Recovery of Bioactive Compounds from Strawberry (Fragaria × ananassa) Pomace by Conventional and Pressurized Liquid Extraction and Assessment Their Bioactivity in Human Cell Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Cell Cultures and Other Materials
2.2. Preparation of Berry Pomace and Determination of Its Proximate Composition
2.3. Extraction of Polar Constituents from Strawberry Pomace
2.4. Evaluation of Antioxidant Properties of Extracts and Solid Materials
2.5. Preparation of Cell Culture and Cellular Assays
2.6. Identification of Bioactive Components Using the Ultra-Performance Liquid Chromatography—Mass Spectrometry (UPLC-MS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Composition of the Strawberry Pomace
3.2. Characterization of Polar Phytochemicals of Strawberry Pomace Extracts
3.3. Antioxidant Properties
3.3.1. Antioxidant Characteristics Measured by the Chemical Methods
3.3.2. Evaluation of Cellular Antioxidant Activity (CCA)
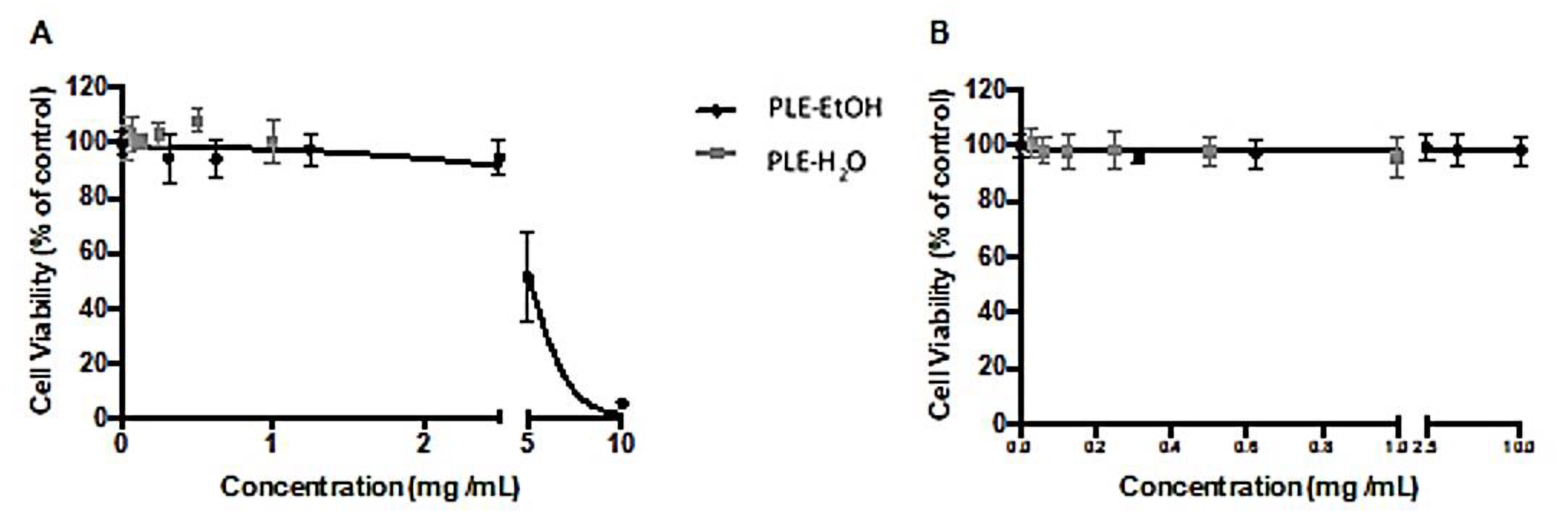
3.4. Antiproliferative Effects and Cytotoxicity of Strawberry Pomace Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gündüz, K. Strawberry: Phytochemical composition of strawberry (Fragaria × ananassa). In Nutritional Composition of Fruits Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 733–752. [Google Scholar] [CrossRef]
- Barkaoui, S.; Mankai, M.; Miloud, N.B.; Kraïem, M.; Madureira, J.; Verde, S.C.; Boudhrioua, N. Gamma radiation coupled to refrigeration may improve antioxidants, sensory properties and extend shelf life of strawberries. LWT Food Sci. Technol. 2021, 150, 112088. [Google Scholar] [CrossRef]
- Šaponjac, V.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.A.; Čanadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Vinčić, M. Chemical composition and potential bioactivity of strawberry pomace. RSC Adv. 2015, 5, 5397–5405. [Google Scholar] [CrossRef]
- Aaby, K.; Skrede, G.; Wrolstad, R.E. Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa). J. Agric. Food Chem. 2005, 53, 4032–4040. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Król, B.; Kosmala, M.; Milala, J.; Zduńczyk, Z.; Żary-Sikorska, E. Physiological properties of dietary ellagitannin-rich preparations obtained from strawberry pomace using different extraction methods. Polish J. Food Nutr. Sci. 2015, 65, 199–209. [Google Scholar] [CrossRef]
- Kadivec, M.; Bornšek, S.M.; Polak, T.; Demšar, L.; Hribar, J.; Požrl, T. Phenolic content of strawberry spreads during processing and storage. J. Agric. Food Chem. 2013, 61, 9220–9229. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Masuero, D.; Guella, G.; Palmieri, L.; Martinatti, P.; Vrhovsek, U. Evolution of ellagitannin content and profile during fruit ripening in Fragaria spp. J. Agric. Food Chem. 2013, 61, 8597–8607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmala, M.; Zduńczyk, Z.; Juśkiewicz, J.; Jurgoński, A.; Karlińska, E.; Macierzyński, J.; Jańczak, R.; Rój, E. Chemical composition of defatted strawberry and raspberry seeds and the effect of these dietary ingredients on polyphenol metabolites, intestinal function, and selected serum parameters in rats. J. Agric. Food Chem. 2015, 63, 2989–2996. [Google Scholar] [CrossRef]
- Akhatou, I.; Fernández-Recamales, A. Nutritional and nutraceutical quality of strawberries in relation to harvest time and crop conditions. J. Agric. Food Chem. 2014, 62, 5749–5760. [Google Scholar] [CrossRef]
- Truchado, P.; Larrosa, M.; García-Conesa, M.T.; Cerdá, B.; Vidal-Guevara, M.L.; Tomás-Barberán, F.A.; Espín, J.C. Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. J. Agric. Food Chem. 2012, 60, 5749–5754. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-Amylase and α-Glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Kosmala, M.; Zdunczyk, Z.; Kołodziejczyk, K.; Klimczak, E.; Juskiewicz, J.; Zdunczyk, P. Chemical composition of polyphenols extracted from strawberry pomace and their effect on physiological properties of diets supplemented with different types of dietary fibre in rats. Eur. J. Nutr. 2014, 53, 521–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaroslawska, J.; Juskiewicz, J.; Wroblewska, M.; Jurgonski, A.; Krol, B.; Zdunczyk, Z. Polyphenol-rich strawberry pomace reduces serum and liver lipids and alters gastrointestinal metabolite formation in fructose-fed rats. J. Nutr. 2011, 20, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Kitrytė, V.; Kraujalienė, V.; Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Chokeberry valorization into food ingredients by enzyme-assisted extraction: Process optimization and product characterization. Food Bioprod. Process. 2017, 105, 36–50. [Google Scholar] [CrossRef]
- Grunovaitė, L.; Pukalskienė, M.; Pukalskas, A.; Venskutonis, P.R. Fractionation of black chokeberry pomace into functional ingredients using high pressure extraction methods and evaluation of their antioxidant capacity and chemical composition. J. Funct. Foods 2016, 24, 85–96. [Google Scholar] [CrossRef]
- Tamkutė, L.; Melero-Gil, B.; Rovira, J.C.; Pukalskienė, M.; Venskutonis, P.R. Effect of cranberry pomace extracts isolated by pressurized ethanol and water on the inhibition of food pathogenic/spoilage bacteria and the quality of pork products. Food Res. Int. 2019, 120, 38–51. [Google Scholar] [CrossRef]
- Kraujalis, P.; Kraujalienė, V.; Kazernavičiūtė, R.; Venskutonis, P.R. Supercritical carbon dioxide and pressurized liquid extraction of valuable ingredients from Viburnum opulus pomace and berries and evaluation of product characteristics. J. Supercrit. Fluids 2017, 112, 99–108. [Google Scholar] [CrossRef]
- Kryževičūtė, N.; Jaime, I.; Diez, A.M.; Rovira, J.; Venskutonis, P.R. Effect of raspberry pomace extracts isolated by high pressure extraction on the quality and shelf-life of beef burgers. Int. J. Food Sci. Technol. 2017, 52, 1852–1861. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Klebson, S.S.; Borges, G.R.; Muniz-Flavia, A.V.C.S.; Mendoca, M.R.; Pinheiro, S.; Padilha, F.F. Separation of antibacterial biocompounds from Hancornia speciosa leaves by a sequential process of pressurized liquid extraction. Sep. Purif. Technol. 2019, 222, 390–395. [Google Scholar] [CrossRef]
- Offcial Methods of Analysis, 15th ed.; The Association of Offcial Analytical Chemists: Arlington, VA, USA, 1990; ISBN 0-935584-42-0.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.W.; Wu, X.L.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.J.; Ou, B.X.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3289. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The ‘QUENCHER’ approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Silva, I.; Estrada, M.F.; Pereira, C.V.; Silva, A.B.; Bronze, M.R.; Alves, P.M.; Duarte, C.M.M.; Brito, C.; Serra, A.T. Polymethoxylated flavones from orange peels inhibit cell proliferation in a 3D cell model of human colorectal cancer. Nutr. Cancer 2018, 70, 257–266. [Google Scholar] [CrossRef]
- Pereira, C.V.; Duarte, M.; Silva, P.; Bento da Silva, A.; Duarte, C.M.M.; Cifuentes, A.; Serra, A.T. Polymethoxylated flavones target cancer stemness and improve the antiproliferative effect of 5-fluorouracil in a 3D cell model of colorectal cancer. Nutrients 2019, 11, 326. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Pieszka, M.; Gogol, P.; Pietras, M.; Pieszka, M. Valuable components of dried pomaces of chokeberry, black currant, strawberry, apple and carrot as a source of natural antioxidants and nutraceuticals in the animal diet. Ann. Anim. Sci. 2015, 15, 475–491. [Google Scholar] [CrossRef] [Green Version]
- Górnaś, P.; Juhneviča-Radenkova, K.; Radenkovs, V.; Mišina, I.; Pugajeva, I.; Soliven, A.; Seglina, D. The impact of different baking conditions on the stability of the extractable polyphenols in muffins enriched by strawberry, sour cherry, raspberry or black currant pomace. LWT Food Sci. Technol. 2016, 65, 946–953. [Google Scholar] [CrossRef]
- Sójka, M.; Macierzyński, J.; Kolodziejczyk, K. Nutrient and polyphenolic composition of industrial strawberry press cake. Eur. Food Res. Technol. 2013, 237, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of volatiles, phenolics, sugars, antioxidant vitamins, and sensory quality of different colored carrot varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–889. [Google Scholar] [CrossRef]
- Bergantin, C.; Maietti, A.; Cavazzini, A.; Pasti, L.; Tedeschi, P.; Brandolini, V.; Marchetti, M. Bioaccessibility and HPLC-MS/MS chemical characterization of phenolic antioxidants in Red Chicory (Cichorium intybus). J. Funct. Foods 2017, 33, 94–102. [Google Scholar] [CrossRef]
- Pukalskienė, M.; Slapšytė, G.; Dedonytė, V.; Lazutka, J.R.; Mierauskienė, J.; Venskutonis, P.R. Genotoxicity and antioxidant activity of five Agrimonia and Filipendula species plant extracts evaluated by comet and micronucleus assays in human lymphocytes and Ames. Salmonella/microsome test. Food Chem. Toxicol. 2018, 113, 303–313. [Google Scholar] [CrossRef]
- Canel, C.; Bailey-Serres, J.N.; Roose, M.L. Pummelo fruit transcript homologous to ripening induced genes. Plant Physiol. 1995, 108, 1323–1324. [Google Scholar] [CrossRef] [Green Version]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars organic acids and total phenolics in 25 wild and cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Reißner, A.M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turnerb, C.; Rohma, H. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Paredes-Lopez, O. Anthocyanins and Betalains. In Natural Colorants for Food and Nutraceutical Uses; CRC Press: Boca Raton, FL, USA, 2003; pp. 167–211. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Wiboonsirikul, J.; Adachi, S. Extraction of functional substances from agricultural products or by-products by subcritical water treatment. Food Sci. Technol. Res. 2008, 14, 319. [Google Scholar] [CrossRef] [Green Version]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–681. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Howard, L.; Pandjaitan, N. Pressurized liquid extraction of flavonoids from spinach. J. Food Sci. 2008, 73, C151–C159. [Google Scholar] [CrossRef] [PubMed]
- Henrion, M.; Servaes, M.; Thielecke, F.; Fogliano, V. Application of the Quencher metodology to the food industry. Food Chem. 2018, 240, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Rui, H.L. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Rekika, D.; Khanizadeh, S.; Deschênes, M.; Levasseur, A.; Charles, M.T.; Tsao, R.; Yang, R. Antioxidant capacity and phenolic content of selected strawberry genotypes. J. Am. Soc. Hortic. Sci. 2005, 40, 1777–1781. [Google Scholar] [CrossRef] [Green Version]
- Ko, M.J.; Jayaramaiah, R.H.; Gupta, R.; Kim, S.W.; An, J.U.; Ziyu, W.J.; Choi, Y.W. Evaluation of bioactive compounds in strawberry fruits by a targeted metabolomic approach. Hortic. Sci. Technol. 2017, 35, 805–819. [Google Scholar] [CrossRef]
- López de las Hazas, M.C.; Mosele, J.I.; Macià, A.; Ludwig, I.A.; Motilva, M.J. Exploring the colonic metabolism of grape and strawberry anthocyanins and their in vitro apoptotic effects in HT-29 colon cancer cells. J. Agric. Food Chem. 2017, 65, 6477–6487. [Google Scholar] [CrossRef]
- Olsson, M.E.; Andersson, C.S.; Oredsson, S.; Berglund, R.H.; Gustavsson, K.E. Antioxidant levels and inhibition of cancer cell proliferation in vitro by extracts from organically and conventionally cultivated strawberries. J. Agric. Food Chem. 2006, 54, 1248–1255. [Google Scholar] [CrossRef]
- Amatori, S.; Mazzoni, L.; Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Battino, M. Polyphenol-rich strawberry extract (PRSE) shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci. Rep. 2016, 6, 30917. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Ghadiri, M.; Ramezani, M.; Askari, V.R. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother. Res. 2020, 34, 685–720. [Google Scholar] [CrossRef]
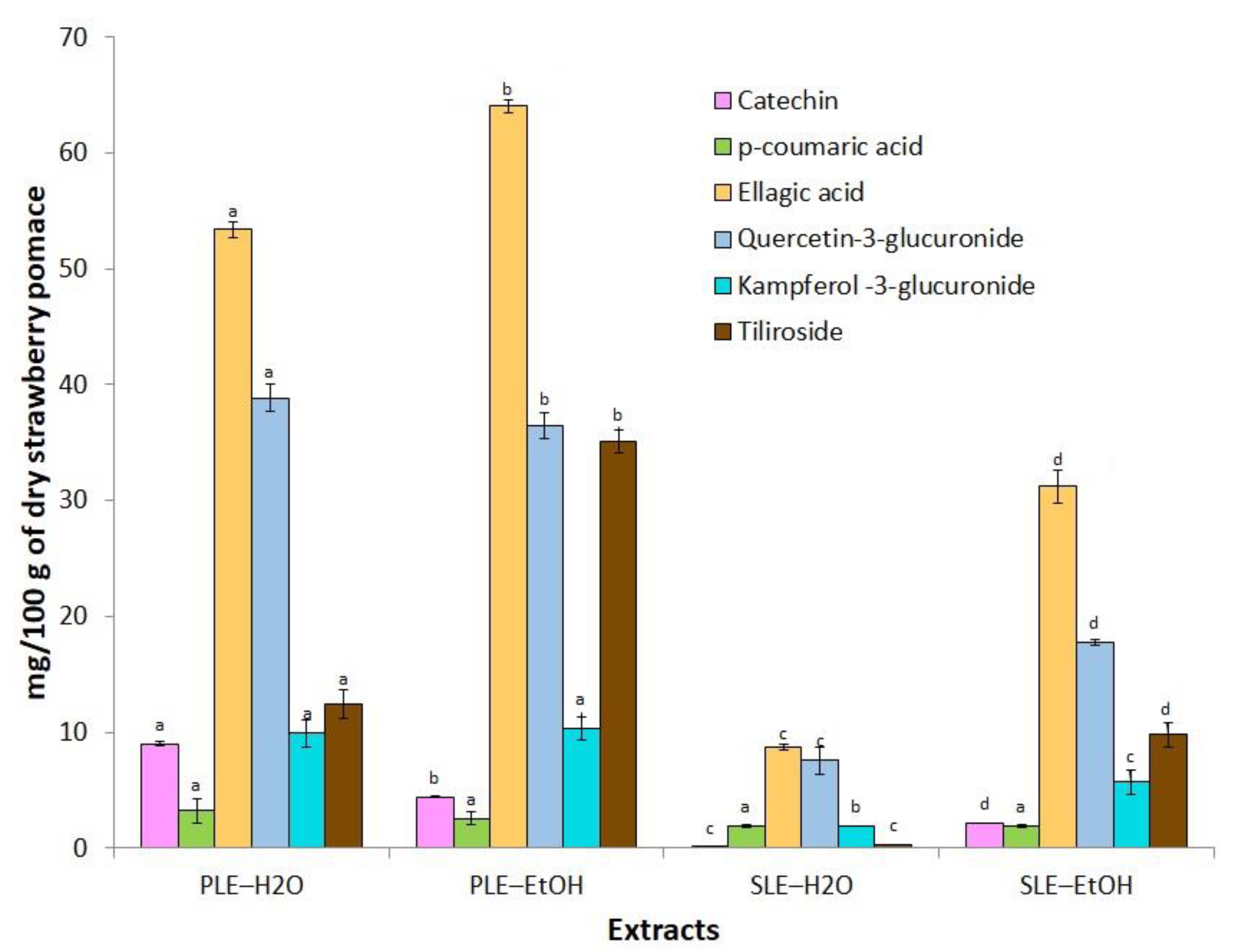

| No. | RT (min) | Precursor Ion [M–H]- | Ion Formula | Negative Ions MS2 m/z (% in MS) | Structural Assignment | Extracts | |||
|---|---|---|---|---|---|---|---|---|---|
| SLE- EtOH | SLE- H2O | PLE- EtOH | PLE- H2O | ||||||
| 1 | 0.5 | 133.0131 a | C4H5O5 | - | Malic acid | + | + | + | + |
| 2 | 0.59 | 191.0186 a | C6H7O7 | - | Citric acid | + | + | + | + |
| 3 | 0.65 | 117.0182 a | C4H5O4 | - | Succinic acid | + | + | + | + |
| 4 | 0.72 | 191.0550 | C7H11O6 | - | Quinic acid | + | + | + | + |
| 5 | 2.35 | 289.0716 a | C15H13O6 | - | Catechin | + | - | + | + |
| 6 | 2.55 | 325.0925 b,c | C15H17O8 | 145.0291 (50.3) | p-coumaric acid hexoside | + | - | + | + |
| 7 | 2.95 | 431.0979 b,c | C21H19O10 | 147.0075 (4.7) 241.0498 (10.2) 269.0448 (100) | Apigenin-7-glucoside | + | - | + | - |
| 8 | 3.65 | 163.0402 a | C9H7O3 | - | p-coumaric acid | + | + | + | + |
| 9 | 3.95 | 447.0571 b,c | C20H15O12 | 300.9986 (100) | Quercetin-3-rhamnoside | + | + | + | + |
| 10 | 4.15 | 300.9987 a | C14H5O8 | - | Ellagic acid | + | + | + | + |
| 11 | 4.35 | 477.0674 a,b | C14H17O13 | 301.0356 (100) 151.0035 (8.4) | Quercetin-3-glucuronide | + | + | + | + |
| 12 | 4.75 | 447.0931 b,c | C21H19O11 | 285.0374 (100) | Kaempferol-hexoside | + | + | + | + |
| 13 | 4.85 | 461.07726 a,b | C21H17O12 | 285.0372 (100) | Kaempferol-3-glucuronide | + | + | + | + |
| 14 | 5.1 | 137.0243 b,c | C7H5O3 | - | Hydroxybenzoic acid | + | + | + | + |
| 15 | 5.6 | 593.1297 a,b | C30H25O13 | 145.0303 (9.3) 285.0420 (100) 447.0932 (49.5) | Tiliroside | + | + | + | + |
| RT, min | Structure Assignment | Precursor Ion [M]+ (m/z) | Molecular Formula | SLE- H2O | SLE-H2O | PLE- EtOH | PLE-H2O |
|---|---|---|---|---|---|---|---|
| mg/100 g DW of Strawberry Pomace | |||||||
| 5.5 | Cyanidin-3- glucoside * | 449.1071 | C21H21O11 | 8.7 ± 0.1 a | 16.1 ± 0.4 b | 16.9 ± 0.4 c | 14.4 ± 0.1 d |
| 6.2 | Pelargonidin-3-glucoside ** | 433.1132 | C21H21O10 | 49.1 ± 0.7 a | 55.5 ± 0.1 b | 78.9 ± 0.9 c | 60.1 ± 1.3 d |
| 6.4 | Pelargonidin-3-rutinoside ** | 579.1696 | C27H31O14 | 9.1 ± 0.1 a | 12.6 ± 0.7 b | 19.9 ± 0.1 c | 14.8 ± 0.2 d |
| 7.6 | Pelargonidin-3-(malonyl)- glucoside ** | 519.1128 | C24H23O13 | - | - | 20.1 ± 0.7 a | 16.6 ± 0.1 a |
| 8.1 | Cyanidin-3- (coumaroyl)- glucoside ** | 595.1438 | C30H27O13 | 13.3 ± 0.1 a | - | 31.9 ± 0.8 b | 20.4 ± 0.8 c |
| Total | 80.2 | 84.2 | 167.7 | 126.3 | |||
| Extract | Yield g/100 g DW | TPC | DPPH• | ABTS•+ | ORAC | ||
|---|---|---|---|---|---|---|---|
| mg GAE/g Extract | mg GAE/g DW of Strawberry Pomace (Quencher Approach) | mg TE/g Extract | mg TE/g Extract | mg TE/g DW of Strawberry Pomace (Quencher Approach) | mg TE/g Extract | ||
| SLE-EtOH | 14.7 ± 1.3 | 46.8 ± 1.8 | 10.2 ± 0.5 | 57.1 ± 2.8 | 180.2 ± 4.2 | 29.3 ± 0.9 | 201.8 ± 2.6 |
| SLE-H2O | 19.5 ± 1.8 | 30.3 ± 0.9 | 14.3 ± 0.8 | 28.3 ± 1.2 | 148.5 ± 1.1 | 42.8 ± 0.7 | 95.4 ± 0.5 |
| PLE-EtOH | 28.6 ± 1.4 | 21.5 ± 0.9 | 6.5 ± 0.4 | 65.3 ± 3.13 | 291.6 ± 3.8 | 19.6 ± 0.4 | 308.9 ± 1.4 |
| PLE-H2O | 24.9 ± 0.6 | 29.6 ± 1.1 | 3.0 ± 0.2 | 117.2 ± 2.7 | 391.95 ± 2.1 | 21.5 ± 0.2 | 222.3 ± 3.5 |
| Dried pomace | - | - | 18.8 ± 1.2 | - | - | 125.4 ± 3.7 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pukalskienė, M.; Pukalskas, A.; Dienaitė, L.; Revinytė, S.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Recovery of Bioactive Compounds from Strawberry (Fragaria × ananassa) Pomace by Conventional and Pressurized Liquid Extraction and Assessment Their Bioactivity in Human Cell Cultures. Foods 2021, 10, 1780. https://doi.org/10.3390/foods10081780
Pukalskienė M, Pukalskas A, Dienaitė L, Revinytė S, Pereira CV, Matias AA, Venskutonis PR. Recovery of Bioactive Compounds from Strawberry (Fragaria × ananassa) Pomace by Conventional and Pressurized Liquid Extraction and Assessment Their Bioactivity in Human Cell Cultures. Foods. 2021; 10(8):1780. https://doi.org/10.3390/foods10081780
Chicago/Turabian StylePukalskienė, Milda, Audrius Pukalskas, Lijana Dienaitė, Simona Revinytė, Carolina V. Pereira, Ana A. Matias, and Petras Rimantas Venskutonis. 2021. "Recovery of Bioactive Compounds from Strawberry (Fragaria × ananassa) Pomace by Conventional and Pressurized Liquid Extraction and Assessment Their Bioactivity in Human Cell Cultures" Foods 10, no. 8: 1780. https://doi.org/10.3390/foods10081780
APA StylePukalskienė, M., Pukalskas, A., Dienaitė, L., Revinytė, S., Pereira, C. V., Matias, A. A., & Venskutonis, P. R. (2021). Recovery of Bioactive Compounds from Strawberry (Fragaria × ananassa) Pomace by Conventional and Pressurized Liquid Extraction and Assessment Their Bioactivity in Human Cell Cultures. Foods, 10(8), 1780. https://doi.org/10.3390/foods10081780







