Use of Food Additive Titanium Dioxide (E171) before the Introduction of Regulatory Restrictions Due to Concern for Genotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and Categorization
2.2. Data Processing and Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ropers, M.-H.; Terrisse, H.; Mercier-Bonin, M.; Humbert, B. Titanium Dioxide as Food Additive; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.J.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.; Marvin, H.J.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.G.; Bouwmeester, H. Characterization of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef]
- Kullavanijaya, P.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2005, 52, 937–958. [Google Scholar] [CrossRef]
- Lautenschlager, S.; Wulf, H.C.; Pittelkow, M.R. Photoprotection. Lancet Lond. Engl. 2007, 370, 528–537. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices. 2017. Available online: https://www.fda.gov/industry/color-additive-inventories/summary-color-additives-use-united-states-foods-drugs-cosmetics-and-medical-devices (accessed on 17 August 2021).
- European Commission. Commission (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Off. J. Eur. Union 2011, L354/16, 1–18. [Google Scholar]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, e05327. [Google Scholar] [CrossRef] [Green Version]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.; Urrutia Ortega, I.M.; Briedé, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis 2017, 32, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Comission. Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re-evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. Off. J. Eur. Union 2010, L80/19, 1–9. [Google Scholar]
- EFSA Panel on Food Additives Nutrient Sources added to Food. Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016, 14, 83. [Google Scholar] [CrossRef]
- Maurici, D.; Aardema, M.; Corvi, R.; Kleber, M.; Krul, C.; Laurent, C.; Loprieno, N.; Pasanen, M.; Pfuhler, S.; Phillips, B.; et al. Genotoxicty and mutagenicity. Altern. Lab. Anim. ATLA 2005, 33 (Suppl. S1), 117–130. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO(2) impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef]
- Chen, X.X.; Cheng, B.; Yang, Y.X.; Cao, A.; Liu, J.H.; Du, L.J.; Liu, Y.; Zhao, Y.; Wang, H. Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum. Small Weinh. Bergstr. Ger. 2013, 9, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Athinarayanan, J.; Alshatwi, A.A.; Periasamy, V.S.; Al-Warthan, A.A. Identification of nanoscale ingredients in commercial food products and their induction of mitochondrially mediated cytotoxic effects on human mesenchymal stem cells. J. Food Sci. 2015, 80, N459–N464. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, R.; Cubadda, F.; Moracci, G.; Aureli, F.; D’Amato, M.; Valeri, M.; De Berardis, B.; Raggi, A.; Mantovani, A.; Passeri, D.; et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology 2014, 8, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, B. Critical review of public health regulations of titanium dioxide, a human food additive. Integr. Environ. Assess. Manag. 2015, 11, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Periasamy, V.S.; Athinarayanan, J.; Al-Hadi, A.M.; Juhaimi, F.A.; Mahmoud, M.H.; Alshatwi, A.A. Identification of titanium dioxide nanoparticles in food products: Induce intracellular oxidative stress mediated by TNF and CYP1A genes in human lung fibroblast cells. Environ. Toxicol. Pharmacol. 2015, 39, 176–186. [Google Scholar] [CrossRef]
- Heringa, M.B.; Geraets, L.; van Eijkeren, J.C.; Vandebriel, R.J.; de Jong, W.H.; Oomen, A.G. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology 2016, 10, 1515–1525. [Google Scholar] [CrossRef] [Green Version]
- Rompelberg, C.; Heringa, M.B.; van Donkersgoed, G.; Drijvers, J.; Roos, A.; Westenbrink, S.; Peters, R.; van Bemmel, G.; Brand, W.; Oomen, A.G. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology 2016, 10, 1404–1414. [Google Scholar] [CrossRef] [Green Version]
- Farrell, T.P.; Magnuson, B. Absorption, Distribution and Excretion of Four Forms of Titanium Dioxide Pigment in the Rat. J. Food Sci. 2017, 82, 1985–1993. [Google Scholar] [CrossRef]
- Guo, Z.; Martucci, N.J.; Moreno-Olivas, F.; Tako, E.; Mahler, G.J. Titanium Dioxide Nanoparticle Ingestion Alters Nutrient Absorption in an In Vitro Model of the Small Intestine. NanoImpact 2017, 5, 70–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Wang, S.; Zhou, L.; Sun, L. The Potential Liver, Brain, and Embryo Toxicity of Titanium Dioxide Nanoparticles on Mice. Nanoscale Res. Lett. 2017, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Food Additives and Nutrient Sources added to Food; Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Evaluation of four new studies on the potential toxicity of titanium dioxide used as a food additive (E 171). EFSA J. 2018, 16, e05366. [Google Scholar] [CrossRef] [PubMed]
- French Agency for Food Environmental and Occupational Health & Safety. AVIS de l’Agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail Relatif aux Risques Liés à L’ingestion de L’additif Alimentaire E171; Saisine n 2019-SA-0036; Anses: Buenos Aires, Argentina, 2019; pp. 1–44. [Google Scholar]
- EFSA. EFSA statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J. 2019, 17, e05714. [Google Scholar] [CrossRef] [Green Version]
- Netherlands Food and Consumer Product Safety Authority (NVWA). Opinion of BuRO on Possible Health Effects of the Food Additive Titanium Dioxide (E171). Available online: https://english.nvwa.nl/documents/consumers/food/safety/documents/opinion-of-buro-on-possible-health-effects-of-the-food-additive-titanium-dioxide-e171 (accessed on 17 August 2021).
- Civil society organisations, Civil Society Organisations Demand the Removal of E171 from the EU List of Permitted Food Additives. Available online: https://www.beuc.eu/publications/beuc-x-2019-031_removal_of_e171_from_the_eu_list_of_permitted_food_additives.pdf (accessed on 17 August 2021).
- EFSA Panel on Food Additives and Flavourings; Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar] [CrossRef]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.-H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; Díaz-Urbina, D.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; González, M.I.; Reyes, J.L.; Villamar-Duque, T.E.; Flores-Sánchez, M.L.O.; Hernández-Pando, R.; et al. Food-grade titanium dioxide (E171) induces anxiety, adenomas in colon and goblet cells hyperplasia in a regular diet model and microvesicular steatosis in a high fat diet model. Food Chem. Toxicol. 2020, 146, 111786. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.; Scarcello, E.; Ibouraadaten, S.; Yakoub, Y.; Leinardi, R.; Ambroise, J.; Bearzatto, B.; Gala, J.-L.; Paquot, A.; Muccioli, G.G.; et al. Dietary nanoparticles alter the composition and function of the gut microbiota in mice at dose levels relevant for human exposure. Food Chem. Toxicol. 2021, 154, 112352. [Google Scholar] [CrossRef]
- Agans, R.T.; Gordon, A.; Hussain, S.; Paliy, O. Titanium Dioxide Nanoparticles Elicit Lower Direct Inhibitory Effect on Human Gut Microbiota Than Silver Nanoparticles. Toxicol. Sci. 2019, 172, 411–416. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef]
- Li, M.; Li, F.; Lu, Z.; Fang, Y.; Qu, J.; Mao, T.; Wang, H.; Chen, J.; Li, B. Effects of TiO2 nanoparticles on intestinal microbial composition of silkworm, Bombyx mori. Sci. Total Environ. 2020, 704, 135273. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, X.; Cheng, S.; Fan, J.; Qin, X.; Wang, T.; Zhang, Y.; Zhang, J.; Qiu, Y.; Qiu, J.; et al. Titanium dioxide nanoparticles via oral exposure leads to adverse disturbance of gut microecology and locomotor activity in adult mice. Arch. Toxicol. 2020, 94, 1173–1190. [Google Scholar] [CrossRef]
- Giorgetti, L.; Spanò, C.; Muccifora, S.; Bellani, L.; Tassi, E.; Bottega, S.; Di Gregorio, S.; Siracusa, G.; Sanità di Toppi, L.; Ruffini Castiglione, M. An integrated approach to highlight biological responses of Pisum sativum root to nano-TiO2 exposure in a biosolid-amended agricultural soil. Sci. Total Environ. 2019, 650, 2705–2716. [Google Scholar] [CrossRef]
- Bellani, L.; Muccifora, S.; Barbieri, F.; Tassi, E.; Ruffini Castiglione, M.; Giorgetti, L. Genotoxicity of the food additive E171, titanium dioxide, in the plants Lens culinaris L. and Allium cepa L. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2020, 849, 503142. [Google Scholar] [CrossRef] [PubMed]
- Tarnavölgyi, G. Analysis of Consumers’ Attitudes Towards Food Additives Using Focus Group Survey. Gábor TARNAVöLGYI 2003, 68, 193–196. [Google Scholar]
- Hansen, J.; Holm, L.; Frewer, L.; Robinson, P.; Sandøe, P. Beyond the knowledge deficit: Recent research into lay and expert attitudes to food risks. Appetite 2003, 41, 111–121. [Google Scholar] [CrossRef]
- Bearth, A.; Cousin, M.-E.; Siegrist, M. The consumer’s perception of artificial food additives: Influences on acceptance, risk and benefit perceptions. Food Qual. Prefer. 2014, 38, 14–23. [Google Scholar] [CrossRef]
- Nutrition Institute. Composition and Labelling Information System as a Tool for Monitoring of the Food Supply. Available online: https://www.nutris.org/en/composition-and-labelling-information-system (accessed on 12 December 2020).
- Dunford, E.; Webster, J.; Metzler, A.B.; Czernichow, S.; Ni Mhurchu, C.; Wolmarans, P.; Snowdon, W.; L’Abbe, M.; Li, N.; Maulik, P.K.; et al. International collaborative project to compare and monitor the nutritional composition of processed foods. Eur. J. Prev. Cardiol. 2012, 19, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Pivk Kupirovič, U.; Miklavec, K.; Hribar, M.; Kušar, A.; Žmitek, K.; Pravst, I. Nutrient Profiling Is Needed to Improve the Nutritional Quality of the Foods Labelled with Health-Related Claims. Nutrients 2019, 11, 287. [Google Scholar] [CrossRef] [Green Version]
- Zupanic, N.; Hribar, M.; Fidler Mis, N.; Pravst, I. Free Sugar Content in Pre-Packaged Products: Does Voluntary Product Reformulation Work in Practice? Nutrients 2019, 11, 2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agresti, A.; Brent, A.C. Approximate is better than “Exact” for interval estimation of binomial proportions. Am. Stat. 1998, 52, 119–126. [Google Scholar] [CrossRef]
- Mintel Group Ltd. Mintel Global New Products Database. Available online: https://www.mintel.com/global-new-products-database/features (accessed on 20 May 2021).
- Mintel. Glossary 2016. Available online: https://www.gnpd.com (accessed on 20 May 2021).
- Watson, E. Food Colors: How Will EFSA’s Decision on Titanium Dioxide Safety Impact the US Market? Available online: foodnavigator.com (accessed on 17 August 2021).
- Huybrechts, I.; Sioen, I.; Boonb, P.E.; De Neve, M.; Amiano, P.; Arganini, C.; Bower, E.; Busk, L.; Christensen, T.; Hilbig, A.; et al. Long-term dietary exposure to different food colours in young children living in different European countries. EFSA Supporting Publ. 2010, 7, 53E. [Google Scholar] [CrossRef]
- Bachler, G.; von Goetz, N.; Hungerbuhler, K. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles. Nanotoxicology 2015, 9, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Sprong, C.; Bakker, M.; Niekerk, M.; Vennemann, M. Exposure Assessment of the Food Additive Titanium Dioxide (E 171) Based on Use Levels Provided by the Industry. Available online: https://rivm.openrepository.com/handle/10029/600597 (accessed on 17 August 2021).
- Yin, C.; Zhao, W.; Liu, R.; Liu, R.; Wang, Z.; Zhu, L.; Chen, W.; Liu, S. TiO2 particles in seafood and surimi products: Attention should be paid to their exposure and uptake through foods. Chemosphere 2017, 188, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Morris, S. Dunkin’ Donuts Drops Titanium Dioxide. Available online: https://sensientfoodcolors.com/en-us/industry-trends/dunkin-donuts-drops-titanium-dioxide/ (accessed on 30 May 2021).
- Sensient Food Colors. Avalanche, Purely Brilliant Titanium Dioxide Alternatives. Available online: https://sensientfoodcolors.com/en-us/color-solutions/avalanche/ (accessed on 30 May 2021).
- Pravst, I.; Lavriša, Ž.; Kušar, A.; Miklavec, K.; Žmitek, K. Changes in Average Sodium Content of Prepacked Foods in Slovenia during 2011–2015. Nutrients 2017, 9, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
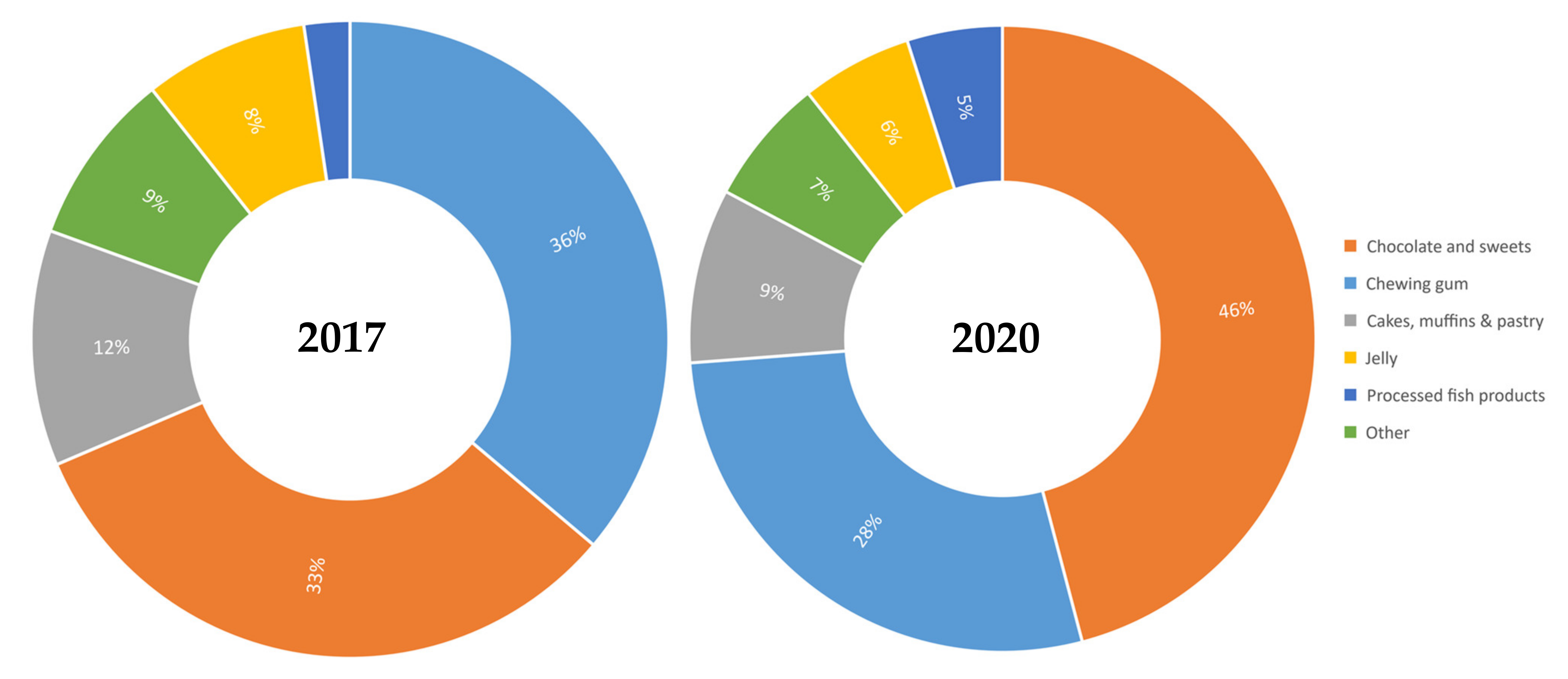
| Food Category | 2017 | 2020 | z-Test Statistic for Proportions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total N | Added TiO2 N | % (95% CI) | Sale-Weighted Proportion (%) | Total N | Added TiO2 N | % (95% CI) | Sale-Weighted Proportion (%) | Proportion Change (95% CI) | p-Value | |
| Chewing gum | 111 | 78 | 70.3 (61.8–78.8) | 85.5 | 138 | 34 | 24.6 (17.4–31.8) | 3.1 | 45.6 (34.5–56.8) | <0.01 |
| Jelly | 185 | 18 | 9.7 (5.5–14.0) | 14.8 | 159 | 7 | 4.4 (1.2–7.6) | 20.2 | 5.3 (0.0–10.6) | 0.03 |
| Processed fish products | 71 | 5 | 7.0 (1.1–13.0) | 19.3 | 87 | 6 | 6.9 (1.6–12.2) | 19.0 | 0.1 (−7.8–8.1) | ns |
| Cakes, muffins and pastry | 569 | 25 | 4.4 (2.7–6.1) | 3.0 | 639 | 11 | 1.7 (0.7–2.7) | 1.1 | 2.7 (0.7–4.6) | <0.01 |
| Chocolate and sweets | 1917 | 70 | 3.7 (2.9–4.5) | 2.8 | 2173 | 56 | 2.6 (1.9–3.2) | 1.1 | 1.1 (0.0–2.1) | 0.02 |
| Canned fish with vegetable | 60 | 1 | 1.7 (0.3–8.9) | * | 60 | 0 | ns | |||
| Sugar | 127 | 2 | 1.6 (0.4–5.6) | * | 108 | 0 | ns | |||
| Ice cream and edible ices | 431 | 6 | 1.4 (0.3–2.5) | 1.6 | 586 | 3 | 0.5 (0.0–1.1) | * | 0.9 (−0.4–2.1) | ns |
| Desserts | 207 | 2 | 1.0 (0.4–2.3) | * | 298 | 0 | ns | |||
| Flavored yogurt | 419 | 3 | 0.7 (0.2–2.1) | * | 386 | 0 | ns | |||
| Cordials | 179 | 1 | 0.6 (0.1–3.1) | * | 190 | 0 | ns | |||
| Soup | 264 | 1 | 0.4 (0.1–2.1) | * | 257 | 1 | 0.4 (0.1–2.2) | * | 0.0 (−1.1–1.1) | ns |
| Biscuits | 1035 | 3 | 0.3 (0.1–0.9) | * | 1122 | 2 | 0.2 (0.1–0.6) | * | 0.1 (−0.2–0.5) | ns |
| Side dishes | 199 | 0 | 224 | 1 | 0.5 (0.1–2.5) | * | ns | |||
| Spreads and processed cheese | 238 | 0 | 205 | 1 | 0.5 (0.1–2.7) | * | ns | |||
| Total | 6012 | 215 | 3.6 (3.1–4.0) | na | 6632 | 122 | 1.8 (1.5–2.2) | na | 1.8 (1.1–2.3) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaznik, U.; Krušič, S.; Hribar, M.; Kušar, A.; Žmitek, K.; Pravst, I. Use of Food Additive Titanium Dioxide (E171) before the Introduction of Regulatory Restrictions Due to Concern for Genotoxicity. Foods 2021, 10, 1910. https://doi.org/10.3390/foods10081910
Blaznik U, Krušič S, Hribar M, Kušar A, Žmitek K, Pravst I. Use of Food Additive Titanium Dioxide (E171) before the Introduction of Regulatory Restrictions Due to Concern for Genotoxicity. Foods. 2021; 10(8):1910. https://doi.org/10.3390/foods10081910
Chicago/Turabian StyleBlaznik, Urška, Sanja Krušič, Maša Hribar, Anita Kušar, Katja Žmitek, and Igor Pravst. 2021. "Use of Food Additive Titanium Dioxide (E171) before the Introduction of Regulatory Restrictions Due to Concern for Genotoxicity" Foods 10, no. 8: 1910. https://doi.org/10.3390/foods10081910
APA StyleBlaznik, U., Krušič, S., Hribar, M., Kušar, A., Žmitek, K., & Pravst, I. (2021). Use of Food Additive Titanium Dioxide (E171) before the Introduction of Regulatory Restrictions Due to Concern for Genotoxicity. Foods, 10(8), 1910. https://doi.org/10.3390/foods10081910











