Dynamics of the Fermentation Process and Chemical Profiling of Pomegranate (Punica granatum L.) Wines Obtained by Different Cultivar×Yeast Combinations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Pomegranate Fruits and Preparation of the Juice
2.2. Pomegranate Juice Fermentations
2.3. Wine Analyses
2.3.1. Organic Acids Analysis
2.3.2. Total Phenolic Compounds Analysis
2.3.3. Spectrophotometric Analyses
2.3.4. Volatile Compounds Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Collection of Pomegranate Fruits and Preparation of the Juice
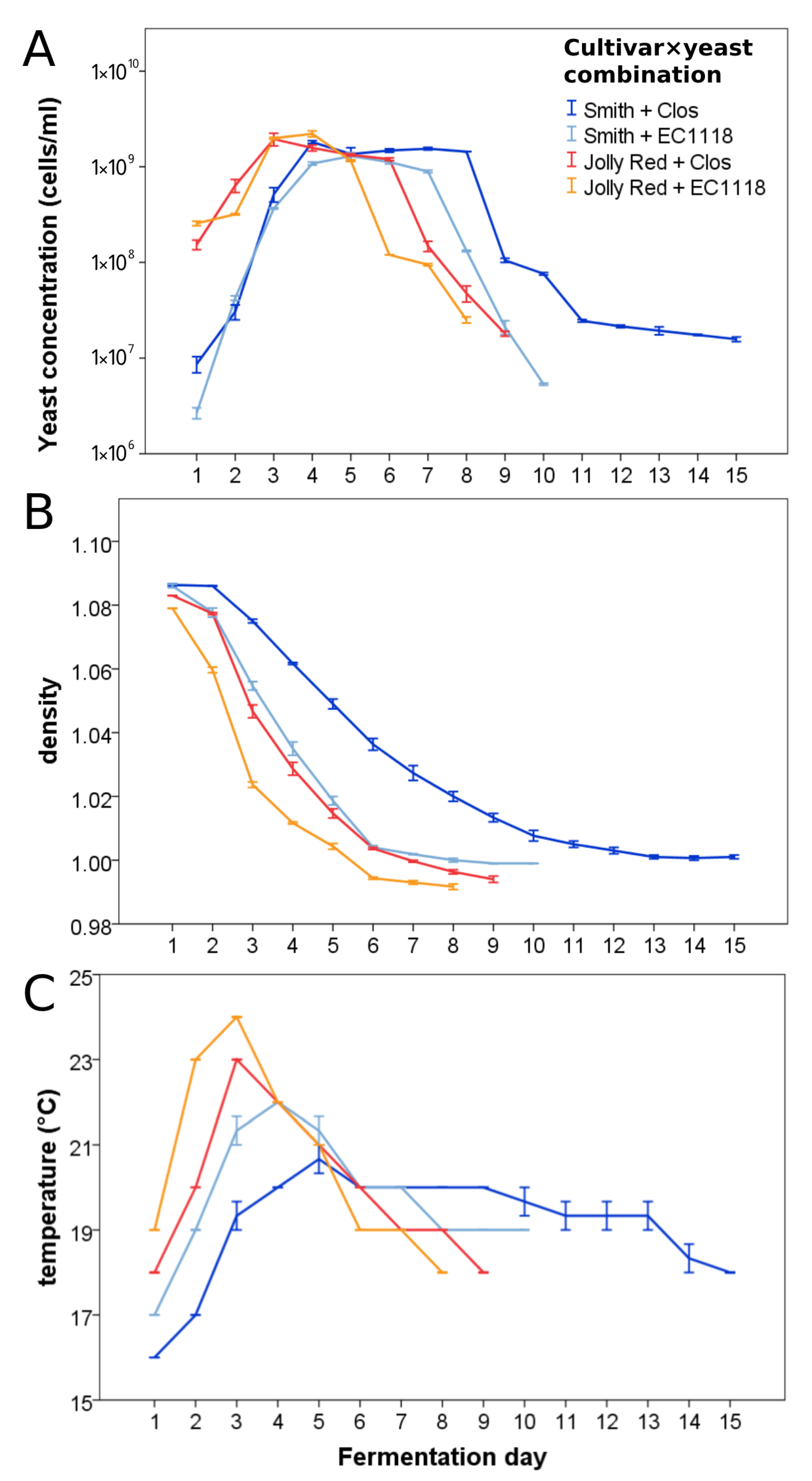
3.2. Pomegranate Juice Fermentations
3.3. Wine Characterization
3.3.1. Basic Parameters
3.3.2. Organic Acids
3.3.3. Phenolic Compounds and Spectrophotometric Analysis
3.3.4. Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stover, E.; Mercure, E.W. The Pomegranate: A New Look at the Fruit of Paradise. HortScience 2007, 42, 1088–1092. [Google Scholar] [CrossRef] [Green Version]
- Jacomet, S.; Kučan, D.; Ritter, A.; Suter, G.; Hagendorn, A. Punica granatum L. (Pomegranates) from Early Roman Contexts in Vindonissa (Switzerland). Veg. Hist. Archaeobot. 2002, 11, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Primavera, M.; Heiss, A.G.; Valamoti, M.S.; Quarta, G.; Masieri, M.; Fiorentino, G. Inside Sacrificial Cakes: Plant Components and Production Processes of Food Offerings at the Demeter and Persephone Sanctuary of Monte Papalucio (Oria, Southern Italy). Archaeol. Anthropol. Sci. 2019, 11, 1273–1287. [Google Scholar] [CrossRef]
- Ciraldi Marina. Food Offerings at the Archaic/Hellenistic Sanctuary of Demeter and Persephone at Monte Papalucio (Oria, Apulia, Southern Italy); Accordia Research Centre: London, UK, 1997. [Google Scholar]
- Bakels, C.; Jacomet, S. Access to Luxury Foods in Central Europe during the Roman Period: The Archaeobotanical Evidence. World Archaeol. 2003, 34, 542–557. [Google Scholar] [CrossRef]
- Goor, A. The History of the Pomegranate in the Holy Land. Econ. Bot. 1967, 21, 215–230. [Google Scholar] [CrossRef]
- Guasch-Jané, M.R.; Andrés-Lacueva, C.; Járegui, O.; Lamuela-Raventós, R.M. The Origin of the Ancient Egyptian Drink Shedeh Revealed Using LC/MS/MS. J. Archaeol. Sci. 2006, 33, 98–101. [Google Scholar] [CrossRef]
- Dhineshkumar, V.; Ramasamy, D. Pomegranate Processing and Value Addition: Review. J. Food Process. Technol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Balmaseda, A.; Bordons, A.; Reguant, C.; Bautista-Gallego, J. Non-Saccharomyces in Wine: Effect upon Oenococcus Oeni and Malolactic Fermentation. Front. Microbiol. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moschetti, G.; Corona, O.; Gaglio, R.; Squadrito, M.; Parrinello, A.; Settanni, L.; Barone, E.; Francesca, N. Use of Fortified Pied de Cuve as an Innovative Method to Start Spontaneous Alcoholic Fermentation for Red Winemaking. Aust. J. Grape Wine Res. 2016, 22, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Bamforth, C.W.; Ward, R.E. The Oxford Handbook of Food Fermentations; Oxford Handbooks: Oxford, UK, 2014. [Google Scholar]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Emam-Djomeh, Z.; Kiani, H. Fermentation of Pomegranate Juice by Probiotic Lactic Acid Bacteria. World J. Microbiol. Biotechnol. 2011, 27, 123–128. [Google Scholar] [CrossRef]
- Filannino, P.; Azzi, L.; Cavoski, I.; Vincentini, O.; Rizzello, C.G.; Gobbetti, M.; Di Cagno, R. Exploitation of the Health-Promoting and Sensory Properties of Organic Pomegranate (Punica granatum L.) Juice through Lactic Acid Fermentation. Int. J. Food Microbiol. 2013, 163, 184–192. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1. [Google Scholar]
- Tarantino, A.; Difonzo, G.; Lopriore, G.; Disciglio, G.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Bioactive Compounds and Quality Evaluation of ‘Wonderful’ Pomegranate Fruit and Juice as Affected by Deficit Irrigation. J. Sci. Food Agric. 2020, 100, 5539–5545. [Google Scholar] [CrossRef]
- Gambacorta, G.; Antonacci, D.; La Gatta, M.; Faccia, M.; La Gatta, B.; Pati, S.; Coletta, A.; La Notte, E. Phenolic Composition of Aglianico and Nero Di Troia Grapes and Wines as Affected by Cover Cropping and Irrigation. Ital. J. Food Sci. 2011, 23, 381–394. [Google Scholar]
- Trani, A.; Verrastro, V.; Punzi, R.; Faccia, M.; Gambacorta, G. Phenols, Volatiles and Sensory Properties of Primitivo Wines from the “Gioia Del Colle” PDO Area. S. Afr. J. Enol. Vitic. 2016, 37, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Gumienna, M.; Szwengiel, A.; Górna, B. Bioactive Components of Pomegranate Fruit and Their Transformation by Fermentation Processes. Eur. Food Res. Technol. 2016, 242, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Mphahlele, R.R.; Fawole, O.A.; Mokwena, L.M.; Opara, U.L. Effect of Extraction Method on Chemical, Volatile Composition and Antioxidant Properties of Pomegranate Juice. S. Afr. J. Bot. 2016, 103, 135–144. [Google Scholar] [CrossRef]
- Berenguer, M.; Vegara, S.; Barrajón, E.; Saura, D.; Valero, M.; Martí, N. Physicochemical Characterization of Pomegranate Wines Fermented with Three Different Saccharomyces cerevisiae Yeast Strains. Food Chem. 2016, 190, 848–855. [Google Scholar] [CrossRef]
- Samson, A.K.S.; Singh, G. Optimum Parameters for Wine Production from Pomegranate Fruit Juice. Int. J. Pharm. Sci. Res. 2017, 8, 4826–4831. [Google Scholar] [CrossRef]
- Mena, P.; Gironés-Vilaplana, A.; Martí, N.; García-Viguera, C. Pomegranate Varietal Wines: Phytochemical Composition and Quality Parameters. Food Chem. 2012, 133, 108–115. [Google Scholar] [CrossRef]
- Ehling, S.; Cole, S. Analysis of Organic Acids in Fruit Juices by Liquid Chromatography-Mass Spectrometry: An Enhanced Tool for Authenticity Testing. J. Agric. Food Chem. 2011, 59, 2229–2234. [Google Scholar] [CrossRef]
- Alcaraz-Mármol, F.; Nuncio-Jáuregui, N.; García-Sánchez, F.; Martínez-Nicolás, J.J.; Hernández, F. Characterization of Twenty Pomegranate (Punica granatum L.) Cultivars Grown in Spain: Aptitudes for Fresh Consumption and Processing. Sci. Hortic. 2017, 219, 152–160. [Google Scholar] [CrossRef]
- Ozgen, M.; Durgaç, C.; Serçe, S.; Kaya, C. Chemical and Antioxidant Properties of Pomegranate Cultivars Grown in the Mediterranean Region of Turkey. Food Chem. 2008, 111, 703–706. [Google Scholar] [CrossRef]
- Türkyilmaz, M. Anthocyanin and Organic Acid Profiles of Pomegranate (Punica granatum L.) Juices from Registered Varieties in Turkey. Int. J. Food Sci. Technol. 2013, 48, 2086–2095. [Google Scholar] [CrossRef]
- Lan, Y.; Wu, J.; Wang, X.; Sun, X.; Hackman, R.M.; Li, Z.; Feng, X. Evaluation of Antioxidant Capacity and Flavor Profile Change of Pomegranate Wine during Fermentation and Aging Process. Food Chem. 2017, 232, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, M.; Yilmaz, H. Organic Acid, Phenolic Profile and Antioxidant Capacities of Pomegranate (Punica granatum L.) Cultivars and Selected Genotypes. Sci. Hortic. 2012, 143, 38–42. [Google Scholar] [CrossRef]
- Poyrazoğlu, E.; Gökmen, V.; Artιk, N. Organic Acids and Phenolic Compounds in Pomegranates (Punica granatum L.) Grown in Turkey. J. Food Compos. Anal. 2002, 15, 567–575. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Calín-Sánchez, Á.; Hernández, F.; Carbonell-Barrachina, Á.A. Pomegranate Juice Adulteration by Addition of Grape or Peach Juices. J. Sci. Food Agric. 2014, 94, 646–655. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Tsiaka, T.; Proestos, C.; Zoumpoulakis, P. Total Phenolic Content, Antioxidant Capacity and Phytochemical Profiling of Grape and Pomegranate Wines. RSC Adv. 2015, 5, 101683–101692. [Google Scholar] [CrossRef]
- Ough, C.S. Sensory Examination of Four Organic Acids Added to Wine. J. Food Sci. 1963, 28, 101–106. [Google Scholar] [CrossRef]
- Liu, P.-T.; Zhang, B.-Q.; Duan, C.-Q.; Yan, G.-L. Pre-Fermentative Supplementation of Unsaturated Fatty Acids Alters the Effect of Overexpressing ATF1 and EEB1 on Esters Biosynthesis in Red Wine. LWT 2020, 120, 108925. [Google Scholar] [CrossRef]
- Hugenholtz, J. Citrate Metabolism in Lactic Acid Bacteria. FEMS Microbiol. Rev. 1993, 12, 165–178. [Google Scholar] [CrossRef]
- OIV (International Organisation of Vine and Wine). International Code of Oenological Practices; International Organisation of Vine and Wine: Paris, France, 2012; Volume 33. [Google Scholar]
- Kokkinomagoulos, E.; Nikolaou, A.; Kourkoutas, Y.; Kandylis, P. Evaluation of Yeast Strains for Pomegranate Alcoholic Beverage Production: Effect on Physicochemical Characteristics, Antioxidant Activity, and Aroma Compounds. Microorganisms 2020, 8, 1583. [Google Scholar] [CrossRef] [PubMed]
- Rios-Corripio, G.; Guerrero-Beltrán, J.Á. Antioxidant and Physicochemical Characteristics of Unfermented and Fermented Pomegranate (Punica granatum L.) Beverages. J. Food Sci. Technol. 2019, 56, 132–139. [Google Scholar] [CrossRef]
- Wang, Q.J.; Spence, C. Drinking through Rosé-Coloured Glasses: Influence of Wine Colour on the Perception of Aroma and Flavour in Wine Experts and Novices. Food Res. Int. 2019, 126, 108678. [Google Scholar] [CrossRef] [PubMed]
- Stávek, J.; Papouskova, B.; Balik, J.; Bednar, P. Effect of Storage Conditions on Various Parameters of Colour and the Anthocyanin Profile of Rosé Wines. Int. J. Food Prop. 2012, 15, 1133–1147. [Google Scholar] [CrossRef]
- Benucci, I. Impact of Post-Bottling Storage Conditions on Colour and Sensory Profile of a Rosé Sparkling Wine. LWT 2020, 118, 108732. [Google Scholar] [CrossRef]
- Wang, X.; Ren, X.; Shao, Q.; Peng, X.; Zou, W.; Sun, Z.; Zhang, L.; Li, H. Transformation of Microbial Negative Correlations into Positive Correlations by Saccharomyces cerevisiae Inoculation during Pomegranate Wine Fermentation. Appl. Environ. Microbiol. 2020, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Sevilla, A.J.; Mena, P.; Martí, N.; García Viguera, C.; Carbonell-Barrachina, Á.A. Volatile Composition and Descriptive Sensory Analysis of Pomegranate Juice and Wine. Food Res. Int. 2013, 54, 246–254. [Google Scholar] [CrossRef]
- Jackson, R. Wine Science; Elsevier: Amsterdam, The Netherlands, 2008; Volume 91. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2020, 10, 51. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial Modulation of Aromatic Esters in Wine: Current Knowledge and Future Prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Cosnett, I.R. Food Flavours Biology and Chemistry. Carbohydr. Polym. 2001, 46, 296. [Google Scholar] [CrossRef]
- Liu, P.; Ivanova-Petropulos, V.; Duan, C.; Yan, G. Effect of Unsaturated Fatty Acids on Intra-Metabolites and Aroma Compounds of Saccharomyces cerevisiae in Wine Fermentation. Foods 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Schreier, P.; Walter, G.J. Flavor Composition of Wines: A Review. C R C Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef] [PubMed]



| Average Fruit Weight (kg) | Extraction Yield (%) | Total Soluble Solids (°Brix) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Jolly Red | 0.275 | 0.004 | 31.733 | 0.236 | 16.167 | 0.09 |
| Smith | 0.211 | 0.004 | 29.624 | 0.324 | 17.028 | 0.086 |
| ANOVA | ||||||
| Significance | <0.001 | <0.001 | <0.001 | |||
| pH | Free SO2 (mg L−1) | Total SO2 (mg L−1) | Glucose + Fructose (g L−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Smith + Clos | 3.12 | 0.01 | 45.67 | 0.33 | 55.00 | 0.58 | 2.41 | 0.60 |
| Smith + EC1118 | 3.14 | 0.00 | 54.67 | 0.67 | 64.67 | 2.91 | 0.29 | 0.04 |
| Jolly Red + Clos | 3.51 | 0.00 | 21.00 | 1.53 | 49.33 | 0.67 | 6.84 | 0.54 |
| Jolly Red + EC1118 | 3.64 | 0.01 | 27.00 | 1.53 | 58.67 | 1.86 | 1.60 | 0.38 |
| ANOVA | ||||||||
| Cultivar Significance | <0.001 | <0.001 | 0.011 | <0.001 | ||||
| Yeast Significance | <0.001 | <0.001 | 0.001 | <0.001 | ||||
| Cultivar×Yeast Significance | <0.001 | 0.226 | 0.928 | 0.008 | ||||
| Cultivar | Yeast | Cultivar×Yeast | |
|---|---|---|---|
| Tartaric acid | <0.001 | 0.414 | 0.047 |
| Ascorbic acid | <0.001 | 0.105 | <0.001 |
| Lactic acid | 0.033 | 0.493 | 0.519 |
| Acetic acid | <0.001 | 0.355 | 0.769 |
| Succinic acid | <0.001 | 0.653 | 0.911 |
| Citric acid | <0.001 | 0.290 | 0.768 |
| Cultivar | Yeast | Cultivar×Yeast | |
|---|---|---|---|
| Phenolic compounds | |||
| TPC ** | 0.002 | 0.928 | 0.212 |
| Total anthocyanins | <0.001 | <0.001 | <0.001 |
| Color | |||
| Intensity | <0.001 | 0.080 | 0.716 |
| Hue | <0.001 | 0.004 | 0.004 |
| RT * | Jolly Red + EC1118 | Jolly Red + Clos | Smith + EC1118 | Smith + Clos | ANOVA Results (p-Values) ** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Cultivar | Yeast | Cultivar×Yeast | ||
| Aldehydes | ||||||||||||
| Acetaldehyde | 4.83 | 0.81 | 0.04 | 1.23 | 0.60 | 0.52 | 0.30 | 0.36 | 0.01 | 0.017 | 0.523 | 0.180 |
| Octanal | 20.8 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.39 | 0.16 | 0.003 | 0.003 | 0.003 |
| Nonanal | 25.36 | 0.97 | 0.15 | 1.35 | 0.73 | 1.78 | 1.08 | 2.14 | 1.07 | 0.139 | 0.475 | 0.985 |
| 3-furaldehyde | 28.56 | 0.00 | 0.00 | 0.00 | 0.00 | 0.34 | 0.19 | 0.30 | 0.06 | <0.001 | 0.733 | 0.733 |
| Benzaldehyde | 31.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.80 | 0.25 | 0.63 | 0.10 | <0.001 | 0.298 | 0.298 |
| 4-Propylbenzaldehyde | 42.73 | 1.42 | 0.01 | 2.08 | 1.77 | 3.42 | 1.07 | 2.07 | 0.27 | 0.136 | 0.582 | 0.135 |
| Total | 3.21 | 0.16 | 4.66 | 1.96 | 6.87 | 2.85 | 5.90 | 1.28 | 0.051 | 0.827 | 0.290 | |
| Alcohols | ||||||||||||
| Isobutanol | 12.41 | 18.57 | 0.78 | 32.34 | 7.49 | 21.31 | 8.37 | 27.30 | 0.96 | 0.735 | 0.016 | 0.267 |
| Isoamyl alcohol | 17.11 | 765.38 | 23.25 | 783.31 | 438.01 | 636.96 | 595.21 | 768.34 | 45.17 | 0.746 | 0.736 | 0.798 |
| Isohexanol | 21.77 | 0.00 | 0.00 | 0.00 | 0.00 | 0.92 | 0.39 | 0.40 | 0.11 | <0.001 | 0.056 | 0.056 |
| 3-methyl-1-pentanol | 22.34 | 1.13 | 0.27 | 0.90 | 0.57 | 1.96 | 0.79 | 1.13 | 0.10 | 0.107 | 0.108 | 0.335 |
| 3-ethoxy-1-propanol | 24.51 | 1.74 | 0.05 | 0.00 | 0.00 | 1.34 | 0.40 | 0.00 | 0.00 | 0.124 | <0.001 | 0.124 |
| 3-hexen-1-ol | 24.88 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.94 | 0.24 | <0.001 | <0.001 | <0.001 |
| 2-nonanol | 30.48 | 0.82 | 0.20 | 0.41 | 0.10 | 1.00 | 0.41 | 0.40 | 0.09 | 0.541 | 0.007 | 0.530 |
| 2-phenylethanol | 45.71 | 377.38 | 9.23 | 514.26 | 254.25 | 422.26 | 142.19 | 306.38 | 34.53 | 0.364 | 0.904 | 0.174 |
| Total | 1165.02 | 32.46 | 1331.20 | 670.92 | 1085.76 | 744.64 | 1105.89 | 80.84 | 0.614 | 0.757 | 0.808 | |
| Acetate esters | ||||||||||||
| Ethyl acetate | 6.81 | 76.42 | 10.10 | 50.19 | 3.69 | 54.56 | 6.51 | 44.69 | 0.62 | 0.005 | 0.001 | 0.054 |
| Isobutyl acetate | 9.84 | 0.90 | 0.16 | 0.56 | 0.32 | 0.36 | 0.30 | 0.23 | 0.04 | 0.012 | 0.123 | 0.469 |
| Isoamyl acetate | 13.72 | 106.46 | 51.76 | 56.62 | 9.48 | 39.46 | 22.05 | 22.46 | 6.23 | 0.016 | 0.078 | 0.351 |
| Hexyl acetate | 20.02 | 0.91 | 0.46 | 0.60 | 0.20 | 0.78 | 0.50 | 0.59 | 0.11 | 0.746 | 0.259 | 0.792 |
| Heptyl acetate | 24.45 | 0.00 | 0.00 | 0.40 | 0.18 | 0.00 | 0.00 | 0.29 | 0.14 | 0.394 | <0.001 | 0.394 |
| Phenethyl acetate | 42.25 | 104.21 | 6.38 | 105.90 | 57.97 | 47.39 | 14.79 | 21.77 | 2.69 | 0.004 | 0.511 | 0.455 |
| Total | 288.89 | 55.47 | 214.28 | 54.55 | 142.54 | 41.05 | 90.03 | 3.59 | <0.001 | 0.037 | 0.675 | |
| Ethyl esters | ||||||||||||
| Ethyl butyrate | 10.59 | 7.87 | 1.68 | 4.71 | 0.23 | 5.57 | 2.36 | 3.29 | 0.42 | 0.059 | 0.013 | 0.615 |
| Ethyl 2-methylbutyrate | 11.11 | 1.46 | 0.94 | 0.46 | 0.10 | 1.12 | 0.95 | 1.11 | 0.09 | 0.695 | 0.227 | 0.239 |
| Ethyl isovalerate | 11.67 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 | 0.06 | 0.001 | 0.001 | 0.001 |
| Ethyl crotonate | 15.41 | 0.41 | 0.02 | 0.18 | 0.07 | 0.20 | 0.08 | 0.06 | 0.05 | 0.001 | <0.001 | 0.253 |
| Ethyl hexanoate | 18.34 | 52.97 | 20.17 | 44.96 | 18.26 | 38.58 | 28.63 | 29.55 | 12.74 | 0.249 | 0.497 | 0.967 |
| Ethyl hex-5-enoate | 20.32 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.22 | 0.07 | <0.001 | <0.001 | <0.001 |
| Ethyl hex-3-enoate | 21.32 | 0.00 | 0.00 | 0.00 | 0.00 | 0.22 | 0.12 | 0.28 | 0.05 | <0.001 | 0.485 | 0.485 |
| Ethyl heptanoate | 22.69 | 0.00 | 0.00 | 0.18 | 0.07 | 0.00 | 0.00 | 0.27 | 0.11 | 0.289 | <0.001 | 0.289 |
| Ethyl lactate | 23.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.61 | 0.11 | <0.001 | <0.001 | <0.001 |
| Ethyl octanoate | 27.05 | 225.17 | 154.14 | 128.64 | 55.10 | 109.05 | 76.25 | 89.35 | 21.07 | 0.177 | 0.300 | 0.485 |
| Ethyl 7-octenoate | 29.22 | 0.00 | 0.00 | 0.62 | 0.15 | 0.00 | 0.00 | 0.63 | 0.23 | 0.938 | <0.001 | 0.938 |
| Ethyl 2-furoate | 34.94 | 0.00 | 0.00 | 0.00 | 0.00 | 1.71 | 0.90 | 1.00 | 0.27 | 0.001 | 0.223 | 0.223 |
| Ethyl decanoate | 35.36 | 246.63 | 104.96 | 40.69 | 10.03 | 96.15 | 41.57 | 47.57 | 5.56 | 0.060 | 0.005 | 0.043 |
| Ethyl 9-decenoate | 37.41 | 66.08 | 7.32 | 194.20 | 30.99 | 11.15 | 1.49 | 56.60 | 5.31 | <0.001 | <0.001 | 0.002 |
| Total | 600.59 | 284.87 | 414.64 | 93.96 | 263.77 | 150.91 | 230.69 | 33.70 | 0.028 | 0.294 | 0.455 | |
| Other esters | ||||||||||||
| Isoamyl octanoate | 36.14 | 3.16 | 0.77 | 0.00 | 0.00 | 1.99 | 0.53 | 1.68 | 0.41 | 0.411 | <0.001 | 0.001 |
| Diethyl succinate | 36.88 | 0.00 | 0.00 | 2.24 | 1.74 | 4.11 | 1.70 | 4.14 | 1.12 | 0.005 | 0.180 | 0.191 |
| Total | 3.16 | 0.77 | 2.24 | 1.74 | 6.10 | 2.14 | 5.82 | 1.51 | ||||
| Ketones | ||||||||||||
| 2-heptanone | 16.16 | 0.30 | 0.04 | 0.10 | 0.10 | 0.09 | 0.16 | 0.03 | 0.05 | 0.037 | 0.043 | 0.247 |
| 2-nonanone | 25.12 | 1.26 | 0.47 | 0.17 | 0.07 | 0.34 | 0.10 | 0.08 | 0.07 | 0.007 | 0.001 | 0.018 |
| Total | 1.57 | 0.50 | 0.26 | 0.15 | 0.43 | 0.13 | 0.11 | 0.02 | 0.003 | <0.001 | 0.013 | |
| Acids | ||||||||||||
| Acetic acid | 28.09 | 3.23 | 0.12 | 6.20 | 1.06 | 9.52 | 3.01 | 9.50 | 0.66 | <0.001 | 0.156 | 0.151 |
| Isobutyric acid | 32.77 | 0.00 | 0.00 | 1.42 | 0.34 | 0.50 | 0.16 | 0.84 | 0.12 | 0.723 | <0.001 | 0.002 |
| Butyric acid | 35.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.81 | 0.36 | 0.48 | 0.03 | <0.001 | 0.160 | 0.160 |
| Caproic acid | 43.24 | 21.37 | 5.00 | 27.35 | 17.81 | 37.54 | 11.10 | 18.08 | 2.07 | 0.597 | 0.313 | 0.076 |
| Caprylic acid | 50.46 | 257.37 | 64.35 | 336.07 | 232.71 | 418.63 | 146.95 | 194.73 | 23.26 | 0.906 | 0.401 | 0.102 |
| Pelargonic acid | 53.82 | 1.78 | 0.41 | 1.71 | 0.34 | 2.69 | 0.85 | 1.59 | 0.36 | 0.235 | 0.094 | 0.129 |
| Capric acid | 57.04 | 45.06 | 11.26 | 23.16 | 15.52 | 116.10 | 44.50 | 31.60 | 9.37 | 0.024 | 0.006 | 0.059 |
| 9-decenoic acid | 58.97 | 2.33 | 0.91 | 31.75 | 25.12 | 4.41 | 2.35 | 9.16 | 3.19 | 0.200 | 0.049 | 0.132 |
| Total | 331.13 | 81.05 | 427.67 | 292.51 | 590.21 | 195.21 | 265.98 | 34.64 | 0.654 | 0.308 | 0.079 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinale, M.; Trinchera, R.; Natrella, G.; Difonzo, G.; De Benedittis, C.; D’amato, I.; Mascellani, M.; Paradiso, V.M.; Rustioni, L. Dynamics of the Fermentation Process and Chemical Profiling of Pomegranate (Punica granatum L.) Wines Obtained by Different Cultivar×Yeast Combinations. Foods 2021, 10, 1913. https://doi.org/10.3390/foods10081913
Cardinale M, Trinchera R, Natrella G, Difonzo G, De Benedittis C, D’amato I, Mascellani M, Paradiso VM, Rustioni L. Dynamics of the Fermentation Process and Chemical Profiling of Pomegranate (Punica granatum L.) Wines Obtained by Different Cultivar×Yeast Combinations. Foods. 2021; 10(8):1913. https://doi.org/10.3390/foods10081913
Chicago/Turabian StyleCardinale, Massimiliano, Roberto Trinchera, Giuseppe Natrella, Graziana Difonzo, Carlo De Benedittis, Ilario D’amato, Marco Mascellani, Vito Michele Paradiso, and Laura Rustioni. 2021. "Dynamics of the Fermentation Process and Chemical Profiling of Pomegranate (Punica granatum L.) Wines Obtained by Different Cultivar×Yeast Combinations" Foods 10, no. 8: 1913. https://doi.org/10.3390/foods10081913
APA StyleCardinale, M., Trinchera, R., Natrella, G., Difonzo, G., De Benedittis, C., D’amato, I., Mascellani, M., Paradiso, V. M., & Rustioni, L. (2021). Dynamics of the Fermentation Process and Chemical Profiling of Pomegranate (Punica granatum L.) Wines Obtained by Different Cultivar×Yeast Combinations. Foods, 10(8), 1913. https://doi.org/10.3390/foods10081913










