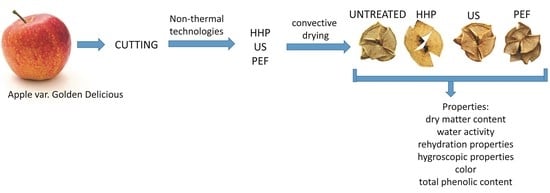
Selected Quality Parameters of Air-Dried Apples Pretreated by High Pressure, Ultrasounds and Pulsed Electric Field—A Comparison Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Technological Methods
2.2.1. High-Pressure Processing (HPP)
2.2.2. Ultrasounds (US)
2.2.3. Pulsed Electric Field (PEF)
2.2.4. Convective Drying
2.3. Analytical Methods
2.3.1. Dry Matter Content (DM)
2.3.2. Water Activity
2.3.3. Rehydration Properties
2.3.4. Hygroscopic Properties
2.3.5. Color
2.3.6. Total Phenolic Content
2.4. Statistical Methods
3. Results and Discussion
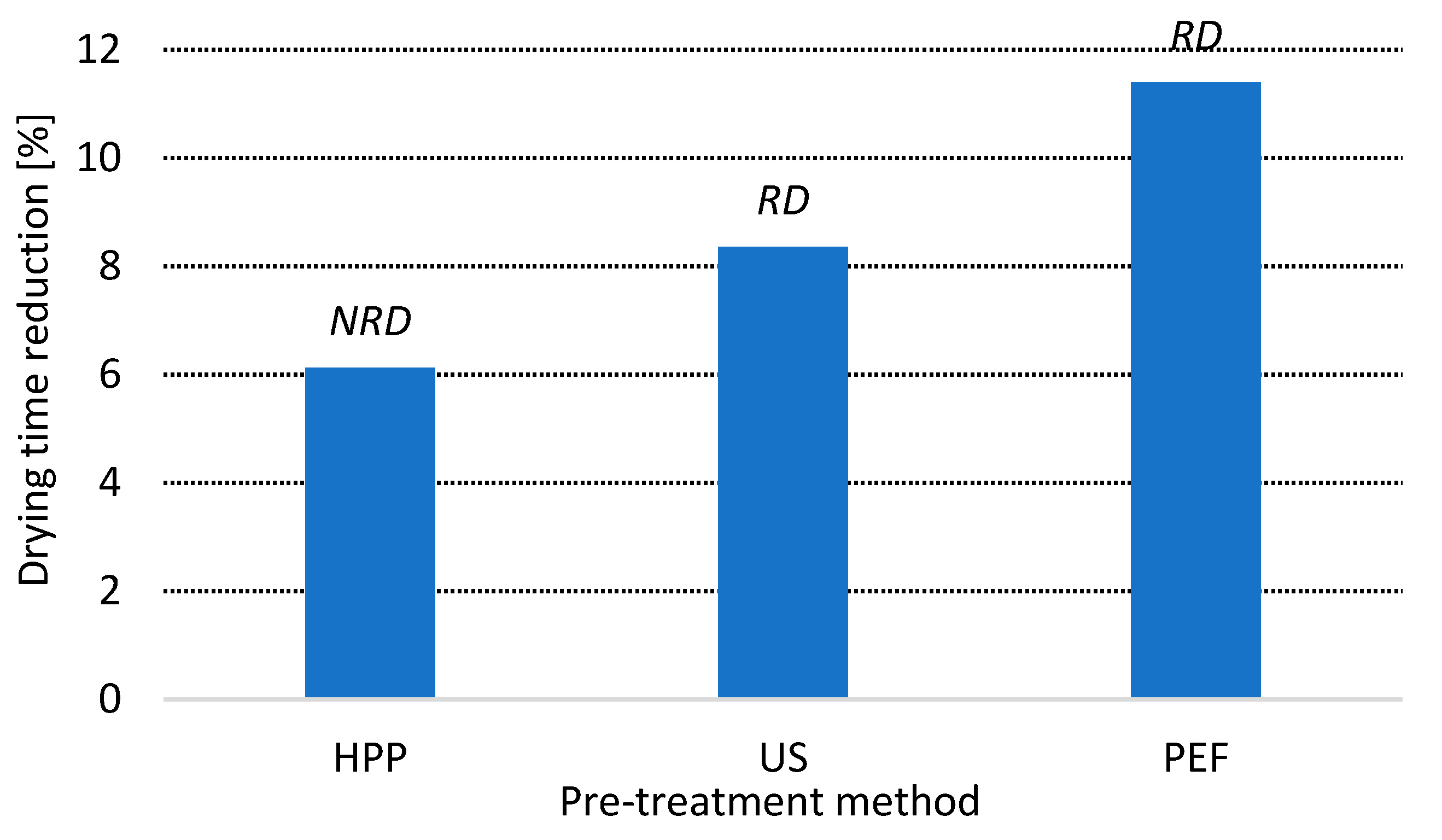
3.1. Drying Time
3.2. Dry Matter, Water Activity, Rehydration, and Hygroscopic Properties
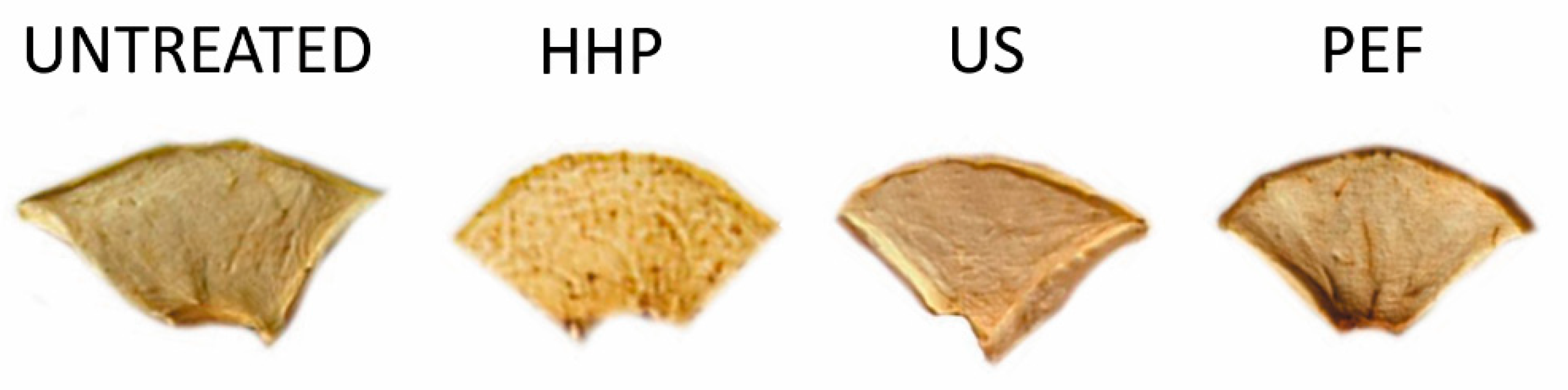
3.3. Color
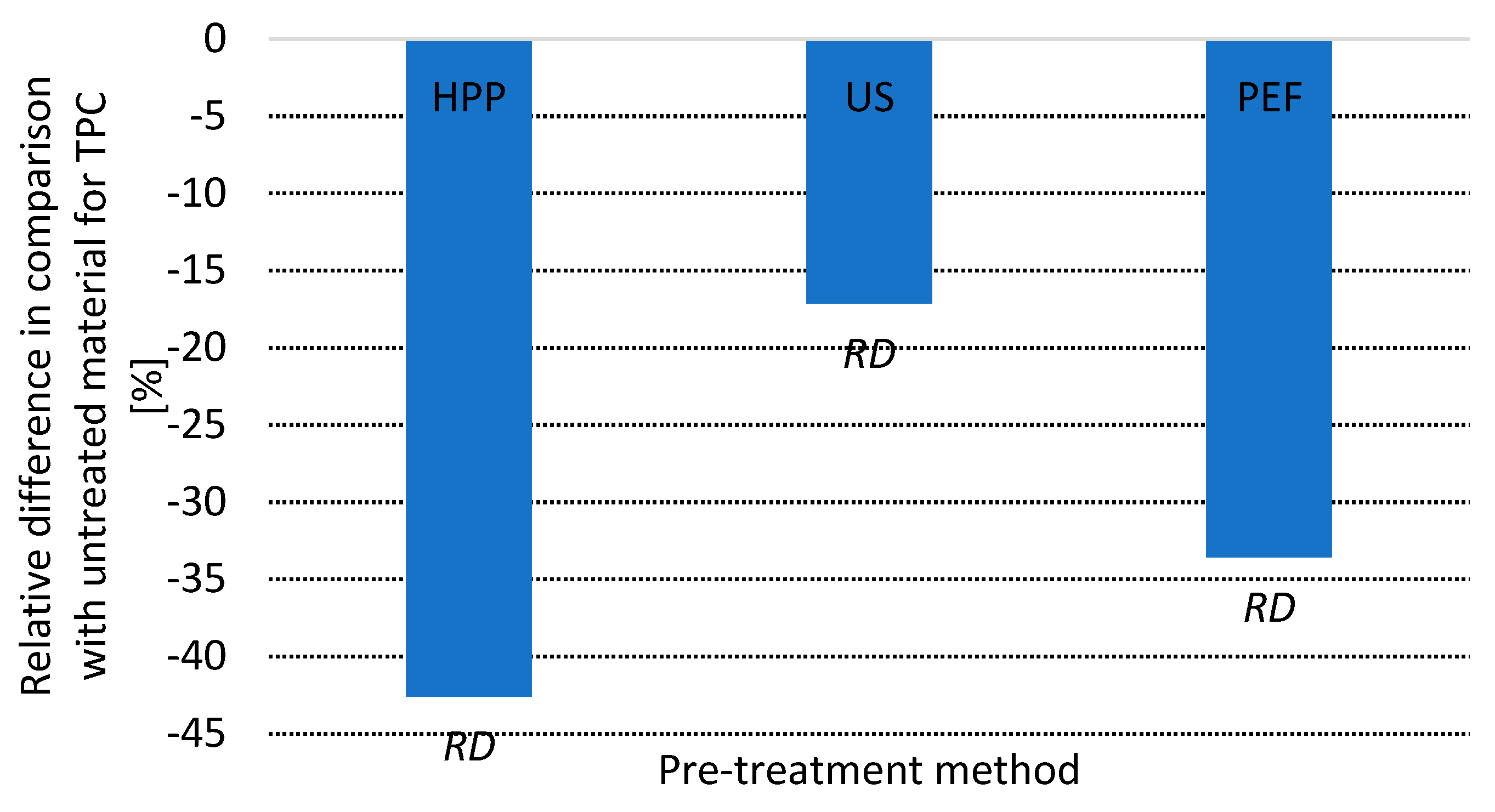
3.4. Total Phenolic Content
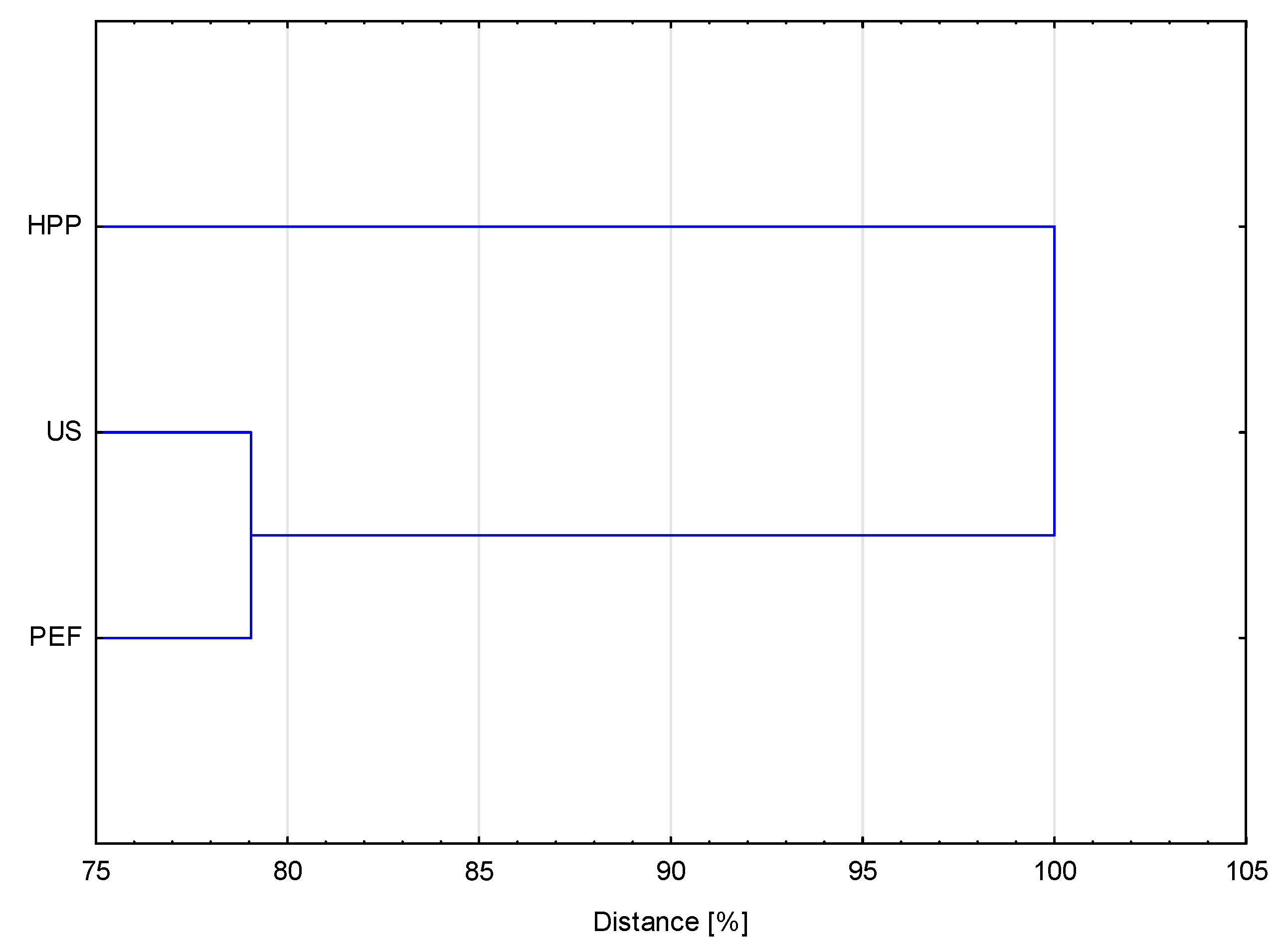
3.5. Principle Component Analysis and Cluster Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Air-Dried Food Market Size, Share & Trends Analysis Report by Product (Coffee Beans, Fruits & Vegetables, Meat, Herbs), by Application (Household, Commercial), by Form, by Region, and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/air-dried-food-market (accessed on 16 May 2021).
- Global Dehydrated Food Market 2021 Is Expected To Register a CAGR of 5.3% with Top Countries Data Industry Growth Analysis, Segmentation, Size, Share, Trend, Future Demand and Leading Players Updates by Forecast. Available online: https://www.wicz.com/story/44156767/global-dehydrated-food-market-2021-is-expected-to-register-a-cagr-of-53-with-top-countries-data-industry-growth-analysis-segmentation-size-share (accessed on 17 May 2021).
- Kemp, I.C. Fundamentals of Energy Analysis of Dryers. In Modern Drying Technology, Energy Savings; Tsotsas, E., Mujumdar, A.S., Eds.; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2012; Volume 4, pp. 1–45. [Google Scholar]
- Strumiłło, C.; Jones, P.L.; Żyłła, R. Energy Aspects in Drying. In Handbook of Industrial Drying; Mujumdar, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1075–1099. [Google Scholar]
- Tao, Y.; Han, M.; Gao, X.; Han, Y.; Show, P.L.; Liu, C.; Ye, X.; Xie, G. Applications of water blanching, surface contacting ultrasound-assisted air drying, and their combination for dehydration of white cabbage: Drying mechanism, bioactive profile, color and rehydration property. Ultrason. Sonochem. 2019, 53, 192–201. [Google Scholar] [CrossRef]
- Szadzińska, J.; Łechtańska, J.; Pashminehazar, R.; Kharaghani, A.; Tsotsas, E. Microwave- and ultrasound-assisted convective drying of raspberries: Drying kinetics and microstructural changes. Dry. Technol. 2019, 37, 1–12. [Google Scholar] [CrossRef]
- Ostermeier, R.; Parniakov, O.; Töpfl, S.; Jäger, H. Applicability of Pulsed Electric Field (PEF) Pre-Treatment for a Convective Two-Step Drying Process. Foods 2020, 9, 512. [Google Scholar] [CrossRef] [Green Version]
- Ling, J.; Xuan, X.; Yu, N.; Cui, Y.; Shang, H.; Liao, X.; Lin, X.; Yu, J. High pressure-assisted vacuum-freeze drying: A novel, efficient way to accelerate moisture migration in shrimp processing. J. Food Sci. 2020, 85, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Witrowa-Rajchert, D.; Wiktor, A.; Sledz, M.; Nowacka, M. Selected Emerging Technologies to Enhance the Drying Process: A Review. Dry. Technol. 2014, 32, 1386–1396. [Google Scholar] [CrossRef]
- Ghafoor, K.; Gavahian, M.; Barba, F.J.; Xia, Q.; Denoya, G.I. An overview of the potential applications based on HPP mechanism. In Present and Future of High Pressure Processing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–11. ISBN 9780128164051. [Google Scholar]
- Sehrawat, R.; Pal, B.; Prabhat, K. Microbial inactivation by high pressure processing: Principle, mechanism and factors responsible. Food Sci. Biotechnol. 2021, 30, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Janowicz, M.; Lenart, A. The impact of high pressure and drying processing on internal structure and quality of fruit. Eur. Food Res. Technol. 2018, 244, 1329–1340. [Google Scholar] [CrossRef]
- Yucel, U.; Alpas, H.; Bayindirli, A. Evaluation of high pressure pretreatment for enhancing the drying rates of carrot, apple, and green bean. J. Food Eng. 2010, 98, 266–272. [Google Scholar] [CrossRef]
- Kingsly, A.R.; Balasubramaniam, V.M.; Rastogi, N.K. Effect of high-pressure processing on texture and drying behavior of pineapple. J. Food Process Eng. 2015, 32, 369–381. [Google Scholar] [CrossRef]
- Wu, J.; Nyborg, W.L. Ultrasound, cavitation bubbles and their interaction with cells. Adv. Drug Deliv. Rev. 2008, 60, 1103–1116. [Google Scholar] [CrossRef]
- Nowacka, M.; Dadan, M.; Tylewicz, U. Current Applications of Ultrasound in Fruit and Vegetables Osmotic Dehydration Processes. Appl. Sci. 2021, 11, 1269. [Google Scholar] [CrossRef]
- Birkin, P.R.; Offin, D.G.; Vian, C.J.B.; Leighton, T.G. Investigation of noninertial cavitation produced by an ultrasonic horn. J. Acoust. Soc. Am. 2014, 130, 3297–3308. [Google Scholar] [CrossRef]
- Meng, L.; Liu, X.; Wang, Y.; Zhang, W.; Zhou, W.; Cai, F.; Li, F.; Wu, J.; Xu, L.; Niu, L.; et al. Sonoporation of Cells by a Parallel Stable Cavitation Microbubble Array. Adv. Sci. 2019, 6, 1900557. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Noguera, J.; Oliveira, F.I.P.; Gallão, M.I.; Weller, C.L.; Rodrigues, S.; Fernandes, F.A.N. Ultrasound-Assisted Osmotic Dehydration of Strawberries: Effect of Pretreatment Time and Ultrasonic Frequency. Dry. Technol. 2010, 28, 294–303. [Google Scholar] [CrossRef]
- Nowacka, M.; Tylewicz, U.; Laghi, L.; Dalla Rosa, M.; Witrowa-Rajchert, D. Effect of ultrasound treatment on the water state in kiwifruit during osmotic dehydration. Food Chem. 2014, 144, 18–25. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Ðordevic, V.B.; Zdunic, G.M.; Pljevljakušic, D.S.; Šavikin, K.P.; Go, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Zhang, J.; Yin, Y.; Zhang, W.; Yang, Y. Effects of Ultrasound-Assisted Emulsification on the Emulsifying and Rheological Properties of Myofibrillar Protein Stabilized Pork Fat Emulsions. Foods 2021, 10, 1201. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Kowalska, H.; Kowalska, J.; Galus, S.; Marzec, A.; Domian, E. The Effect of Pre-Treatment (Blanching, Ultrasound and Freezing) on Quality of Freeze-Dried Red Beets. Foods 2021, 10, 132. [Google Scholar] [CrossRef]
- Cichowska, J.; Witrowa-Rajchert, D.; Stasiak-Rózánska, L.; Figiel, A. Ultrasound-assisted osmotic dehydration of apples in polyols and dihydroxyacetone (DHA) solutions. Molecules 2019, 24, 3429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozkir, H.; Rayman, A.; Serdar, E.; Metin, G.; Baysal, T. Influence of ultrasound and osmotic dehydration pretreatments on drying and quality properties of persimmon fruit. Ultrason. Sonochem. 2019, 54, 135–141. [Google Scholar] [CrossRef]
- Tao, Y.; Li, D.; Siong, W.; Loke, P.; Yang, X.; Manickam, S.; Xie, G.; Han, Y. Comparison between airborne ultrasound and contact ultrasound to intensify air drying of blackberry: Heat and mass transfer simulation, energy consumption and quality evaluation. Ultrason. Sonochem. 2021, 72, 105410. [Google Scholar] [CrossRef] [PubMed]
- Önal, B.; Adiletta, G.; Di Matteo, M.; Russo, P.; Ramos, N.; Silva, C.L.M. Microwave and Ultrasound Pre-Treatments for Drying of the “Rocha” Pear: Impact on Phytochemical Parameters, Color Changes and Drying Kinetics. Foods 2021, 10, 853. [Google Scholar] [CrossRef] [PubMed]
- Dadan, M.; Nowacka, M. The Assessment of the Possibility of Using Ethanol and Ultrasound to Design the Properties of Dried Carrot Tissue. Appl. Sci. 2021, 11, 689. [Google Scholar] [CrossRef]
- Schössler, K.; Thomas, T.; Knorr, D. Modification of cell structure and mass transfer in potato tissue by contact ultrasound. Food Res. Int. 2012, 49, 425–431. [Google Scholar] [CrossRef]
- Mahnic-Kalamiza, S.; Vorobiev, E.; Miklavcic, D. Electroporation in Food Processing and Biorefinery. J. Membr. Biol. 2014, 247, 1279–1304. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zeng, X.A.; Ngadi, M. Enhanced extraction of phenolic compounds from onion by pulsed electric field (PEF). J. Food Process. Preserv. 2018, 42, e13755. [Google Scholar] [CrossRef]
- Wiktor, A.; Schulz, M.; Voigt, E.; Witrowa-Rajchert, D.; Knorr, D. The effect of pulsed electric field treatment on immersion freezing, thawing and selected properties of apple tissue. J. Food Eng. 2015, 146, 8–16. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Dalla Rosa, M. Effect of pulsed electric field (PEF) pre-treatment coupled with osmotic dehydration on physico-chemical characteristics of organic strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Mastwijk, H.C.; Berendsen, L.B.J.M.; Nederhoff, A.L.; Matser, A.M.; Van Boekel, M.A.J.S.; Groot, M.N.N. Moderate intensity Pulsed Electric Fields (PEF) as alternative mild preservation technology for fruit juice. Int. J. Food Microbiol. 2019, 298, 63–73. [Google Scholar] [CrossRef]
- Fauster, T.; Schlossnikl, D.; Rath, F.; Ostermeier, R.; Teufel, F.; Toep, S.; Jaeger, H. Impact of pulsed electric field (PEF) pretreatment on process performance of industrial French fries production. J. Food Eng. 2018, 235, 16–22. [Google Scholar] [CrossRef]
- Alam, R.; Lyng, J.G.; Frontuto, D.; Marra, F.; Cinquanta, L. Effect of Pulsed Electric Field Pretreatment on Drying Kinetics, Color, and Texture of Parsnip and Carrot. J. Food Sci. 2018, 83, 2159–2166. [Google Scholar] [CrossRef]
- Ostermeier, R.; Hill, K.; Dingis, A.; Töpfl, S.; Jäger, H. Influence of pulsed electric field (PEF) and ultrasound treatment on the frying behavior and quality of potato chips. Innov. Food Sci. Emerg. Technol. 2020, 67, 102553. [Google Scholar] [CrossRef]
- Lammerskitten, A.; Shorstkii, I.; Parniakov, O.; Mykhailyk, V.; Toepfl, S.; Rybak, K.; Dadan, M.; Nowacka, M.; Wiktor, A. The effect of different methods of mango drying assisted by a pulsed electric field on chemical and physical properties. J. Food Process. Preserv. 2020, 44, e14973. [Google Scholar] [CrossRef]
- Pataro, G.; Falcone, M.; Donsì, G.; Ferrari, G. Metal release from stainless steel electrodes of a PEF treatment chamber: Effects of electrical parameters and food composition. Innov. Food Sci. Emerg. Technol. 2014, 21, 58–65. [Google Scholar] [CrossRef]
- Lammerskitten, A.; Mykhailyk, V.; Wiktor, A.; Toepfl, S.; Nowacka, M.; Bialik, M.; Czyżewski, J.; Witrowa-Rajchert, D.; Parniakov, O. Impact of pulsed electric fields on physical properties of freeze-dried apple tissue. Innov. Food Sci. Emerg. Technol. 2019, 57, 102211. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Rockville, MD, USA, 2002. [Google Scholar]
- Fijalkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The physical, optical and reconstitution properties of apples subjected to ultrasound before drying. Ital. J. Food Sci. 2017, 29, 343–356. [Google Scholar]
- Rybak, K.; Wiktor, A.; Witrowa-rajchert, D.; Parniakov, O.; Nowacka, M. The Quality of Red Bell Pepper Subjected to Freeze-Drying Preceded by Traditional and Novel Pretreatment. Foods 2021, 10, 226. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Arevalo, P.; Ngadi, M.O.; Bazhal, M.I.; Raghavan, G.S.V. Impact of Pulsed Electric Fields on the Dehydration and Physical Properties of Apple and Potato Slices. Dry. Technol. 2007, 22, 1233–1246. [Google Scholar] [CrossRef]
- Wiktor, A.; Iwaniuk, M.; Śledź, M.; Nowacka, M.; Chudoba, T.; Witrowa-Rajchert, D. Drying Kinetics of Apple Tissue Treated by Pulsed Electric Field. Dry. Technol. 2013, 31, 112–119. [Google Scholar] [CrossRef]
- Taylor, P.; Gachovska, T.K.; Adedeji, A.A.; Ngadi, M.; Raghavan, G.V.S.; Gachovska, T.K.; Adedeji, A.A.; Ngadi, M.; Raghavan, G.V.S. Drying Characteristics of Pulsed Electric Field-Treated Carrot. Dry. Technol. 2008, 26, 1244–1250. [Google Scholar] [CrossRef]
- Galvão, A.M.M.T.; Rodrigues, S.; Fernandes, F.A.N. Kinetics of ultrasound pretreated apple cubes dried in fluidized bed dryer. Dry. Technol. 2019, 38, 1367–1377. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Śledź, M.; Jurek, N.; Witrowa-Rajchert, D.; Nowacka, M.; Wiktor, A.; Magdalena, S. Drying of ultrasound pretreated apple and its selected physical properties. J. Food Eng. 2012, 113, 427–433. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, Y.; Kaveh, M.; Fatemi, H.; Aziz, M. Combined Hot Air, Microwave, and Infrared Drying of on Drying Time, Energy, Qualitative, and Bioactive Compounds’ Properties. Foods 2021, 10, 1006. [Google Scholar] [CrossRef]
- Taghinezhad, E.; Kaveh, M.; Szumny, A. Thermodynamic and Quality Performance Studies for Drying Kiwi in Hybrid Hot Air-Infrared Drying with Ultrasound Pretreatment. Appl. Sci. 2021, 11, 1297. [Google Scholar] [CrossRef]
- Fijalkowska, A.; Nowacka, M.; Wiktor, A.; Sledz, M.; Witrowa-Rajchert, D. Ultrasound as a Pretreatment Method to Improve Drying Kinetics and Sensory Properties of Dried Apple. J. Food Process Eng. 2016, 39, 256–265. [Google Scholar] [CrossRef]
- Witrowa-Rajchert, D.; Lewicki, P.P. Rehydration properties of dried plant tissues. Int. J. Food Sci. Technol. 2006, 41, 1040–1046. [Google Scholar] [CrossRef]
- Kaveh, M.; Abbaspour-Gilandeh, Y.; Taghinezhad, E.; Witrowa-Rajchert, D.; Nowacka, M. The Quality of Infrared Rotary Dried Terebinth (Pistacia atlantica L.)-Optimization and Prediction Approach Using Response Surface Methodology. Molecules 2021, 26, 1999. [Google Scholar] [CrossRef]
- Belmiro, R.H.; Tribst, A.A.L.; Cristianini, M. Impact of high pressure processing in hydration and drying curves of common beans (Phaseolus vulgaris L.). Innov. Food Sci. Emerg. Technol. 2018, 47, 279–285. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Paniwnyk, L.; Lelas, V. Accelerated drying of button mushrooms, Brussels sprouts and cauliflower by applying power ultrasound and its rehydration properties. J. Food Eng. 2007, 81, 88–97. [Google Scholar] [CrossRef]
- Fauster, T.; Giancaterino, M.; Pittia, P.; Jaeger, H. Effect of pulsed electric field pretreatment on shrinkage, rehydration capacity and texture of freeze-dried plant materials. LWT Food Sci. Technol. 2020, 121, 108937. [Google Scholar] [CrossRef]
- Rybak, K.; Parniakov, O.; Samborska, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Energy and Quality Aspects of Freeze-Drying Preceded by Traditional and Novel Pre-Treatment Methods as Exemplified by Red Bell Pepper. Sustainability 2021, 13, 2035. [Google Scholar] [CrossRef]
- Parniakov, O.; Bals, O.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted vacuum freeze-drying of apple tissue. Innov. Food Sci. Emerg. Technol. 2016, 35, 52–57. [Google Scholar] [CrossRef]
- Zubernik, J.; Dadan, M.; Cichowska, J.; Witrowa-Rajchert, D. The Impact of the Pre-Treatment in Ethanol Solution on the Drying Kinetics and Selected Properties of Convective Dried Apples. Int. J. Food Eng. 2020, 16, 1–11. [Google Scholar] [CrossRef]
- Zhu, D.; Ji, B.; Eum, H.L.; Zude, M. Evaluation of the non-enzymatic browning in thermally processed apple juice by front-face fluorescence spectroscopy. Food Chem. 2009, 113, 272–279. [Google Scholar] [CrossRef]
- Dadan, M.; Nowacka, M.; Wiktor, A.; Sobczynska, A.; Witrowa-Rajchert, D. Ultrasound to improve drying processes and prevent thermolabile nutrients degradation. In Design and Optimization of Innovative Food Processing Techniques Assisted by Ultrasound; Barba, F.J., Cravotto, G., Chemat, F., Lorenzo Rodriguez, J.M., Munekata, P.E.S., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 55–110. ISBN 978-0-12-818275-8. [Google Scholar]
- Wibowo, S.; Aba, E.; De Man, S.; Bernaert, N.; Van Droogenbroeck, B.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Comparing the impact of high pressure, pulsed electric field and thermal pasteurization on quality attributes of cloudy apple juice using targeted and untargeted analyses. Innov. Food Sci. Emerg. Technol. 2019, 54, 64–77. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Chudoba, T.; Lojkowski, W.; Witrowa-Rajchert, D. The impact of pulsed electric field treatment on selected bioactive compound content and color of plant tissue. Innov. Food Sci. Emerg. Technol. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Janositz, A.; Semrau, J.; Knorr, D. Impact of PEF treatment on quality parameters of white asparagus (Asparagus officinalis L.). Innov. Food Sci. Emerg. Technol. 2011, 12, 269–274. [Google Scholar] [CrossRef]
- Subhashree, S.N.; Sunoj, S.; Xue, J.; Bora, G.C. Quantification of browning in apples using colour and textural features by image analysis. Food Qual. Saf. 2017, 1, 221–226. [Google Scholar] [CrossRef]
- Marszałek, K.; Szczepańska, J.; Starzonek, S.; Woźniak, Ł.; Trych, U.; Skąpska, S.; Rzoska, S.; Saraiva, J.A.; Lorenzo, J.M.; Barba, F.J. Enzyme inactivation and evaluation of physicochemical properties, sugar and phenolic profile changes in cloudy apple juices after high pressure processing, and subsequent refrigerated storage. J. Food Process Eng. 2019, 42, 1–8. [Google Scholar] [CrossRef]
- Castro, M.; Moreira, S.A.; Machado, M.F.; Sousa, A.; Saraiva, J.A. Effect of a HPP pretreatment on thermal inactivation kinetics of polyphenoloxidase obtained from three apple cultivars. J. Food Process Eng. 2017, 40, e12570. [Google Scholar] [CrossRef]
- Denoya, G.I.; Vaudagna, S.R.; Polenta, G. Effect of high pressure processing and vacuum packaging on the preservation of fresh-cut peaches. LWT Food Sci. Technol. 2014, 62, 801–806. [Google Scholar] [CrossRef]
- Bambace, M.F.; Moreira, M.R.; Sanchez-Moreno, C.; De Ancos, B. Effects of combined application of high-pressure processing and active coatings on phenolic com-pounds and microbiological and physicochemical quality of apple cubes. J. Sci. Food Agric. 2021, 101, 4256–4265. [Google Scholar] [CrossRef] [PubMed]
- Baslar, M.; Ertugay, M.F. The effect of ultrasound and photosonication treatment on polyphenoloxidase (PPO) activity, total phenolic component and colour of apple juice. Int. J. Food Sci. Technol. 2013, 48, 886–892. [Google Scholar] [CrossRef]


| Pretreatment Method | Parameters |
|---|---|
| HPP | 400 MPa, 15 min |
| US | 21 kHz, 180 W, 45 min |
| PEF | 1 kV/cm, 3.5 kJ/kg |
| Pretreatment Method | Relative Difference in Comparison with Control Material [%] | ||||
|---|---|---|---|---|---|
| X | SSL | H72h | aw | DM [%] | |
| HPP | −2.13 RD | 9.14 RD | −5.51 RD | 4.82 RD | 0.45 NRD |
| US | −2.84 NRD | 0.4 NRD | 0.75 RD | −4.42 RD | −0.54 NRD |
| PEF | 28.57 RD | −6.76 RD | 0.84 NRD | −7.23 RD | 0.76 NRD |
| Pretreatment Method | Relative Difference in Comparison with Untreated Material [%] | |||
|---|---|---|---|---|
| L* | a* | b* | TCD | |
| HPP | −21.52 RD | 180.84 RD | 11.02 RD | 103.64 RD |
| US | −6.33 NRD | 28.57 RD | 12.14 NRD | 3.08 NRD |
| PEF | −10.55 RD | −352.38 RD | 1.43 NRD | 3.85 NRD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiktor, A.; Landfeld, A.; Matys, A.; Novotná, P.; Dadan, M.; Kováříková, E.; Nowacka, M.; Mulenko, M.; Witrowa-Rajchert, D.; Strohalm, J.; et al. Selected Quality Parameters of Air-Dried Apples Pretreated by High Pressure, Ultrasounds and Pulsed Electric Field—A Comparison Study. Foods 2021, 10, 1943. https://doi.org/10.3390/foods10081943
Wiktor A, Landfeld A, Matys A, Novotná P, Dadan M, Kováříková E, Nowacka M, Mulenko M, Witrowa-Rajchert D, Strohalm J, et al. Selected Quality Parameters of Air-Dried Apples Pretreated by High Pressure, Ultrasounds and Pulsed Electric Field—A Comparison Study. Foods. 2021; 10(8):1943. https://doi.org/10.3390/foods10081943
Chicago/Turabian StyleWiktor, Artur, Aleš Landfeld, Aleksandra Matys, Pavla Novotná, Magdalena Dadan, Eliška Kováříková, Malgorzata Nowacka, Martin Mulenko, Dorota Witrowa-Rajchert, Jan Strohalm, and et al. 2021. "Selected Quality Parameters of Air-Dried Apples Pretreated by High Pressure, Ultrasounds and Pulsed Electric Field—A Comparison Study" Foods 10, no. 8: 1943. https://doi.org/10.3390/foods10081943
APA StyleWiktor, A., Landfeld, A., Matys, A., Novotná, P., Dadan, M., Kováříková, E., Nowacka, M., Mulenko, M., Witrowa-Rajchert, D., Strohalm, J., & Houška, M. (2021). Selected Quality Parameters of Air-Dried Apples Pretreated by High Pressure, Ultrasounds and Pulsed Electric Field—A Comparison Study. Foods, 10(8), 1943. https://doi.org/10.3390/foods10081943