Evaluation of Physicochemical Properties of Beetroot-Based Dietary Supplements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Average Weight
2.2. Friability Test
2.3. Hardness Test
2.4. Disintegration Time
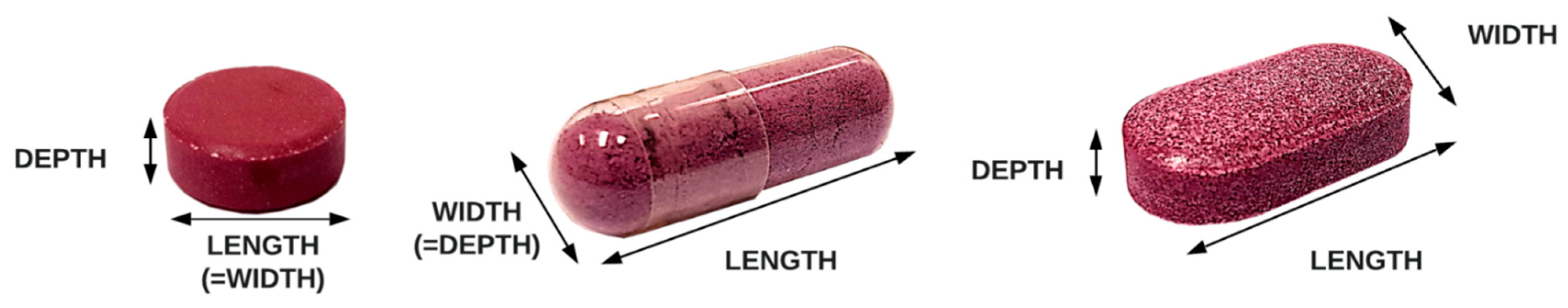
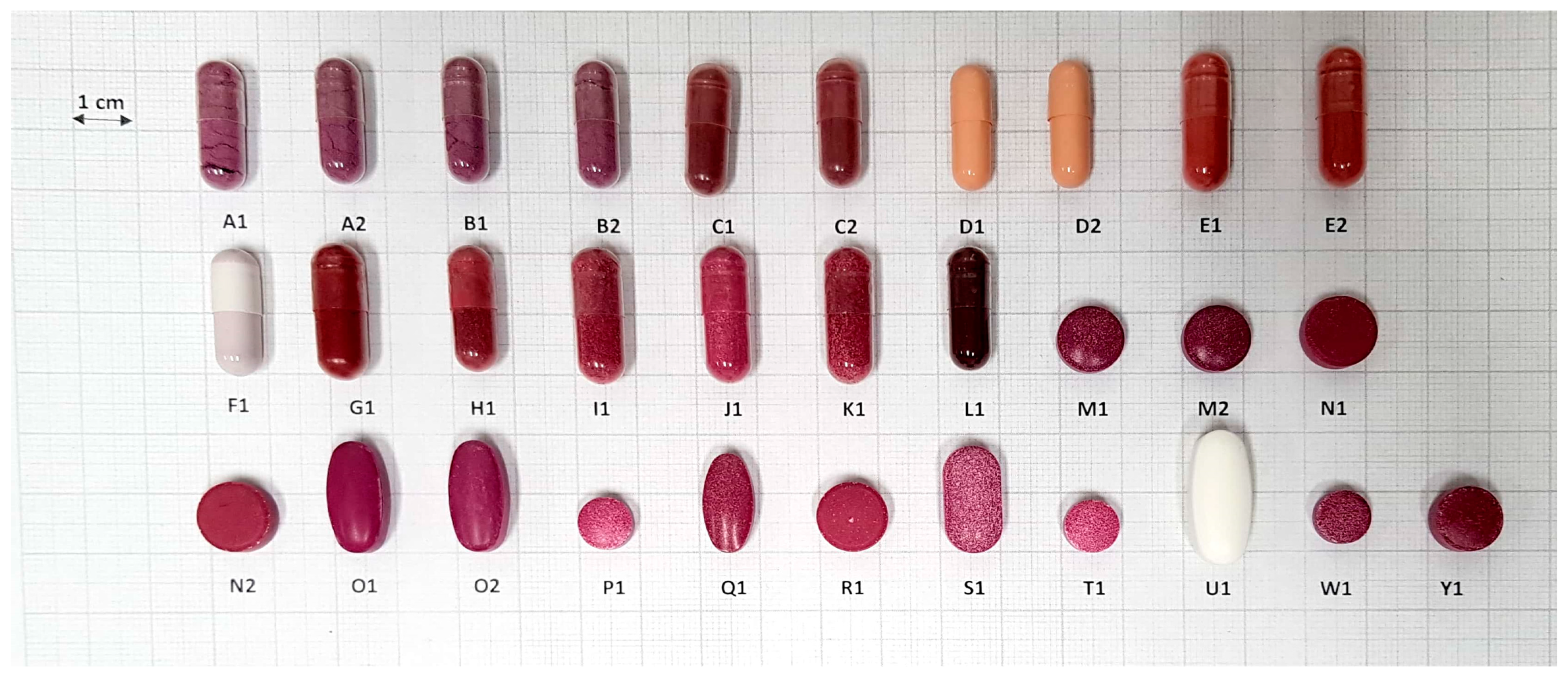
2.5. Analysis of Size and Shape
2.6. Preparation of Samples to Flame Atomic Absorption Spectroscopy (FAAS) Analysis
2.7. Analysis of Iron and Zinc
2.8. Statistical Analysis
3. Results and Discussion
3.1. Weight Variation of the Dosage Form
3.2. Hardness and Friability Tests
3.3. Disintegration Test
3.4. Analysis of Size and Shape
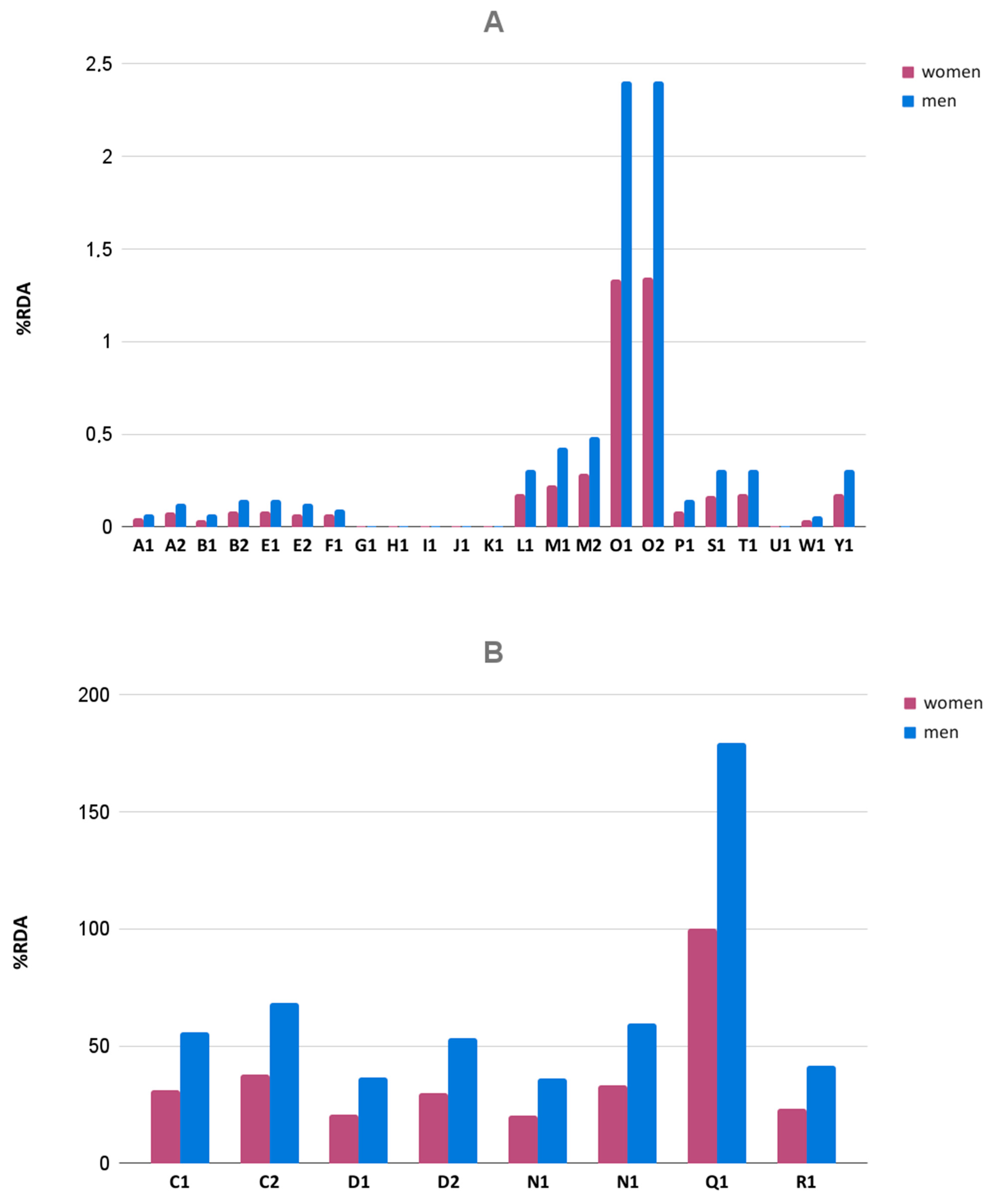
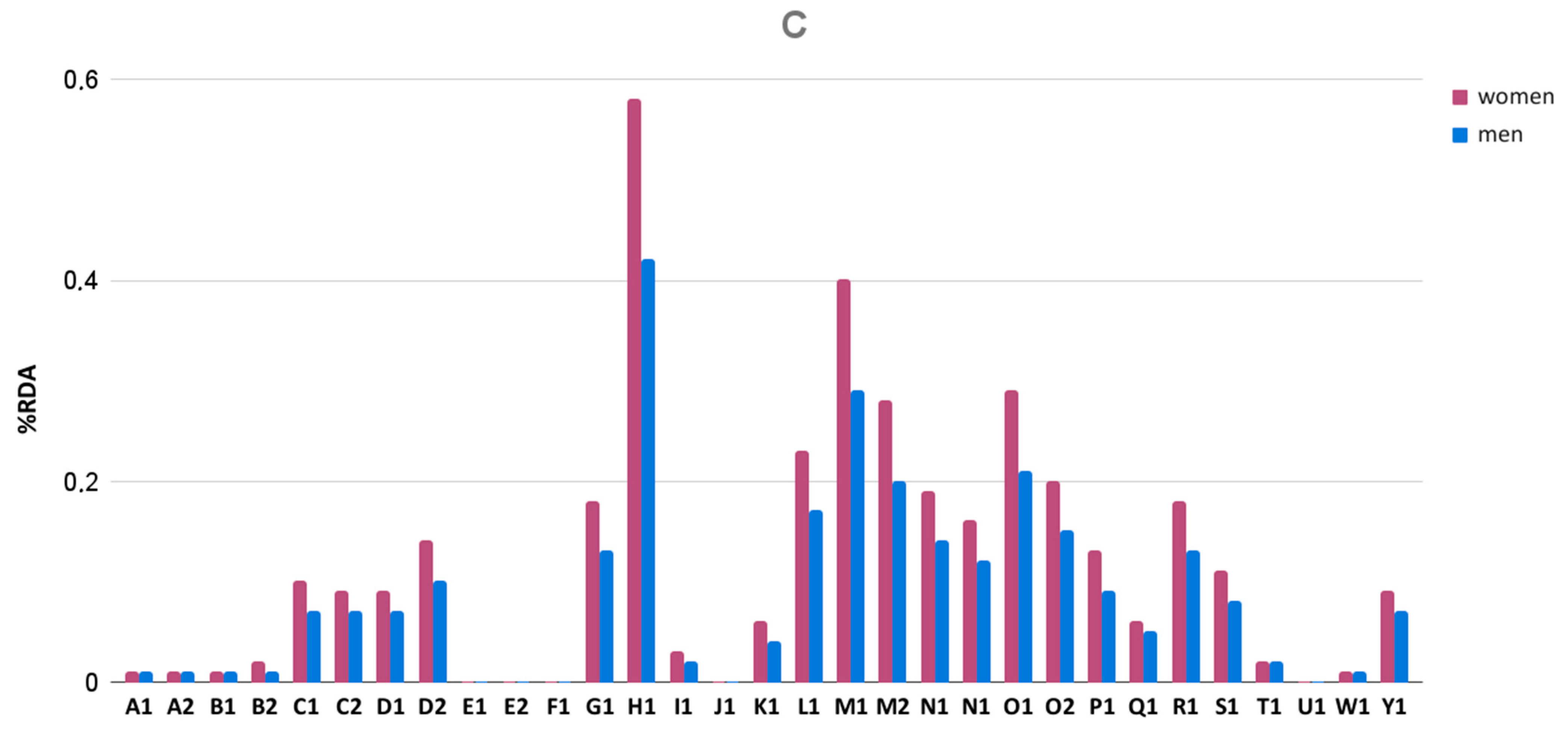
3.5. Iron and Zinc Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dziennik Ustaw. Ustawa z dnia 25 sierpnia 2006 r. o bezpieczeństwie żywności i żywienia (z późn. zm.). In 2006 Nr 171 poz. 1225; ISAP: Warsaw, Poland, 2006. [Google Scholar]
- European Food Safety Authority. Food Supplements. Available online: https://www.efsa.europa.eu/en/topics/topic/food-supplements (accessed on 25 November 2020).
- Brzezińska, J.; Grembecka, M. Suplementy diety—Specyficzna żywność. PHMD 2021. accepted. [Google Scholar]
- Kowalska, A.; Bieniek, M.; Manning, L. Food supplements’non-conformity in europe—Poland: A case study. Trends Food Sci. Technol. 2019, 93, 262–270. [Google Scholar] [CrossRef]
- FDA. Dietary Supplement Health and Education Act of 1994; FDA: Montgomery, MD, USA, 1994.
- GIS Zasady Wprowadzania do Obrotu Suplementów Diety. Available online: http://www.wsse.gda.pl/nadzor-sanitarny/oddzial-bezpieczenstwa-zywnosci-zywienia-i-produktow-kosmetycznych/suplementy-diety/56-zasady-wprowadzania-do-obrotu-suplementow-diety (accessed on 8 July 2020).
- GIS Rejestr Produktów Objętych Powiadomieniem o Pierwszym Wprowadzeniu do Obrotu. Available online: https://powiadomienia.gis.gov.pl/ (accessed on 14 July 2020).
- United States Pharmacopeial Convention. <2040> Disintegration and dissolution of dietary supplements. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- United States Pharmacopeial Convention. <2091> Weight variation of dietary supplement. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Baião, D.D.S.; da Silva, D.V.T.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. In Food Additives; IntechOpen: London, UK, 2017; pp. 21–43. [Google Scholar]
- Chawla, H.; Parle, M.; Sharma, K.; Yadav, M. Beetroot: A health promoting functional food. Inven. Rapid Nutraceuticals 2016, 1, 1–5. [Google Scholar]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Baião, D.D.S.; da Silva, D.V.T.; Paschoalin, V.M.F. Beetroot, a remarkable vegetable: Its nitrate and phytochemical contents can be adjusted in novel formulations to benefit health and support cardiovascular disease therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef]
- Odoh, U.E.; Ezugwu, C.O.; Okoro, E.C. Quantitative phytochemical, proximate/nutritive composition analysis of Beta vulgaris Linnaeus (Chenopodiaceae). Planta Med. 2012, 78, 3723–3728. [Google Scholar] [CrossRef]
- Gheith, I.; El-Mahmoudy, A. Laboratory evidence for the hematopoietic potential of Beta vulgaris leaf and stalk extract in a phenylhydrazine model of anemia. Braz. J. Med. Biol. Res. 2018, 51, 1–8. [Google Scholar] [CrossRef]
- Lotfi, M.; Azizi, M.; Tahmasbi, W.; Bashiri, P. The effects of consuming 6 weeks of beetroot juice (Beta vulgaris L.) on hematological parameters in female soccer players. J. Kermanshah Univ. Med. Sci. 2018, 22, 1–5. [Google Scholar] [CrossRef]
- Thompson Coon, J. Goodman and Gilman’s the Pharmacological Basis of Therapeutics; McGraw-Hill Education: New York, NY, USA, 2010; Volume 7, ISBN 9780071769396. [Google Scholar]
- Platel, K.; Srinivasan, K. Bioavailability of micronutrients from plant foods: An update. Crit. Rev. Food Sci. Nutr. 2016, 56, 1608–1619. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164. [Google Scholar] [PubMed]
- Olivares, M.; Pizarro, F.; Ruz, M.; De Romaña, D.L. Acute inhibition of iron bioavailability by zinc: Studies in humans. BioMetals 2012, 25, 657–664. [Google Scholar] [CrossRef]
- Ritz, T.; Werchan, C.A.; Kroll, J.L.; Rosenfield, D. Beetroot juice supplementation for the prevention of cold symptoms associated with stress: A proof-of-concept study. Physiol. Behav. 2019, 202, 45–51. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A. Inorganic nitrite and nitrate in cardiovascular therapy: A better alternative to organic nitrates as nitric oxide donors? Vascul. Pharmacol. 2018, 102, 1–10. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002. Off. J. Eur. Communities 2002, L183, 51–57. [Google Scholar]
- European Parliament. Regulation (EC) No 1925/2006 of The European Parliament and of The Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. Off. J. Eur. Union 2006, L404, 26–38. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32006R1925&from=EN (accessed on 20 July 2021).
- Brzezicha, J.; Błażejewicz, D.; Brzezińska, J.; Grembecka, M. Green coffee VS dietary supplements: A comparative analysis of bioactive compounds and antioxidant activity. Food Chem. Toxicol. 2021, 155, 112377. [Google Scholar] [CrossRef] [PubMed]
- Bureau Européen des Unions de Consommateurs Food Supplements—Challenges & Risks for Consumers. Available online: http://www.beuc.eu/publications/beuc-x-2016-092_ipa_beuc_position_on_food_supplements.pdf (accessed on 18 May 2021).
- Biesterbos, J.W.H.; Sijm, D.T.H.M.; van Dam, R.; Mol, H.G.J. A health risk for consumers: The presence of adulterated food supplements in the Netherlands. Food Addit. Contam. Part A 2019, 36, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Stępień, K.A.; Niewiarowski, J.; Harasimiuk, A. Powszechność suplementów diety a zagrożenia związane z ich stosowaniem. Biul. Wydz. Farm. WUM 2019, 9, 51–59. [Google Scholar]
- Palmer, M.E.; Haller, C.; McKinney, P.E.; Klein-Schwartz, W.; Tschirgi, A.; Smolinske, S.C.; Woolf, A.; Sprague, B.M.; Ko, R.; Everson, G.; et al. Adverse events associated with dietary supplements: An observational study. Lancet 2003, 361, 101–106. [Google Scholar] [CrossRef]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab. 2020, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- United States Pharmacopeial Convention. <1216> Tablet friability. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- United States Pharmacopeial Convention. <1217> Tablet breaking force. In States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- Overgaard, A.B.A.; Hojsted, J.; Hansen, R.; Moller-Sonnergaard, J.; Christrup, L.L. Patients’ evaluation of shape, size and colour of solid dosage forms. Pharm. World Sci. 2001, 23, 185–188. [Google Scholar] [CrossRef]
- Brzezicha-Cirocka, J.; Grembecka, M.; Ciesielski, T.; Flaten, T.P.; Szefer, P. Evaluation of macro- and microelement levels in black tea in view of its geographical origin. Biol. Trace Elem. Res. 2017, 176, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Konieczka, P.; Namiesnik, J. Quality Assurance and Quality Control in the Analytical Chemical Laboratory; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Anselmo, C.D.S.; Mendes, T.C.; Cabral, L.M.; De Sousa, V.P. Physicochemical quality profiles of commercial oral tablets and capsule containing lutein—Impact of insufficient specific sanitary regulations. An. Acad. Bras. Cienc. 2018, 90, 3063–3073. [Google Scholar] [CrossRef] [Green Version]
- Geller, A.I.; Shehab, N.; Weidle, N.J.; Lovegrove, M.C.; Wolpert, B.J.; Timbo, B.B.; Mozersky, R.P.; Budnitz, D.S. Emergency department visits for adverse events related to dietary supplements. N. Engl. J. Med. 2015, 373, 1531–1540. [Google Scholar] [CrossRef]
- Kelly, J.; D’Cruz, G.; Wright, D. Patients with dysphagia: Experiences of taking medication. J. Adv. Nurs. 2010, 66, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Gusev, P.A.; Andrews, K.W.; Savarala, S.; Tey, P.T.; Han, F.; Oh, L.; Pehrsson, P.R.; Dwyer, J.T.; Betz, J.M.; Kuszak, A.J.; et al. Disintegration and dissolution testing of green tea dietary supplements: Application and evaluation of United States Pharmacopeial standards. J. Pharm. Sci. 2020, 109, 1933–1942. [Google Scholar] [CrossRef]
- Hahm, H.; Kujawa, J.; Augsburger, L. Comparison of melatonin products against USP’s nutritional supplements standards and other criteria. J. Am. Pharm. Assoc. 1999, 39, 27–31. [Google Scholar] [CrossRef]
- FDA Size, Shape and Other Physical Attributes of Generic Tablets and Capsules. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.html (accessed on 3 May 2021).
- Channer, K.S.; Virjee, J.P. The effect of size and shape of tablets on their esophageal transit. J. Clin. Pharmacol. 1986, 26, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Brotherman, D.P.; Bayraktaroglu, T.O.; Garofalo, R.J. Comparison of ease of swallowing of dietary supplement products for age-related eye disease. J. Am. Pharm. Assoc. 2004, 44, 587–593. [Google Scholar] [CrossRef]
- O’Donnell, C.; Tandon, P.; Govardhanam, V.; Habal, F. Pill-induced esophagitis from intake of dietary supplements. ACG Case Reports J. 2019, 6, e00106. [Google Scholar] [CrossRef]
- Bliss, J.M. Tablets and capsules that stick in the oesophagus. J. R. Coll. Gen. Pract. 1984, 34, 301. [Google Scholar]
- Vallet, T.; Michelon, H.; Orlu, M.; Jani, Y.; Leglise, P.; Laribe-Caget, S.; Piccoli, M.; Fur, A.L.; Liu, F.; Ruiz, F.; et al. Acceptability in the older population: The importance of an appropriate tablet size. Pharmaceutics 2020, 12, 746. [Google Scholar] [CrossRef]
- Kabeya, K.; Satoh, H.; Hori, S.; Miura, Y.; Sawada, Y. Threshold size of medical tablets and capsules: Based on information collected by Japanese medical wholesaler. Patient Prefer. Adherence 2020, 14, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Murray-Kolbe, L.E.; Beard, J.I. Iron. In Encyclopedia of Dietary Supplements, 2nd ed.; Coates, P.M., Betz, J.M., Blackman, M.R., White, J.D., Levine, M., Cragg, G.M., Moss, J., Eds.; Informa Healthcare: London, UK; New York, NY, USA, 2010. [Google Scholar]
- Cancelo-Hidalgo, M.J.; Castelo-Branco, C.; Palacios, S.; Haya-Palazuelos, J.; Ciria-Recasens, M.; Manasanch, J.; Pérez-Edo, L. Tolerability of different oral iron supplements: A systematic review. Curr. Med. Res. Opin. 2013, 29, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny: Warszawa, Poland, 2020; ISBN 9788365870285. [Google Scholar]
- Iron-Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/ (accessed on 16 May 2021).
- Zinc-Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 16 May 2021).
- DeLoughery, T.G. Iron deficiency anemia. Med. Clin. N. Am. 2017, 101, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Gera, T.; Sachdev, H.S.; Boy, E. Effect of iron-fortified foods on hematologic and biological outcomes: Systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 309–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Gao, J.; Li, N.; Han, S.; Wu, L.; Zhang, Y.; Han, T.; Shan, R.; Li, Y.; Sun, C.; et al. Dietary iron and vitamins in association with mortality. Clin. Nutr. 2021, 40, 2401–2409. [Google Scholar] [CrossRef]
| Code | Weight Uniformity | Disintegration Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Declared Weight of the Dosage Unit (mg) | Average Gross Weight (mg) | Average Net Weight ± SD (mg) 1 | Min% 2 | Max% 3 | Pharmacopeia Criteria | Ratio of Average Weight to Declared Weight (%) | Disintegration Time (min) | Pharmacopeia Criteria | |
| A1 | 500 | 496.75 ± 15.25 | 397.15 ± 13.81 | −5.07 | +6.26 | passed | 99.35 | 5:00 | passed |
| A2 | 500 | 486.15 ± 22.5 | 392.95 ± 25.75 | −14.26 | 16.02 | failed | 97.23 | 4:30 | passed |
| B1 | 500 | 490.05 ± 14.59 | 403.45 ± 15.32 | −9.53 | +5.59 | passed | 98.01 | 5:00 | passed |
| B2 | 500 | 492.55 ± 11.88 | 399.75 ± 10.93 | −5.82 | +5.19 | passed | 98.51 | 4:00 | passed |
| C1 | 596 | 608.05 ± 12.48 | 512.9 ± 12.37 | −5.98 | +5.25 | passed | 102.02 | 5:30 | passed |
| C2 | 596 | 599.7 ± 18.17 | 502.95 ± 18.18 | −9.04 | +7.81 | passed | 100.62 | 6:00 | passed |
| D1 | 376 | 388.1 ± 9.06 | 311.65 ± 7.86 | −5.05 | +4.11 | passed | 103.22 | 8:00 | passed |
| D2 | 376 | 379.35 ± 13.9 | 302.65 ± 13.65 | −9.95 | +6.95 | passed | 100.89 | 6:30 | passed |
| E1 | 690 | 735.3 ± 26.69 | 599.8 ± 26.66 | −6.84 | +4.72 | passed | 106.57 | 9:00 | passed |
| E2 | 690 | 751.65 ± 25.94 | 614.45 ± 26.64 | −6.34 | +7.10 | passed | 108.93 | 9:00 | passed |
| F1 | NA* | 601.96 ± 11.96 | 506.59 ± 11.58 | −6.26 | +3.62 | passed | ND* | 12:00 | passed |
| G1 | NA* | 832.55 ± 12.8 | 713.54 ± 13.62 | −3.77 | +2.90 | passed | ND* | 14:00 | passed |
| H1 | NA* | 634.80 ± 10.65 | 539.90 ± 11.24 | −3.82 | +3.61 | passed | ND* | 11:00 | passed |
| I1 | NA* | 750.14 ± 24.84 | 625.28 ± 23.45 | −8.14 | +5.43 | passed | ND* | 17:00 | passed |
| J1 | NA* | 719.70 ± 19.12 | 584.43 ± 18.69 | −11.93 | +3.08 | passed | ND* | 11:00 | passed |
| K1 | NA* | 748.52 ± 14.87 | 628.28 ± 16.07 | −4.37 | +4.60 | passed | ND* | 13:00 | passed |
| L1 | 300 | 382.65 ± 18.82 | 286.11 ± 18.25 | −10.04 | +14.92 | failed | 127.55 | 31:00 | failed |
| Weight Uniformity | Disintegration Time | Friability | Hardness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Declared Weight of the Dosage Unit (mg) | Average Weight ± SD (mg) | Min% 1 | Max% 2 | Pharmacopeia Criteria | Ratio of Average Weight to Declared Weight (%) | Disintegration Time (min) | Pharmacopeia Criteria | Value (%) | Pharmacopeia Criteria | Value ± SD (N) | Min (N) | Max (N) |
| M1 | 550 | 547.65 ± 11.21 | −4.68 | +2.80 | passed | 99.57 | 17:30 | passed | 0.24 | passed | 204.50 ± 12.28 | 175 | 224 |
| M2 | 550 | 553.20 ± 18.19 | −7.81 | +3.40 | passed | 100.58 | 13:30 | passed | 0.27 | passed | 155.70 ± 12.37 | 138 | 172 |
| N1 | 650 | 671.15 ± 3.73 | −0.92 | +0.87 | passed | 103.25 | 13:00 | passed | 0.06 | passed | 59.10 ± 14.27 | 39 | 82 |
| N2 | 650 | 659.60 ± 5.43 | −1.76 | +1.27 | passed | 101.48 | 15:50 | passed | 0.18 | passed | 164.40 ± 45.05 | 55 | 211 |
| O1 | 925 | 1033.45 ± 8.49 | −1.01 | +2.18 | passed | 111.72 | 22:50 | passed | 0.97 | failed | 119.80 ± 9.53 | 105 | 136 |
| O2 | 925 | 1036.00 ± 8.30 | −1.25 | +1.74 | passed | 112.00 | 44:00 | failed | 0.88 | failed | 121.50 ± 14.57 | 100 | 148 |
| P1 | 350 | 352.33 ± 13.37 | −11.88 | +5.17 | passed | 100.67 | 30:40 | failed | 0.16 | passed | 180.5 ± 77.75 | 26 | 322 |
| Q1 | 630 | 725.28 ± 3.53 | −0.89 | +0.66 | passed | 115.12 | 16:50 | passed | 0.21 | passed | 125.3 ± 3.74 | 118 | 132 |
| R1 | 650 | 653.15 ± 7.40 | −2.77 | +2.00 | passed | 100.48 | 15:10 | passed | 0.25 | passed | 198.7 ± 19.93 | 169 | 235 |
| S1 | NA* | 1063.88 ± 31.54 | −9.88 | +3.27 | passed | ND* | 40:00 | failed | 0.06 | passed | 455.8 ± 31.67 | 386 | 488 |
| T1 | 350 | 355.28 ± 6.05 | −2.77 | +3.51 | passed | 101.51 | 20:30 | passed | 0.22 | passed | 104 ± 16.29 | 84 | 138 |
| U1 | 1460 | 1528.94 ± 29.93 | −2.44 | +7.50 | passed | 104.72 | 48:00 | failed | 0.07 | passed | 241.6 ± 74.74 | 21 | 286 |
| W1 | 350 | 353.88 ± 3.96 | −1.71 | +1.80 | passed | 101.11 | 37:20 | failed | 0.15 | passed | 420.5 ± 167 | 381 | 440 |
| Y1 | NA* | 812.42 ± 7.70 | −1.54 | +2.22 | passed | ND* | >1 h | failed | 0.44 | passed | 216.5 ± 15.04 | 193 | 250 |
| Form | Product | Shape | Shield/ Coating 1 | Width ± SD (mm) | Length ± SD (mm) | Depth ± SD (mm) | W + L + D (mm) 4 | FDA Recommendation 5 |
|---|---|---|---|---|---|---|---|---|
| CAPSULES | A1 | cylindrical capsule | gelatin | 7.44 ± 0.06 | 21.49 ± 0.16 | 7.44 ± 0.06 | 36.36 | acceptable |
| A2 | cylindrical capsule | gelatin | 7.40 ± 0.06 | 21.46 ± 0.18 | 7.40 ± 0.06 | 36.26 | ||
| B1 | cylindrical capsule | gelatin | 7.48 ± 0.05 | 21.40 ± 0.26 | 7.48 ± 0.05 | 36.355 | ||
| B2 | cylindrical capsule | gelatin | 7.48 ± 0.05 | 21.39 ± 0.22 | 7.48 ± 0.05 | 36.34 | ||
| C1 | cylindrical capsule | gelatin | 7.57 ± 0.02 | 21.62 ± 0.27 | 7.57 ± 0.02 | 36.75 | ||
| C2 | cylindrical capsule | gelatin | 7.56 ± 0.02 | 21.69 ± 0.22 | 7.56 ± 0.02 | 36.81 | ||
| D1 | cylindrical capsule | gelatin | 6.70 ± 0.05 | 20.74 ± 0.21 | 6.70 ± 0.05 | 34.125 | ||
| D2 | cylindrical capsule | gelatin | 6.70 ± 0.03 | 20.85 ± 0.18 | 6.70 ± 0.03 | 34.25 | ||
| E1 | cylindrical capsule | pullulan | 8.34 ± 0.05 | 23.52 ± 0.02 | 8.34 ± 0.05 | 40.185 | ||
| E2 | cylindrical capsule | pullulan | 8.40 ± 0.06 | 23.55 ± 0.02 | 8.40 ± 0.06 | 40.34 | ||
| F1 | cylindrical capsule | gelatin | 7.49 ± 0.05 | 21.69 ± 0.16 | 7.49 ± 0.05 | 36.66 | ||
| G1 | cylindrical capsule | HPMC 2 | 8.40 ± 0.07 | 23.13 ± 0.05 | 8.40 ± 0.07 | 39.92 | ||
| H1 | cylindrical capsule | HPMC 2 | 7.47 ± 0.08 | 21.22 ± 0.12 | 7.47 ± 0.08 | 36.15 | ||
| I1 | cylindrical capsule | cellulose | 8.34 ± 0.05 | 23.06 ± 0.1 | 8.34 ± 0.05 | 39.725 | ||
| J1 | cylindrical capsule | HPMC 2 | 8.35 ± 0.04 | 23.31 ± 0.09 | 8.35 ± 0.04 | 40 | ||
| K1 | cylindrical capsule | HPMC 2 | 8.34 ± 0.05 | 23.39 ± 0.17 | 8.34 ± 0.05 | 40.055 | ||
| L1 | cylindrical capsule | cellulose | 7.51 ± 0.03 | 21.02 ± 0.14 | 7.51 ± 0.03 | 36.025 | ||
| TABLETS | M1 | arched circular | uncoated | 11.19 ± 0.03 | 11.19 ± 0.03 | 5.80 ± 0.05 | 28.17 | |
| M2 | arched circular | uncoated | 11.19 ± 0.02 | 11.19 ± 0.02 | 5.77 ± 0.03 | 28.14 | ||
| N1 | flat circular | uncoated | 12.12 ± 0.03 | 12.12 ± 0.03 | 4.84 ± 0.02 | 29.075 | ||
| N1 | flat circular | uncoated | 12.11 ± 0.02 | 12.11 ± 0.02 | 4.83 ± 0.02 | 29.05 | ||
| O1 | arched oblong | uncoated | 9.19 ± 0.02 | 19.09 ± 0.02 | 7.17 ± 0.08 | 35.435 | acceptable | |
| O2 | arched oblong | uncoated | 9.19 ± 0.02 | 19.09 ± 0.02 | 7.15 ± 0.08 | 35.425 | ||
| P1 | arched circular | uncoated | 9.13 ± 0.02 | 9.13 ± 0.02 | 4.71 ± 0.13 | 22.965 | ||
| Q1 | arched oval | uncoated | 8.60 ± 0.01 | 17.17 ± 0.03 | 6.01 ± 0.02 | 31.775 | ||
| R1 | flat circular | uncoated | 12.09 ± 0.02 | 12.09 ± 0.02 | 4.78 ± 0.02 | 28.95 | ||
| S1 | arched oblong | uncoated | 9.66 ± 0.02 | 18.46 ± 0.03 | 6.47 ± 0.07 | 34.585 | ||
| T1 | arched circular | uncoated | 9.15 ± 0.01 | 9.15 ± 0.01 | 4.80 ± 4.8 | 23.09 | ||
| U1 | arched oval | uncoated | 9.92 ± 0.03 | 22.91 ± 0.04 | 8.45 ± 0.05 | 41.275 | unacceptable | |
| W1 | arched circular | uncoated | 9.14 ± 0.02 | 9.14 ± 0.02 | 4.69 ± 0.06 | 22.97 | acceptable | |
| Y1 | arched circular | Glaze 3 | 11.17 ± 0.07 | 11.17 ± 0.07 | 6.48 ± 0.03 | 28.805 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzezińska, J.; Szewczyk, A.; Brzezicha, J.; Prokopowicz, M.; Grembecka, M. Evaluation of Physicochemical Properties of Beetroot-Based Dietary Supplements. Foods 2021, 10, 1693. https://doi.org/10.3390/foods10081693
Brzezińska J, Szewczyk A, Brzezicha J, Prokopowicz M, Grembecka M. Evaluation of Physicochemical Properties of Beetroot-Based Dietary Supplements. Foods. 2021; 10(8):1693. https://doi.org/10.3390/foods10081693
Chicago/Turabian StyleBrzezińska, Joanna, Adrian Szewczyk, Justyna Brzezicha, Magdalena Prokopowicz, and Małgorzata Grembecka. 2021. "Evaluation of Physicochemical Properties of Beetroot-Based Dietary Supplements" Foods 10, no. 8: 1693. https://doi.org/10.3390/foods10081693
APA StyleBrzezińska, J., Szewczyk, A., Brzezicha, J., Prokopowicz, M., & Grembecka, M. (2021). Evaluation of Physicochemical Properties of Beetroot-Based Dietary Supplements. Foods, 10(8), 1693. https://doi.org/10.3390/foods10081693








