Comparative Study of Physicochemical Properties and Starch Granule Structure in Seven Ginkgo Kernel Flours
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ginkgo Samples and Growth Conditions
2.2. Morphology and Weight of Ginkgo Kernels
2.3. Major Component Analysis of Ginkgo Kernels
2.4. Isolation of Ginkgo Starch
2.5. Morphology Analysis of Ginkgo Starch
2.6. Raman Spectroscopy Analysis of Ginkgo Starch
2.7. Analysis of Ginkgo Flour Thermal and Pasting Properties
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphology of Ginkgo Kernels
3.2. Major Components of Ginkgo Kernel
3.3. Starch Granule Structures in Ginkgo Kernel
3.4. Thermal Properties of Kernel Flours
3.5. Pasting Properties of Kernel Flours
3.6. Effects of α-Amylase and Protein Structure on Pasting Properties of Kernel Flours
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.; Zheng, S. The missing link in ginkgo evolution. Nat. Cell Biol. 2003, 423, 821–822. [Google Scholar] [CrossRef]
- Del Tredici, P.D. The phenology of sexual reproduction in Ginkgo biloba: Ecological and evolutionary implications. Bot. Rev. 2007, 73, 267–278. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Y.; Wang, Y.P.; Wang, Q.; Xu, X.Y.; Chen, P. Observation on amyloplast in endosperm during development of seeds in Ginkgo biloba. J. Fruit Sci. 2007, 24, 692–695. [Google Scholar]
- Singh, B.; Kaur, P.; Singh, R.; Ahuja, P. Biology and chemistry of Ginkgo biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Jin, B.; Lin, M.M.; Chen, P. Gametophyte development and embryogenesis in Ginkgo biloba: A current view. Chin. Bull. Bot. 2010, 45, 119–127. [Google Scholar] [CrossRef]
- Jin, B.; Xie, Y.; Lu, Y.; Wang, D.; Zhang, M.; Wang, L. Starch granule and protein accumulation during seed development of Ginkgo biloba L. ISRN Bot. 2012, 2012, 653796. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.G.; He, W.S.; Chen, G.; Jia, C.S.; Zhang, X.M.; Fang, B. Enzymatic digestion characteristics and structure analysis of ginkgo (Ginkgo biloba L.) starch noodles. Food Sci. Technol. Res. 2014, 20, 997–1004. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Zhang, T.; Jiang, B.; Mu, W.M.; Miao, M. Characterization and antioxidant activity of Ginkgo biloba exocarp poly-saccharides. Carbohyd. Polym. 2012, 87, 40–45. [Google Scholar] [CrossRef]
- Shan, S.-J.; Luo, J.; Xu, D.-R.; Niu, X.-L.; Xu, D.-Q.; Zhang, P.-P.; Kong, L.-Y. Elucidation of micromolecular phenylpropanoid and lignan glycosides as the main antioxidants of ginkgo seeds. Ind. Crop Prod. 2018, 112, 830–838. [Google Scholar] [CrossRef]
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.; Hayes, L.D.; Milic, M.; Padulo, J. Herbal medicine for sports: A review. J. Int. Soc. Sport Nutr. 2018, 15, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Wang, L.; Pan, Y.; Chen, P.; Wang, D.; Xie, Y.; Jin, X.X. Research on starch and protein accumulation and metabolism during the development of the Ginkgo biloba female gametophyte. Acta Hortic. Sin. 2011, 38, 15–24. [Google Scholar]
- Deng, Q.; Wang, L.; Wei, F.; Xie, B.; Huang, F.; Huang, W.; Shi, J.; Huang, Q.; Tian, B.; Xue, S. Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem. 2011, 124, 1458–1465. [Google Scholar] [CrossRef]
- Sado, T.; Nakata, S.; Tsuno, T.; Sato, M.; Misawa, Y.; Yamauchi, S.; Inaba, Y.; Kobayashi, D.; Wada, K. Concentrations of various forms of vitamin B6 in ginkgo seed poisoning. Brain Dev. 2019, 41, 292–295. [Google Scholar] [CrossRef]
- Zhou, M.; Hua, T.; Ma, X.; Sun, H.; Xu, L. Protein content and amino acids profile in 10 cultivars of ginkgo (Ginkgo biloba L.) nut from China. R. Soc. Open Sci. 2019, 6, 181571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wang, C.; Ye, J.; Zhou, H.; Tao, R.; Li, W. Preparation of starch-hard carbon spherules from ginkgo seeds and their phenol-adsorption characteristics. Molecules 2018, 23, 96. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Ye, C.; Huang, Y.; Zhang, N.; Zhang, X.; Xiao, M. Ginkgo biloba sarcotesta polysaccharide inhibits inflammatory responses through suppressing both NF-κB and MAPK signaling pathway. J. Sci. Food Agric. 2018, 99, 2329–2339. [Google Scholar] [CrossRef]
- Spence, K.; Jane, J. Chemical and physical properties of ginkgo (Ginkgo biloba) starch. Carbohydr. Polym. 1999, 40, 261–269. [Google Scholar] [CrossRef]
- Cai, J.W.; Cai, C.H.; Man, J.M.; Xu, B.; Wei, C.X. Physicochemical properties of ginkgo kernel starch. Int. J. Food Prop. 2015, 18, 380–391. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, H.; Jiang, B.; Cui, S.; Jin, Z.; Zhang, T. Structure and functional properties of starches from Chinese ginkgo (Ginkgo biloba L.) nuts. Food Res. Int. 2012, 49, 303–310. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.; Yang, Y.; Qi, Y.; Hao, W.; Wang, L.; Liu, Q.; Ling, Y.; Zhang, C. Relationship between structure and physicochemical properties of ginkgo starches from seven cultivars. Food Chem. 2020, 314, 125082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, L.; Shao, K.; Gu, M.; Liu, Q. Toward underlying reasons for rice starches having low viscosity and high amylose: Physiochemical and structural characteristics. J. Sci. Food Agric. 2012, 93, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-J.; Liu, Q.-Q.; Sang, Y.; Gu, M.-H.; Shi, Y.-C. Underlying reasons for waxy rice flours having different pasting properties. Food Chem. 2010, 120, 94–100. [Google Scholar] [CrossRef]
- Bravo-Núñez, A.; Gómez, M. Physicochemical properties of native and extruded maize flours in the presence of animal proteins. J. Food Eng. 2019, 243, 49–56. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Wang, D.; Wang, Y.; Zhang, M.; Jin, B.; Chen, P. Male cone morphogenesis, pollen development and pollen dispersal mechanism in Ginkgo biloba L. Can. J. Plant Sci. 2011, 91, 971–981. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Qi, Y.; Zhang, X.M.; Chen, Y.F.; Wang, L.; Lin, Y.P. Optimization of water-rich starch sample preparation methods for scanning electron microscopy. Plant Sci. J. 2018, 36, 119–126. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Zhu, Z.; Lu, H.; Zhou, X.; Qian, Y.; Li, Q.; Lu, Y.; Gu, M.; Liu, Q. Characterization of grain quality and starch fine structure of two Japonica rice (Oryza Sativa) cultivars with good sensory properties at different temperatures during the filling stage. J. Agric. Food Chem. 2016, 64, 4048–4057. [Google Scholar] [CrossRef] [PubMed]
- Hirwitz, W.; Latimer, G. Official methods of analysis of AOAC International (16th ed.). Trends Food Sci. Tech. 1995, 6, 382. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhang, C.Q.; Chan, M.L.; Zhao, D.S.; Chen, J.Z.; Wang, Q.; Li, Q.F.; Yu, H.X.; Gu, M.H.; Sun, S.S.M.; et al. Biofortification of rice with the essential amino acid lysine: Molecular characterization, nutritional evaluation, and field performance. J. Exp. Bot. 2016, 67, 4285–4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naguleswaran, S.; Li, J.; Vasanthan, T.; Bressler, D. Distribution of granule channels, protein, and phospholipid in triticale and con starches as revealed by confocal laser scanning microscopy. Cereal Chem. 2011, 88, 87–94. [Google Scholar] [CrossRef]
- Burrell, M.M. Starch: The need for improved quality or quantity—An overview. J. Exp. Bot. 2003, 54, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Ch’Ng, P.; Abdullah, M.; Mathai, E.; Yunus, N. Some physical properties of ginkgo nuts and kernels. Int. Agrophys. 2013, 27, 485–489. [Google Scholar] [CrossRef]
- Chen, P.; He, F.R.; Qian, B.; Wei, J.; Wang, L. Seed types and their relative characteristics in Ginkgo biloba of China. Sci. Silvae Sin. 2004, 40, 66–70. [Google Scholar]
- Wellner, N.; Georget, D.M.R.; Parker, M.L.; Morris, V.J. In situ Raman microscopy of starch granule structures in wild type and ae mutant maize kernels. Starch Stärke 2010, 63, 128–138. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Gilian, G.S. Amino acid analysis. Curr. Protoc. Protein Sci. 2009, 58, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, D.E.; Shippy, S. Regulation of synaptic transmission by ambient extracellular glutamate. Neuroscience 2007, 14, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, S.M. Arginine metabolism: Boundaries of our knowledge. J. Nutr. 2007, 137, 1602S–1609S. [Google Scholar] [CrossRef] [Green Version]
- Pérez, S.; Baldwin, P.M.; Gallant, D.J. Structural features of starch granules I. In Starch, 3rd ed.; BeMiller, J.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 149–192. [Google Scholar]
- Cai, C.; Wei, C. In situ observation of crystallinity disruption patterns during starch gelatinization. Carbohydr. Polym. 2013, 92, 469–478. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.X.; Zhang, J.; Cai, X.L.; Liu, Q.Q.; Wei, C.X. Relationships between transparency, amylose content, starch cavity and moisture of brown rice kernels. J. Cereal Sci. 2019, 90, 102854. [Google Scholar] [CrossRef]
- Naguleswaran, S.; Vasanthan, T.; Hoover, R.; Bressler, D. The susceptibility of large and small granules of waxy, normal and high-amylose genotypes of barley and corn starches toward amylolysis at sub-gelatinization temperatures. Food Res. Int. 2013, 51, 771–782. [Google Scholar] [CrossRef]
- Fechner, P.M.; Wartewig, S.; Kleinebudde, P.; Neubert, R.H. Studies of the retrogradation process for various starch gels using Raman spectroscopy. Carbohydr. Res. 2005, 340, 2563–2568. [Google Scholar] [CrossRef]
- Dupuy, N.; Laureyns, J. Recognition of starches by Raman spectroscopy. Carbohyd. Polym. 2002, 49, 83–90. [Google Scholar] [CrossRef]
- Popov, D.; Buléon, A.; Burghammer, M.; Chanzy, H.; Montesanti, N.; Putaux, J.-L.; Potocki-Véronèse, G.; Riekel, C. Crystal structure of A-amylose: A revisit from synchrotron microdiffraction analysis of single crystals. Macromolecules 2009, 42, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.H.; Huang, J.; Zhao, L.X.; Liu, Q.Q.; Zhang, C.Q.; Wei, C.X. Heterogeneous structure and spatial distribution in endosperm of high-amylose rice starch granules with different morphologies. J. Agric. Food Chem. 2014, 62, 10143–10152. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Physicochemical properties of quinoa flour as affected by starch interactions. Food Chem. 2017, 221, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jane, J. Characterization of barley starches of waxy, normal and high amylose varieties. Carbohydr. Polym. 2000, 41, 365–377. [Google Scholar] [CrossRef]
- Xu, A.; Lin, L.S.; Guo, K.; Liu, T.X.; Yin, Z.T.; Wei, C.X. Physicochemical properties of starches from vitreous and floury endosperms from the same maize kernels. Food Chem. 2019, 291, 149–156. [Google Scholar] [CrossRef]
- Ahmed, S.; Ru, W.D.; Cheng, L.R.; Bian, X.B.; Zhang, L.; Jin, L.P.; Bao, J.S. Genetic diversity and stability in starch physico-chemical property traits of potato breeding lines. Food Chem. 2019, 290, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Grace, N.C.F.; Henry, C.J. The physicochemical characterization of unconventional starches and flours used in Asia. Foods 2020, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Chen, N.; Duan, B.; Zhu, Z.; Liao, X. Impact of proteins on pasting and cooking properties of waxy and non-waxy rice. J. Cereal Sci. 2008, 47, 372–379. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P.; Chibbar, R.N.; Han, H.L.; Demeke, T. Physiochemical and structural characteristics of flours and starches from waxy and nonwaxy wheats. Cereal Chem. 2002, 79, 458–464. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Yan, S.; Lei, N.; Wang, J.; Sun, B. Molecular causes for the increased stickiness of cooked non-glutinous rice by enzymatic hydrolysis of the grain surface protein. Carbohydr. Polym. 2019, 216, 197–203. [Google Scholar] [CrossRef] [PubMed]
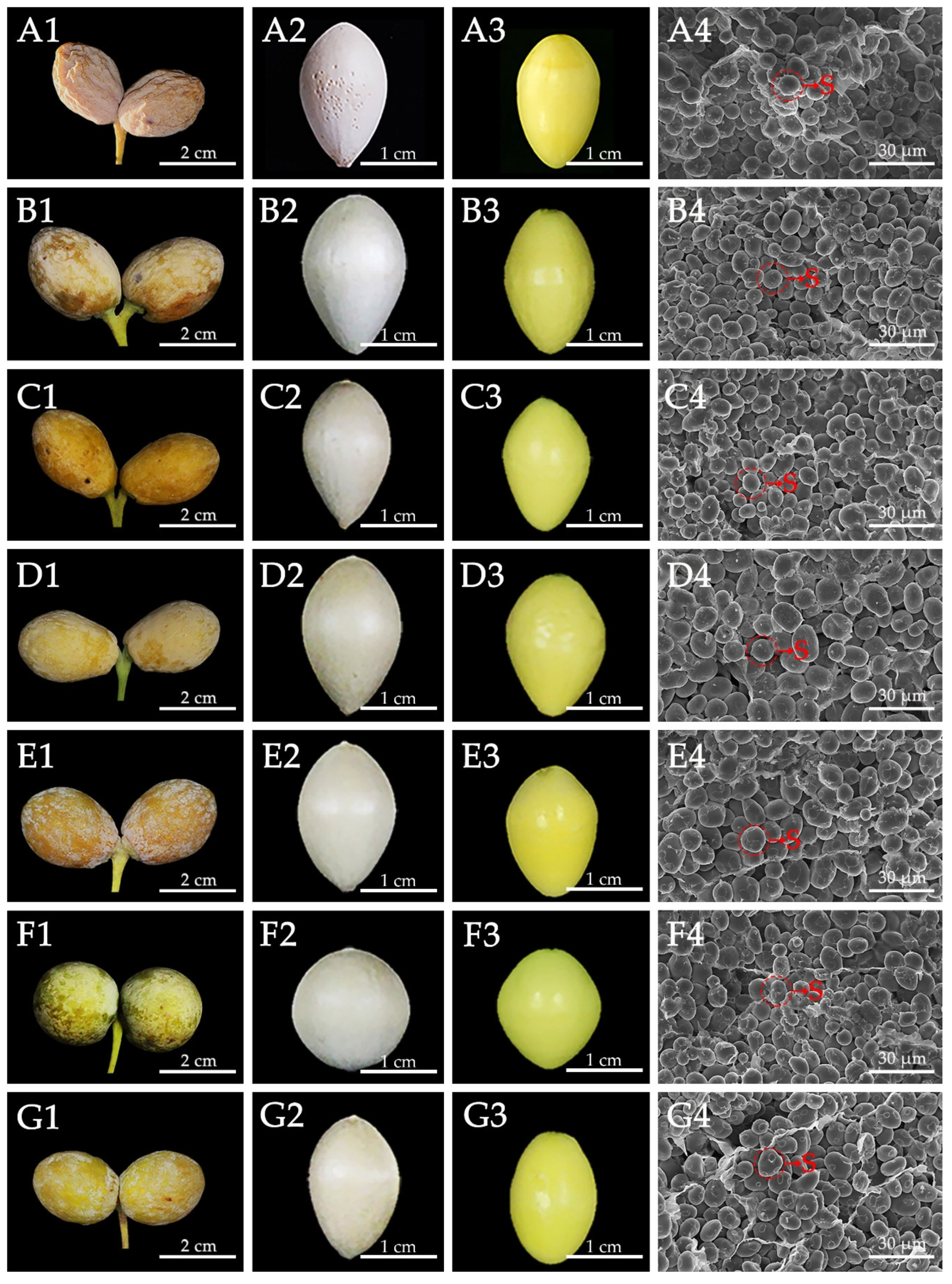
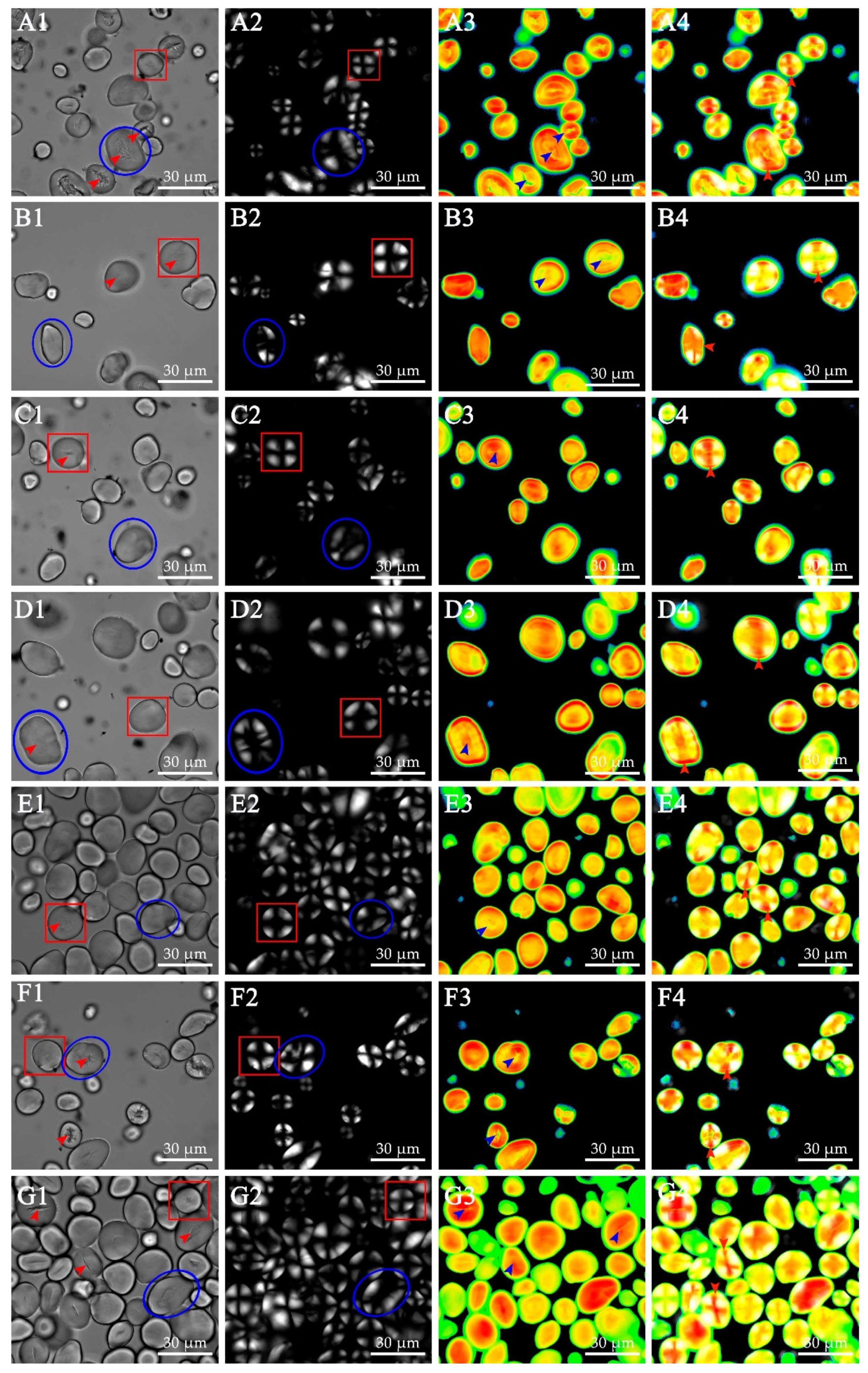


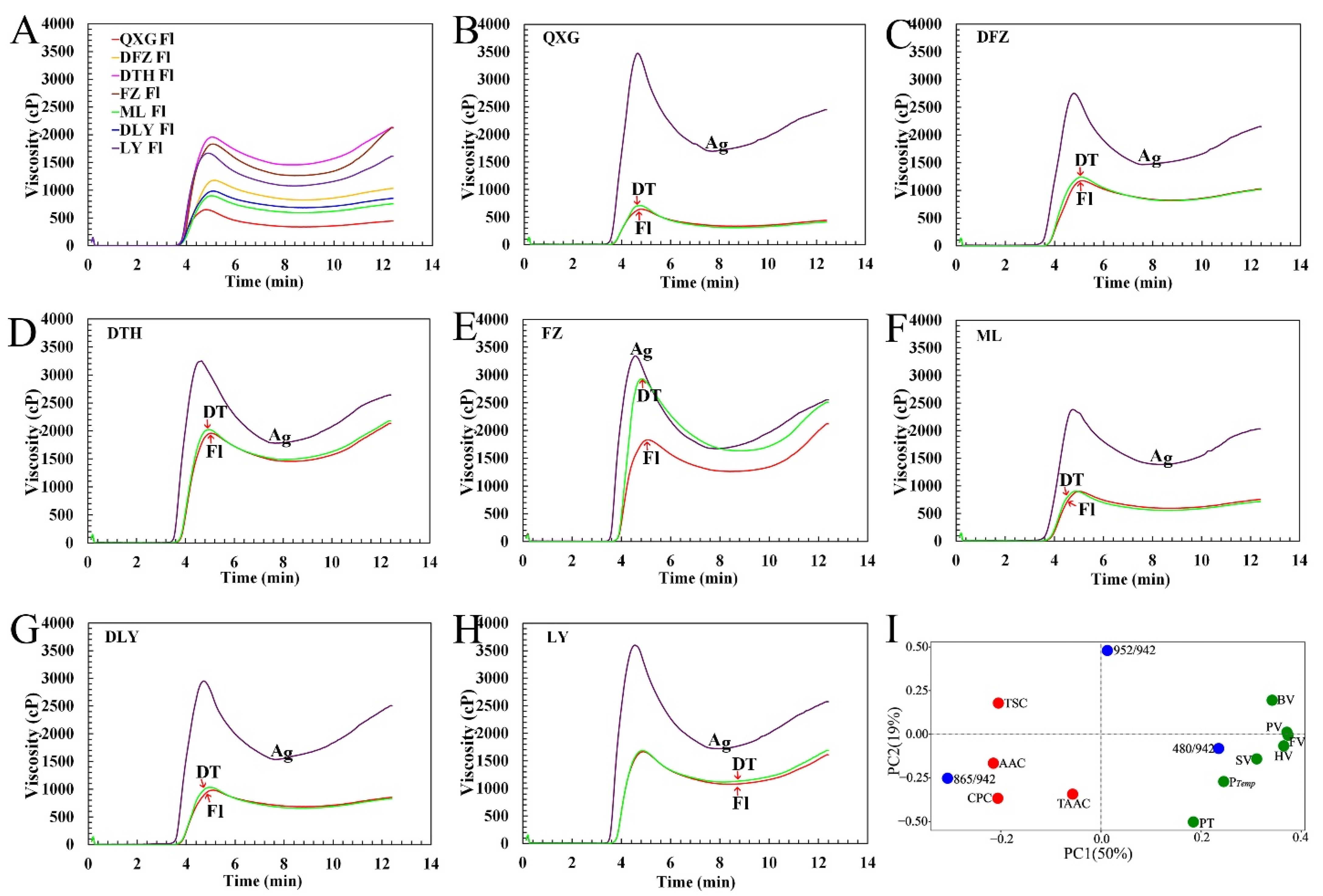
| Measured Parameters | Ginkgo Cultivars | ||||||
|---|---|---|---|---|---|---|---|
| QXG | DFZ | DTH | FZ | ML | DLY | LY | |
| Shape | Oval | Oval | Oval | Oval | Oval | Spherical | Oval |
| Width (mm) | 15.24 ± 0.36 b | 16.04 ± 0.39 b | 14.01 ± 0.29 c | 15.20 ± 0.27 b | 15.17 ± 0.48 b | 17.84 ± 0.72 a | 13.53 ± 0.17 c |
| Length (mm) | 26.21 ± 0.45 a | 26.44 ± 0.34 a | 23.63 ± 0.20 b | 25.67 ± 0.67 a | 24.50 ± 0.39 b | 20.85 ± 0.50 c | 22.38 ± 0.48 b |
| Weight (g) | 0.89 ± 0.11 d | 1.15 ± 0.30 bc | 1.03 ± 0.17 c | 1.72 ± 0.34 a | 1.35 ± 0.40 b | 1.16 ± 0.28 bc | 0.86 ± 0.07 d |
| MC (%, w/w) | 7.01 ± 0.26 a | 6.61 ± 0.05 a | 6.53 ± 0.18 a | 7.39 ± 0.76 a | 7.42 ± 0.26 a | 6.40 ± 0.12 a | 6.71 ± 0.11 a |
| TSC (%, w/w) | 70.72 ± 2.20 b | 70.45 ± 1.55 b | 70.24 ± 0.70 b | 69.40 ± 2.03 c | 71.83 ± 1.57 a | 68.95 ± 1.70 c | 70.45 ± 2.86 b |
| AAC (%, w/w) | 19.52 ± 1.03 bc | 20.26 ± 0.70 b | 20.25 ± 0.67 b | 18.96 ± 0.62 c | 25.61 ± 0.30 a | 19.44 ± 0.43 b | 18.58 ± 0.25 c |
| CPC (%, w/w) | 9.94 ± 0.03 b | 12.57 ± 0.04 a | 10.39 ± 0.01 b | 6.88 ± 0.02 d | 10.20 ± 0.13 b | 12.03 ± 0.01 a | 8.25 ± 0.03 c |
| TAAC (mg/g) | 7.43 ± 0.33 c | 7.94 ± 3.31 c | 8.80 ± 1.03 b | 6.82 ± 0.52 c | 7.77 ± 0.13 c | 9.30 ± 0.47 a | 7.47 ± 0.40 c |
| DSC | Cultivars | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|---|
| Gelatinization | QXG | 75.85 ± 0.24 bc | 81.90 ± 0.21 ab | 91.70 ± 0.02 a | 10.39 ± 0.61 b |
| DFZ | 76.55 ± 0.14 b | 82.60 ± 0.06 a | 91.55 ± 0.28 a | 9.42 ± 0.15 c | |
| DTH | 75.70 ± 0.09 bc | 81.50 ± 0.15 ab | 89.95 ± 0.28 b | 10.43 ± 0.01 b | |
| FZ | 76.70 ± 0.05 b | 81.95 ± 0.03 ab | 89.95 ± 0.12 b | 10.54 ± 0.15 b | |
| ML | 74.90 ± 0.28 bc | 82.10 ± 0.28 a | 90.05 ± 0.35 b | 9.15 ± 0.46 c | |
| DLY | 75.95 ± 0.09 bc | 82.55 ± 0.08 a | 90.85 ± 0.27 a | 9.33 ± 0.04 c | |
| LY | 79.30 ± 1.75 a | 82.65 ± 0.78 a | 89.05 ± 0.16 b | 11.76 ± 0.71 a | |
| Retrogradation | QXG | 45.80 ± 0.04 a | 55.80 ± 0.04 b | 66.90 ± 0.04 b | 4.24 ± 0.05 a |
| DFZ | 44.40 ± 1.10 b | 56.95 ± 0.15 ab | 67.60 ± 0.10 ab | 4.04 ± 0.51 a | |
| DTH | 45.80 ± 0.05 a | 56.50 ± 0.11 b | 67.00 ± 0.07 b | 3.54 ± 0.23 ab | |
| FZ | 45.30 ± 0.20 ab | 56.10 ± 0.71 b | 67.45 ± 0.34 ab | 3.97 ± 0.16 a | |
| ML | 44.15 ± 0.49 b | 58.80 ± 1.98 a | 68.45 ± 0.92 a | 4.12 ± 0.23 a | |
| DLY | 46.70 ± 0.04 a | 56.70 ± 0.03 b | 67.20 ± 0.02 ab | 3.11 ± 0.04 b | |
| LY | 44.00 ± 0.02 b | 54.80 ± 0.04 b | 68.00 ± 0.08 a | 3.86 ± 0.03 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Hao, W.; Zhang, X.; Zhao, Y.; Xu, Y.; Luo, J.; Liu, Q.; Liu, Q.; Wang, L.; Zhang, C. Comparative Study of Physicochemical Properties and Starch Granule Structure in Seven Ginkgo Kernel Flours. Foods 2021, 10, 1721. https://doi.org/10.3390/foods10081721
Lu Y, Hao W, Zhang X, Zhao Y, Xu Y, Luo J, Liu Q, Liu Q, Wang L, Zhang C. Comparative Study of Physicochemical Properties and Starch Granule Structure in Seven Ginkgo Kernel Flours. Foods. 2021; 10(8):1721. https://doi.org/10.3390/foods10081721
Chicago/Turabian StyleLu, Yan, Weizhuo Hao, Xiaomin Zhang, Yue Zhao, Yang Xu, Jixun Luo, Qing Liu, Qiaoquan Liu, Li Wang, and Changquan Zhang. 2021. "Comparative Study of Physicochemical Properties and Starch Granule Structure in Seven Ginkgo Kernel Flours" Foods 10, no. 8: 1721. https://doi.org/10.3390/foods10081721
APA StyleLu, Y., Hao, W., Zhang, X., Zhao, Y., Xu, Y., Luo, J., Liu, Q., Liu, Q., Wang, L., & Zhang, C. (2021). Comparative Study of Physicochemical Properties and Starch Granule Structure in Seven Ginkgo Kernel Flours. Foods, 10(8), 1721. https://doi.org/10.3390/foods10081721







