375–385. [Google Scholar] [CrossRef]
- Vidal, J.-C.; Moutounet, M. Monitoring of oxygen in the gas and liquide phases of bottles of wine at bottling and during storage. OENO One 2006, 40, 35–45. [Google Scholar] [CrossRef]
- Bunner, D. La mesure de l’oxygène dans les bouteilles par chimiluminescence. Vign. Champen. 2010, 131, 84–101. [Google Scholar]
- Hart, A.; Kleinig, A. The role of oxygen in the aging of bottled wine. Aust. N. Z. Wine Ind. J. 2005, 20, 46–50. [Google Scholar]
- Godden, P.; Lattey, K.; Francis, L.; Gishen, M.; Cowey, G.; Holdstock, M.; Robinson, E.; Waters, E.; Skouroumounis, G.; Sefton, M. Towards offering wine to the consumer in optimal condition-the wine, the closures and other packaging variables: A review of AWRI research examining the changes that occur in wine after bottling. Wine Ind. J. 2005, 20, 20–30. [Google Scholar]
- Vidal, J.-C.; Moutounet, M. Impact des conditions opératoires au conditionnement et de la perméabilité du bouchon sur l’oxygène et l’évolution d’un vin blanc de sauvignon en bouteille. Infowine 2011, 3, 16p. [Google Scholar]
- Chevalier, V.; Pons, A.; Loisel, C. Caracterización de las transferencias de oxígeno de tapones de corcho Impacto del cierre en el envejecimiento de los vinos en botella. SeVi N° 3.535 2019, 70–77. Available online: http://www.sevi.net/attachment/4552/d4276.pdf?g_download=1 (accessed on 1 July 2021).
- He, J.; Zhou, Q.; Peck, J.; Soles, R.; Qian, M.C. The effect of wine closures on volatile sulfur and other compounds during post-bottle ageing. Flavour Fragr. J. 2013, 28, 118–128. [Google Scholar] [CrossRef]
- Vinventions. Available online: https://www.vinventions.com/assets/0d44a56e-6b54-444f-9e85-39cebacbf0f5/brochure-nomacorc-greenline-us.pdf (accessed on 10 May 2021).
- ASTM F1927-20. Standard Test Method for Determination of Oxygen Gas Transmission Rate, Permeability and Permeance at Controlled Relative Humidity through Barrier Materials Using a Colorimetric Detector; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Peck, J. Science of Closures, Oxygen Transmission, Measurement, Variability; American Society for Enology and Viticulture Communication: Seattle, WA, USA, 24 June 2005. [Google Scholar]
- Rabiot, D.; Sanchez, J.; Aracil, J.M. Study of the Oxygen Transfer through Synthetic Corks for Wine Conservation. In Proceedings of the 2nd European Congress of Chemical Engineering, Montpellier, France, 4–7 October 1999. [Google Scholar]
- Lagorce-Tachon, A.l.; Karbowiak, T.; Simon, J.-M.; Gougeon, R.G.; Bellat, J.-P. Diffusion of oxygen through cork stopper: Is it a Knudsen or a Fickian mechanism? J. Agric. Food Chem. 2014, 62, 9180–9185. [Google Scholar] [CrossRef] [PubMed]
- Chanut, J.; Lagorce, A.; Lequin, S.; Gougeon, R.D.; Simon, J.-M.; Bellat, J.-P.; Karbowiak, T. Fast manometric method for determining the effective oxygen diffusion coefficient through wine stopper. Polym. Test. 2021, 93, 106924. [Google Scholar] [CrossRef]
- Lequin, S.; Chassagne, D.; Karbowiak, T.; Simon, J.-M.; Paulin, C.; Bellat, J.-P. Diffusion of oxygen in cork. J. Agric. Food Chem. 2012, 60, 3348–3356. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Bellat, J.-P.; Gougeon, R.D.; Karbowiak, T. Gas transfer through wine closures: A critical review. Trends Food Sci. Technol. 2018, 78, 255–269. [Google Scholar] [CrossRef]
- Lopes, P. The importance of closures for managing the oxygenation of wines during bottle aging. In Proceedings of the Œnofutur 13: Control of Oxidation and Sulfites in Red Wines, Montpellier, France, 16 April 2021. [Google Scholar]
- Karbowiak, T.; Gougeon, R.D.; Alinc, J.-B.; Brachais, L.; Debeaufort, F.; Voilley, A.; Chassagne, D. Wine oxidation and the role of cork. Crit. Rev. Food Sci. Nutr. 2009, 50, 20–52. [Google Scholar] [CrossRef]
- Karbowiak, T.; Crouvisier-Urion, K.; Lagorce, A.; Ballester, J.; Geoffroy, A.; Roullier-Gall, C.; Chanut, J.; Gougeon, R.D.; Schmitt-Kopplin, P.; Bellat, J.-P. Wine aging: A bottleneck story. NPJ Sci. Food 2019, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Faria, D.P.; Fonseca, A.L.; Pereira, H.; Teodoro, O.M. Permeability of cork to gases. J. Agric. Food Chem. 2011, 59, 3590–3597. [Google Scholar] [CrossRef] [PubMed]
- Brazinha, C.; Fonseca, A.P.; Pereira, H.; Teodoro, O.M.; Crespo, J.G. Gas transport through cork: Modelling gas permeation based on the morphology of a natural polymer material. J. Membr. Sci. 2013, 428, 52–62. [Google Scholar] [CrossRef]
- Oliveira, V.; Lopes, P.; Cabral, M.; Pereira, H. Kinetics of oxygen ingress into wine bottles closed with natural cork stoppers of different qualities. Am. J. Enol. Vitic. 2013, 64, 395–399. [Google Scholar] [CrossRef]
- Oliveira, V.; Lopes, P.; Cabral, M.; Pereira, H. Influence of cork defects in the oxygen ingress through wine stoppers: Insights with X-ray tomography. J. Food Eng. 2015, 165, 66–73. [Google Scholar] [CrossRef]
- Keenan, C.; Gözükara, M.; Christie, G.; Heyes, D. Oxygen permeability of macrocrystalline paraffin wax and relevance to wax coatings on natural corks used as wine bottle closures. Aust. J. Grape Wine Res. 1999, 5, 66–70. [Google Scholar] [CrossRef]
- Lopes, P.; Silva, M.A.; Pons, A.; Tominaga, T.; Lavigne, V.R.; Saucier, C.D.; Darriet, P.; Teissedre, P.-L.; Dubourdieu, D. Impact of oxygen dissolved at bottling and transmitted through closures on the composition and sensory properties of a Sauvignon blanc wine during bottle storage. J. Agric. Food Chem. 2009, 57, 10261–10270. [Google Scholar] [CrossRef] [PubMed]
- Skouroumounis, G.K.; Kwiatkowski, M.; Francis, I.; Oakey, H.; Capone, D.; Duncan, B.; Sefton, M.; Waters, E. The impact of closure type and storage conditions on the composition, colour and flavour properties of a Riesling and a wooded Chardonnay wine during five years’ storage. Aust. J. Grape Wine Res. 2005, 11, 369–377. [Google Scholar] [CrossRef]
- Godden, P.; Francis, L.; Field, J.; Gishen, M.; Coulter, A.; Valente, P.; Hoj, P.; Robinson, E. Wine bottle closures: Physical characteristics and effect on composition and sensory properties of a Semillon wine 1. Performance up to 20 months post-bottling. Aust. J. Grape Wine Res. 2001, 7, 64–105. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Furtado, I.; Bastos, M.d.L.; Guedes de Pinho, P.; Pinto, J. The influence of different closures on volatile composition of a white wine. Food Packag. Shelf Life 2020, 23, 100465. [Google Scholar] [CrossRef]
- Coquet, C.; Ferré, E.; Peyronel, D.; Dal Farra, C.; Farnet, A.M. Identification of new molecules extracted from Quercus suber L. cork. Comptes Rendus Biol. 2008, 331, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; Meireles, S.; Brandão, T.; Guedes de Pinho, P. Optimization of the HS-SPME–GC–IT/MS method using a central composite design for volatile carbonyl compounds determination in beers. Talanta 2013, 117, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; Lopes, P.; Cabral, M.; Guedes de Pinho, P. HS-SPME/GC-MS methodologies for the analysis of volatile compounds in cork material. Eur. Food Res. Technol. 2016, 242, 457–466. [Google Scholar] [CrossRef]
- Culleré, L.; Cacho, J.; Ferreira, V. Comparative study of the aromatic profile of different kinds of wine cork stoppers. Food Chem. 2009, 112, 381–387. [Google Scholar] [CrossRef]
- Juanola, R.; Subira, D.; Salvadó, V.; Regueiro, J.G.; Anticó, E. Migration of 2,4,6-trichloroanisole from cork stoppers to wine. Eur. Food Res. Technol. 2005, 220, 347–352. [Google Scholar] [CrossRef]
- Sefton, M.A.; Simpson, R.F. Compounds causing cork taint and the factors affecting their transfer from natural cork closures to wine–a review. Aust. J. Grape Wine Res. 2005, 11, 226–240. [Google Scholar] [CrossRef]
- Soleas, G.J.; Yan, J.; Seaver, T.; Goldberg, D.M. Method for the gas chromatographic assay with mass selective detection of trichloro compounds in corks and wines applied to elucidate the potential cause of cork taint. J. Agric. Food Chem. 2002, 50, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.; Rauhut, D.; Jung, R. “Cork taint” responsible compounds. Determination of haloanisoles and halophenols in cork matrix: A review. Talanta 2017, 175, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Slabizki, P.; Legrum, C.; Wegmann-Herr, P.; Fischer, C.; Schmarr, H.-G. Quantification of cork off-flavor compounds in natural cork stoppers and wine by multidimensional gas chromatography mass spectrometry. Eur. Food Res. Technol. 2016, 242, 977–986. [Google Scholar] [CrossRef]
- Neto, P.V.; Rocha, S.M.; Silvestre, A.J. Simultaneous headspace solid phase microextraction analysis of off-flavour compounds from Quercus suber L. cork. J. Sci. Food Agric. 2007, 87, 632–640. [Google Scholar] [CrossRef]
- Mislata, A.M.; Puxeu, M.; Ferrer-Gallego, R. Aromatic Potential and Bioactivity of Cork Stoppers and Cork By-Products. Foods 2020, 9, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, J.; Oliveira, A.S.; Lopes, P.; Roseira, I.; Cabral, M.; Bastos, M.d.L.; Guedes de Pinho, P. Characterization of chemical compounds susceptible to be extracted from cork by the wine using GC-MS and 1H NMR metabolomic approaches. Food Chem. 2019, 271, 639–649. [Google Scholar] [CrossRef]
- Prat, C.; Besalú, E.; Bañeras, L.; Anticó, E. Multivariate analysis of volatile compounds detected by headspace solid-phase microextraction/gas chromatography: A tool for sensory classification of cork stoppers. Food Chem. 2011, 126, 1978–1984. [Google Scholar] [CrossRef]
- Juanola, R.; Guerrero, L.; Subira, D.; Salvadó, V.; Insa, S.; Regueiro, J.G.; Anticó, E. Relationship between sensory and instrumental analysis of 2, 4, 6-trichloroanisole in wine and cork stoppers. Anal. Chim. Acta 2004, 513, 291–297. [Google Scholar] [CrossRef]
- Moreira, N.; Lopes, P.; Ferreira, H.; Cabral, M.; Guedes de Pinho, P. Influence of packaging and aging on the red wine volatile composition and sensory attributes. Food Packag. Shelf Life 2016, 8, 14–23. [Google Scholar] [CrossRef]
- FoodB. FooDB Version 1.0. Available online: http://foodb.ca/ (accessed on 12 March 2019).
- Karbowiak, T.; Mansfield, A.K.; Barrera-García, V.D.; Chassagne, D. Sorption and diffusion properties of volatile phenols into cork. Food Chem. 2010, 122, 1089–1094. [Google Scholar] [CrossRef]
- Sajilata, M.; Savitha, K.; Singhal, R.; Kanetkar, V. Scalping of flavors in packaged foods. Compr. Rev. Food Sci. Food Saf. 2007, 6, 17–35. [Google Scholar] [CrossRef]
- Buck, K.; Bussey, R. Product safety and consumer acceptance in food-packaging applications. Cereal Foods World 2002, 47, 425. [Google Scholar]
- Risch, S.J.; Hotchkiss, J.H. Food and Packaging Interactions II; ACS Publications: Washington, DC, USA, 1991. [Google Scholar]
- Choi, S.J.; Kim, J.K.; Kim, H.K.; Harris, K.; Kim, C.-J.; Park, G.G.; Park, C.-S.; Shin, D.-H. 2,4-Di-tert-butylphenol from sweet potato protects against oxidative stress in PC12 cells and in mice. J. Med. Food 2013, 16, 977–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prata, J.C.; Paço, A.; Reis, V.; da Costa, J.P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Identification of microplastics in white wines capped with polyethylene stoppers using micro-Raman spectroscopy. Food Chem. 2020, 331, 127323. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Jourdes, M.; Darriet, P.; Teissedre, P.-L. Scalping of light volatile sulfur compounds by wine closures. J. Agric. Food Chem. 2012, 60, 10952–10956. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.; Jägerstad, M. Flavour scalping by food packaging. Trends Food Sci. Technol. 1994, 5, 353–356. [Google Scholar] [CrossRef]
- Capone, D.L.; Skouroumounis, G.; Barker, D.; McLean, H.; Pollnitz, A.; Sefton, M. Absorption of chloroanisoles from wine by corks and by other materials. Aust. J. Grape Wine Res. 1999, 5, 91–98. [Google Scholar] [CrossRef]
- Blake, A.; Kotseridis, Y.; Brindle, I.D.; Inglis, D.; Sears, M.; Pickering, G.J. Effect of closure and packaging type on 3-alkyl-2-methoxypyrazines and other impact odorants of Riesling and Cabernet Franc wines. J. Agric. Food Chem. 2009, 57, 4680–4690. [Google Scholar] [CrossRef]
- Capone, D.L.; Sefton, M.; Pretoius, I.; Høj, P. Flavour ‘scalping’ by wine bottle closures—The ‘winemaking’ continues post vineyard and winery. Aust. N. Z. Wine Ind. J. 2003, 18, 16–20. [Google Scholar]
- Skurray, G.; Castets, E.; Holland, B. Permeation of vanillin through natural and synthetic corks. Aust. Grapegrow. Winemak. 2000, 438a, 121–124. [Google Scholar]
- Skouroumounis, G.K.; Kwiatkowski, M.; Francis, I.; Oakey, H.; Capone, D.; Peng, Z.; Duncan, B.; Sefton, M.; Waters, E. The influence of ascorbic acid on the composition, colour and flavour properties of a Riesling and a wooded Chardonnay wine during five years’ storage. Aust. J. Grape Wine Res. 2005, 11, 355–368. [Google Scholar] [CrossRef]
- Tarasov, A.; Giuliani, N.; Dobrydnev, A.; Müller, N.; Volovenko, Y.; Rauhut, D.; Jung, R. Absorption of 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) from wine by bottle closures. Eur. Food Res. Technol. 2019, 245, 2343–2351. [Google Scholar] [CrossRef]
- Schneider, V. Chemical and sensory discrimination of different kinds of white wine aging and enological measures to improve white wine flavor stability: A review. In Recent Advances in Wine Stabilization and Conservation; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 162–207. [Google Scholar]
- Gallardo-Chacón, J.-J.; Karbowiak, T. Sorption of 4-ethylphenol and 4-ethylguaiacol by suberin from cork. Food Chem. 2015, 181, 222–226. [Google Scholar] [CrossRef]
- Pickering, G.J.; Blake, A.J.; Soleas, G.J.; Inglis, D.L. Remediation of wine with elevated concentrations of 3-alkyl-2-methoxypyrazines using cork and synthetic closures. J. Food Agric. Environ. 2010, 8, 97–101. [Google Scholar]
- van Willige, R.W.G. Effects of Flavour Absorption on Foods and Their Packaging Materials. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 31 May 2002. [Google Scholar]
| Closures | Colorimetric * | Chemiluminescence | Coulometric | |
|---|---|---|---|---|
| Cork stoppers | Technical | 0.003 to 0.004 | 0.025 e 0.050 e | 0.03 to 0.05 b |
| Natural | 0.007 to 0.010 | NA | 0.004 to 5.0 b 0.001 to 0.051 d | |
| Synthetic | Co-extruded | 0.033 0.065 | 0.050 f 0.091 f 0.141 f | 0.040 b 0.17 to 0.31 d 0.7 to 1.4 e |
| Screw caps | Saran-tin | 0.001 | 0.010 to 0.012 c | <0.001 a 0.008 b 0.003 to 0.0039 c |
| Saranex | 0.02 to 0.03 | 0.045 to 0.065 c | 0.03 b 0.02 to 0.03 c | |
| Compound | CAS | Odor Descriptor | References |
|---|---|---|---|
| Alcohols | |||
| 3-Methyl-1-butanol | 123-51-3 | Whiskey a | [59] |
| 1-Octen-3-ol | 3391-86-4 | Mushrooms a | [59] |
| 1-Octanol | 111-87-5 | Wax a | [59] |
| Benzyl alcohol | Roses, almond | [60] | |
| Geosmin | 19700-21-1 | Earth, musty | [58,59] |
| Isobutanol | 78-83-1 | Flowery, anise | [53] |
| Phenylethyl alcohol | 60-12-8 | Flowers, honey | [60] |
| Aldehydes | |||
| Propanal | 123-38-6 | Fruity, fresh green b | [52] |
| Butanal | 123-72-8 | Fruity, burnt, sweet b | [52] |
| Pentanal | 110-62-3 | Dry fruit, nutty | [52] |
| Hexanal | 66-25-1 | Grass, herbaceous b | [52,61] |
| Heptanal | 111-71-7 | Fatty, rancid b | [52,61] |
| Octanal | 124-13-0 | Lemon | [52,53,61] |
| Nonanal | 124-19-6 | Herbal, citrus b | [52,61] |
| Decanal | 112-31-2 | Fruity, citrus b | [52,61] |
| Undecanal | 112-44-7 | Citrus, floral c | [52] |
| 2-Propenal | 107-02-8 | Almond, cherry c | [52] |
| (E)-2-Butenal | 123-73-9 | Flower c | [52] |
| (E)-2-Pentenal | 1576-87-0 | Fruity, green c | [52] |
| (E)-2-Hexenal | 6728-26-3 | Almond, fruity c | [52] |
| (E)-2-Heptenal | 18829-55-5 | Fatty, green c | [52] |
| (E)-2-Octenal | 2548-87-0 | Fatty, herbal c | [52] |
| (E)-2-Nonenal | 18829-56-6 | Green, cucumber c | [52,60] |
| (E)-2-Decenal | 3913-81-3 | Fatty, oily c | [52] |
| 2-Methyl-1-propanal | 78-84-2 | Fruity, malty b | [52] |
| 2-Methyl-1-butanal | 96-17-3 | Almond, nutty c | [52] |
| 3-Methyl-1-butanal | 590-86-3 | Fruity, cheesy b | [52] |
| Benzaldehyde | 100-52-7 | Bitter almonds b | [52,61] |
| Phenylacetaldehyde | 122-78-1 | Floral, honey b | [52] |
| Benzenoids | |||
| o-Cymene | 527-84-4 | - | [61] |
| Naphthalene | 91-20-3 | Pungent, tarry c | [61] |
| Guaiacol | 90-05-1 | Phenolic, spicy | [53,59,62] |
| 4-Vinylguaiacol | 7786-61-0 | Wood, spice, curry | [60] |
| Methyl guaiacol | 91-16-7 | Leather, spicy d | [62] |
| Eugenol | 97-53-0 | Spice, cloves, honey | [60] |
| Isoeugenol | 97-54-1 | Carmination | [60] |
| Cerulignol | 2785-87-7 | Spicy | [60] |
| 2,4,6-Trichloroanisole (TCA) | 87-40-1 | Musty, earthy, moldy | [54,56,58,59,62,63] |
| m-Cresol | 108-39-4 | Leather | [53] |
| Vanillin | 121-33-5 | Vanillin | [53,60] |
| Methyl vanillate | 3943-74-6 | Vanillin | [53] |
| Pyrazines | |||
| MDMP | - | Musty, dusty | [58] |
| IPMP | 25773-40-4 | Green, vegetative | [58] |
| IBMP | 24683-00-9 | Green bell pepper | [58] |
| Dicarbonyls | |||
| Diacetyl | 431-03-8 | Buttery, cream | [52,53] |
| Glyoxal | 107-22-2 | - | [52] |
| Methylglyoxal | 78-98-8 | - | [52] |
| Ketones | |||
| Propan-2-one | 67-64-1 | Apple, ethereal c | [52] |
| 2-Butanone | 78-93-3 | Fruity, acetone b | [52] |
| 3-Methyl-2-butanone | 563-80-4 | Camphor c | [52] |
| 2-Pentanone | 107-87-9 | Fruity b | [52] |
| 2-Hexanone | 591-78-6 | Ether c | [52] |
| 2-Heptanone | 110-43-0 | Fruity, herbal c | [52] |
| 3-Penten-2-one | 625-33-2 | Fishy, phenolic c | [52] |
| 4-Heptanone | 123-19-3 | Fruity, sweet c | [52] |
| 2-Cyclohexen-1-one | 930-68-7 | Green, roasted c | [52] |
| 6-Methyl-5-heptanone | 13019-20-0 | Fruity, green c | [52] |
| 2-Octanone | 111-13-7 | Bitter, earthy c | [52] |
| 2-Nonanone | 821-55-6 | Fresh, herbal c | [52] |
| 2-Decanone | 693-54-9 | Fatty, floral c | [52] |
| 2-Undecanone | 112-12-9 | Fresh, floral c | [52] |
| 1-Octen-3-one | 4312-99-6 | Mushroom | [53] |
| Acids | |||
| Octanoic acid | 124-07-2 | Coconut, rancid, cheese | [60] |
| Vanillic acid | 121-34-6 | Vanilla | [60] |
| Nonanoic acid | 112-05-0 | Wax, dry, fatty | [60] |
| Dodecanoic acid | 143-07-7 | Coconut, fatty, metallic | [60] |
| Benzeneacetic acid | 103-82-2 | Honey, fruity, sour | [60] |
| Furans | |||
| Furfural | 98-01-1 | Toasty, caramel b | [52,60] |
| 5-Methyl-2-furfural | 620-02-0 | Spicy, toasty b | [52] |
| Esters | |||
| Ethyl hexanoate | 123-66-0 | Fruity, brandy b | [61] |
| Ethyl heptanoate | 106-30-9 | Fruity, nutty b | [61] |
| Ethyl nonanoate | 123-29-5 | Fruity, waxy c | [61] |
| Fenchyl acetate | 13851-11-1 | Citrus, herbal c | [61] |
| Isobornyl acetate | 125-12-2 | Herbal, woody c | [61] |
| Ethyl isobutyrate | 97-62-1 | Fruity, strawberry | [53] |
| Ethyl 2-methylbutyrate | 7452-79-1 | Fruity, green apple | [53] |
| Ethyl isovalerate | 108-64-5 | Fruity, anise | [53] |
| 3-Methylbutyl acetate | 123-92-2 | Fruity, anise | [53] |
| Ethyl butyrate | 105-54-4 | Fruity | [53] |
| Butyl acetate | 123-86-4 | Grass | [53] |
| Monoterpenes | |||
| α-Pinene | 80-56-8 | Minty c | [52,61] |
| Camphene | 79-92-5 | Herbal, woody c | [61] |
| β-Pinene | 80-56-8 | Green, hay c | [61] |
| 1,4-Cineole | 470-67-7 | Minty, pine c | [52,61] |
| Citronellol | 106-22-9 | Citrus, floral c | [62] |
| α-Terpinene | 99-86-5 | Citrus, herbal c | [52,61] |
| Limonene | 5989-54-8 | Lemon, orange c | [52,61,62] |
| Eucalyptol | 470-82-6 | Mint, herbal c | [52,61] |
| Terpinolene | 586-62-9 | Pine, woody c | [61,62] |
| Fenchone | 1195-79-5 | Earthy, herbal c | [52,61] |
| Fenchol | 1632-73-1 | Lemon, pine c | [52,61,62] |
| α-Campholenal | 4501-58-1 | Green, leafy c | [61] |
| L-Camphor | 464-49-3 | Camphor c | [52,60,61,62] |
| trans-β-Terpineol | 7299-40-3 | - | [61] |
| trans-3-Pinanone | 547-60-4 | Spicy c | [61] |
| Isoborneol | 124-76-5 | Herbal, woody c | [52,61] |
| 2-Methylisoborneol | 2371-42-8 | Musty, muddy | [58] |
| L-Borneol | 464-45-9 | Camphor, anise | [52,53,61,62] |
| 2-Methylisoborneol | 2371-42-8 | Earth, musty a | [59] |
| cis-3-Pinanone | 15358-88-0 | Camphoreous, cedar c | [52,61] |
| α-Terpineol | 98-55-5 | Floral, mint c | [52,53,61] |
| 1-Terpineol | 7785-53-7 | Floral, lilac c | [52] |
| 4-Terpineol | 562-74-3 | Earth, musty c | [52,61] |
| Linalool | 78-70-6 | Flowery, muscat | [53,62] |
| cis-Linalool oxide | 11063-77-7 | Earthy, sweet c | [52] |
| L-(-)-Menthol | 2216-51-5 | Minty, peppermint c | [52] |
| 2-Pinen-4-one | 18309-32-5 | Menthol c | [52] |
| Sesquiterpenes | |||
| α-Copaene | 3856-25-5 | Spice, woody c | [61] |
| D-Longifolene | 475-20-7 | Rose, sweet c | [61] |
| β-Cadinene | 523-47-7 | Green, woody c | [61] |
| L-Calamenene | 483-77-2 | Herb, spice c | [61] |
| Eremophila ketone | 158930-41-7 | - | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtado, I.; Lopes, P.; Oliveira, A.S.; Amaro, F.; Bastos, M.d.L.; Cabral, M.; Guedes de Pinho, P.; Pinto, J. The Impact of Different Closures on the Flavor Composition of Wines during Bottle Aging. Foods 2021, 10, 2070. https://doi.org/10.3390/foods10092070
Furtado I, Lopes P, Oliveira AS, Amaro F, Bastos MdL, Cabral M, Guedes de Pinho P, Pinto J. The Impact of Different Closures on the Flavor Composition of Wines during Bottle Aging. Foods. 2021; 10(9):2070. https://doi.org/10.3390/foods10092070
Chicago/Turabian StyleFurtado, Isabel, Paulo Lopes, Ana Sofia Oliveira, Filipa Amaro, Maria de Lourdes Bastos, Miguel Cabral, Paula Guedes de Pinho, and Joana Pinto. 2021. "The Impact of Different Closures on the Flavor Composition of Wines during Bottle Aging" Foods 10, no. 9: 2070. https://doi.org/10.3390/foods10092070
APA StyleFurtado, I., Lopes, P., Oliveira, A. S., Amaro, F., Bastos, M. d. L., Cabral, M., Guedes de Pinho, P., & Pinto, J. (2021). The Impact of Different Closures on the Flavor Composition of Wines during Bottle Aging. Foods, 10(9), 2070. https://doi.org/10.3390/foods10092070








