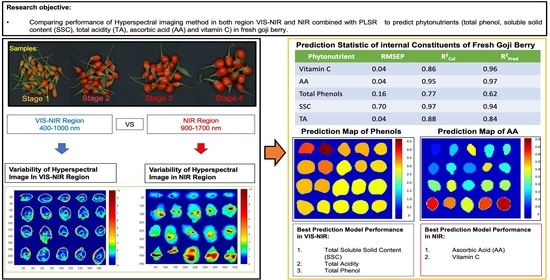
Comparison Performance of Visible-NIR and Near-Infrared Hyperspectral Imaging for Prediction of Nutritional Quality of Goji Berry (Lycium barbarum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Spectral Acquisition
2.2. Hyperspectral Image Acquisition
2.3. Chemical Analysis, and Partial Least Square Regression (PLSR)
2.3.1. Determination of Vitamin C
2.3.2. Determination of Anthocyanin
2.3.3. Total Polyphenol and Antioxidant Activity
2.3.4. Maturity Indexes
2.3.5. Partial Least Squares Regression (PLSR)
2.3.6. Mapping of Internal Constituents
3. Results and Discussion
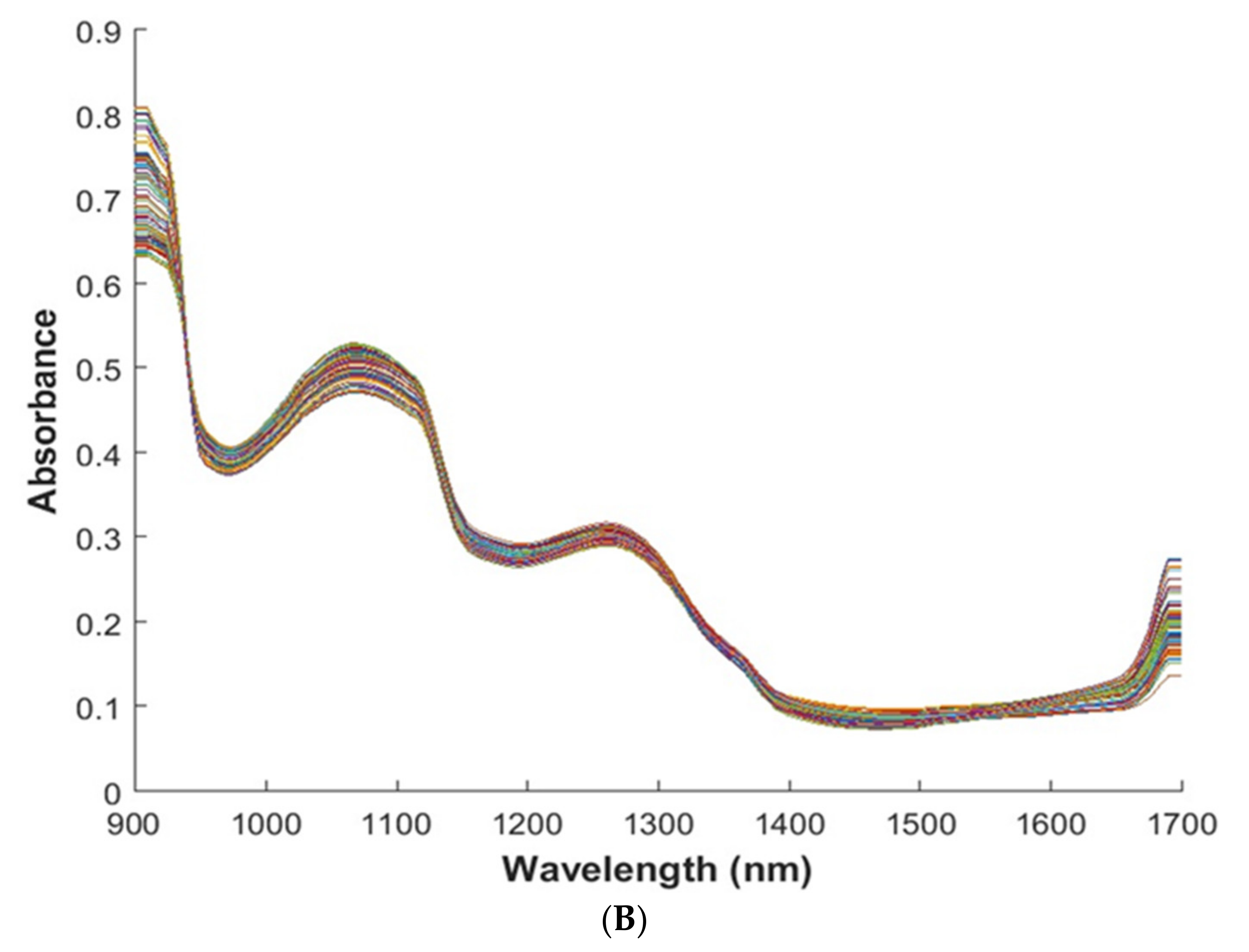
3.1. Spectral and Spatial Profile
3.2. Comparison of Prediction Model between Spectra Range VIS-NIR and NIR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jatoi, M.A.; Fruk, M.; Buhin, J.; Vinceković, M.; Vuković, M.; Jemrić, T. Effect of Different Storage Temperatures on Storage Life, Physico-chemical and Sensory Attributes of Goji Berry (Lycium barbarum L.) Fruits. Erwerbs Obstbau 2018, 60, 119–126. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. Goji Berry (Lycium barbarum): Composition and Health Effects—A. Review. Polish J. Food Nutr. Sci. 2016, 66, 67–75. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, H.; Quan, J.; An, W.; Zhao, J.; Xi, W. Identification of characteristic aroma volatiles of Ningxia goji berries (Lycium barbarum L.) and their developmental changes. Int. J. Food Prop. 2017, 20, S214–S227. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef]
- Baca-Bocanegra, B.; Nogales-Bueno, J.; Hernández-Hierro, J.M.; Heredia, F.J. Evaluation of extractable polyphenols released to wine from cooperage byproduct by near infrared hyperspectral imaging. Food Chem. 2018, 244, 206–212. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Chu, B.; Zhang, C. Determination of total polysaccharides and total flavonoids in chrysanthemum morifolium using near-infrared hyperspectral imaging and multivariate analysis. Molecules 2018, 23, 2395. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, Q.; Liu, F.; He, Y.; Xiao, Y. Rapid and non-destructive measurement of spinach pigments content during storage using hyperspectral imaging with chemometrics. Meas. J. Int. Meas. Confed. 2017, 97, 149–155. [Google Scholar] [CrossRef]
- Amodio, M.L.; Chaudhry, M.M.A.; Colelli, G. Spectral and hyperspectral technologies as an additional tool to increase information on quality and origin of horticultural crops. Agronomy 2020, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wu, W.; Zhou, L.; Cheng, H.; Ye, X.; He, Y. Developing deep learning based regression approaches for determination of chemical compositions in dry black goji berries (Lycium ruthenicum Murr.) using near-infrared hyperspectral imaging. Food Chem. 2020, 319, 2019. [Google Scholar] [CrossRef]
- Dai, Q.; Cheng, J.-H.; Sun, D.-W.; Zeng, X.-A. Advances in Feature Selection Methods for Hyperspectral Image Processing in Food Industry Applications: A. Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1368–1382. [Google Scholar] [CrossRef]
- Xu, J.-L.; Esquerre, C.; Sun, D. Methods for performing dimensionality reduction in hyperspectral image classification. J. Near Infrared Spectrosc. 2018, 26, 61–75. [Google Scholar] [CrossRef]
- Guan, J.L.X.; Huang, J.K. Evaluation of moisture content in processed apple chips using NIRS and wavelength selection techniques. Infrared Phys. Technol. 2019, 98, 305–310. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Prediction of firmness parameters of tomatoes by portable visible and near-infrared spectroscopy. J. Food Eng. 2018, 222, 185–198. [Google Scholar] [CrossRef]
- Amodio, M.L.; Capotorto, I.; Chaudhry, M.M.A.; Colelli, G. The use of hyperspectral imaging to predict the distribution of internal constituents and to classify edible fennel heads based on the harvest time. Comput. Electron. Agric. 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Arslan, M.; Zou, X.; Tahir, H.E.; Hu, X.; Rakha, A.; Basheer, S.; Hao, Z. Near-infrared spectroscopy coupled chemometric algorithms for prediction of antioxidant activity of black goji berries (Lycium ruthenicum Murr.). J. Food Meas. Charact. 2018, 12, 2366–2376. [Google Scholar] [CrossRef]
- Zapata, S.; Dufour, J. Ascorbic, dehydroascorbic and isoascorbic acid simultaneous determinations by reverse phase ion interaction HPLC. J. Food Sci. 1992, 57, 506–511. [Google Scholar] [CrossRef]
- Cefola, M.; Amodio, M.L.; Cornacchia, R.; Rinaldi, R.; Vanadia, S.; Colelli, G. Effect of atmosphere composition on the quality of ready-to-use broccoli raab (Brassica rapa L.). J. Sci. Food Agric. 2010, 90, 789–797. [Google Scholar] [CrossRef]
- Proctor, J.T.A. Color Stimulation in Attached Apples with Supplementary Light. Can. J. Plant. Sci. 1974, 54, 499–503. [Google Scholar] [CrossRef]
- Wells, R. Photosynthetic responses to cutout. In Proceeding of Beltwide Cotton Conference; National Cotton Council: Memphis, TN, USA, 1995; pp. 62–64. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mobaraki, N. Chemometrics and Intelligent Laboratory Systems HYPER-Tools. A graphical user-friendly interface for hyperspectral image analysis. Chemom. Intell. Lab. Syst. 2018, 172, 174–187. [Google Scholar] [CrossRef]
- Haaland, D.M.; Thomas, E.V. Partial least-squares methods for spectral analyses. 1. Relation to other quantitative calibration methods and the extraction of qualitative information. Anal. Chem. 1988, 60, 1193–1202. [Google Scholar] [CrossRef]
- Olivieri, A. Introduction to Multivariate Calibration. A Practical Approach; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Brown, A.; Anderson, D.; Racicot, K.; Pilkenton, S.J.; Apostolidis, E. Evaluation of Phenolic Phytochemical Enriched Commercial Plant Extracts on the In Vitro Inhibition of α-Glucosidase. Front. Nutr. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Bordbar, M.; Sharifi-Zarchi, Z.; Khodadadi, B. Green synthesis of copper oxide nanoparticles/clinoptilolite using Rheum palmatum L. root extract: High catalytic activity for reduction of 4-nitro phenol, rhodamine B, and methylene blue. J. Sol Gel Sci. Technol. 2017, 81, 724–733. [Google Scholar] [CrossRef]
- Travers, S.; Bertelsen, M.G.; Petersen, K.K.; Kucheryavskiy, S.V. Predicting pear (cv. Clara Frijs) dry matter and soluble solids content with near infrared spectroscopy. LWT Food Sci. Technol. 2014, 59, 1107–1113. [Google Scholar] [CrossRef]
- Escribano, S.; Biasi, W.V.; Lerud, R.; Slaughter, D.C.; Mitcham, E.J. Non-destructive prediction of soluble solids and dry matter content using NIR spectroscopy and its relationship with sensory quality in sweet cherries. Postharvest Biol. Technol. 2017, 128, 112–120. [Google Scholar] [CrossRef]
- Hernández-Sánchez, N.; Gómez-Del-Campo, M. From NIR spectra to singular wavelengths for the estimation of the oil and water contents in olive fruits. Grasas Aceites 2018, 69, 4. [Google Scholar] [CrossRef] [Green Version]
- Badaró, A.T.; Morimitsu, F.L.; Ferreira, A.R.; Clerici, M.T.P.S.; Barbin, D.F. Identification of fiber added to semolina by near infrared (NIR) spectral techniques. Food Chem. 2019, 289, 195–203. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J. Rapid determination of vitamin C by NIR, MIR and FT-Raman techniques. J. Pharm. Pharmacol. 2002, 54, 1247–1255. [Google Scholar] [CrossRef]
- Chaudhry, M.M.A.; Amodio, M.L.; Amigo, J.M.; de Chiara, M.L.V.; Babellahi, F.; Colelli, G. Feasibility study for the surface prediction and mapping of phytonutrients in minimally processed rocket leaves (Diplotaxis tenuifolia) during storage by hyperspectral imaging. Comput. Electron. Agric. 2020, 175, 105575. [Google Scholar] [CrossRef]
- Kafkas, N.E.; Oğuz, H.İ.; Oğuz, İ. Evaluation of fruit characteristics of various organically-grown goji berry (Lycium barbarum L., Lycium chinense Miller) species during ripening stages. J. Food Compos. Anal. 2021, 101, 103846. [Google Scholar]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Benchennouf, A.; Grigorakis, S.; Loupassaki, S.; Kokkalou, E. Phytochemical analysis and antioxidant activity of Lycium barbarum (GOJI) cultivated in Greece. Pharm. Biol. 2017, 55, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Fatchurrahman, D.; Kuramoto, M.; al Riza, D.F.; Ogawa, Y.; Suzuki, T.; Kondo, N. Fluorescence time series monitoring of different parts of green pepper (Capsicum annuum L.) under different storage temperatures. Comput. Electron. Agric. 2020, 179, 2020. [Google Scholar] [CrossRef]
- Marques, E.J.N.; de Freitas, S.T.; Pimentel, M.F.; Pasquini, C. Rapid and non-destructive determination of quality parameters in the ‘Tommy Atkins’ mango using a novel handheld near infrared spectrometer. Food Chem. 2016, 197, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Ignat, T.; Schmilovitch, Z.; Fefoldi, J.; Steiner, B.; Alkalai-Tuvia, S. Non-destructive measurement of ascorbic acid content in bell peppers by VIS-NIR and SWIR spectrometry. Postharvest Biol. Technol. 2012, 74, 91–99. [Google Scholar] [CrossRef]
- Azadshahraki, F.; Jamshidi, B.; Sharabiani, V.R. Non-destructive determination of vitamin C and lycopene contents of intact cv. Newton tomatoes using NIR spectroscopy. Yuz. Yil Univ. J. Agric. Sci. 2018, 28, 389–397. [Google Scholar]
- Wang, X.; Xue, L.; He, X.; Liu, M. Vitamin C content estimation of chilies using Vis/NIR spectroscopy. In Proceedings of the ICEICE 2011, International Conference on Electric Information and Control Engineering, Wuhan, China, 25–27 March 2011; pp. 1894–1897. [Google Scholar]
- Pissard, A.; Pierna, J.A.F.; Baeten, V.; Sinnaeve, G.; Lognay, G.; Mouteau, A.; Dupont, P.; Rondia, A.; Lateur, M. Non-destructive measurement of vitamin C, total polyphenol and sugar content in apples using near-infrared spectroscopy. J. Sci. Food Agric. 2013, 93, 238–244. [Google Scholar] [CrossRef]
- Borba, K.R.; Spricigo, P.C.; Aykas, D.P.; Mitsuyuki, M.C.; Colnago, L.A.; Ferreira, M.D. Non-invasive quantification of vitamin C, citric acid, and sugar in ‘Valência’ oranges using infrared spectroscopies. J. Food Sci. Technol. 2020, 58, 731–738. [Google Scholar] [CrossRef]
- Wu, T.; Lv, H.; Wang, F.; Wang, Y. Characterization of Polyphenols from Lycium ruthenicum Fruit by UPLC-Q-TOF/MSE and Their Antioxidant Activity in Caco-2 Cells. J. Agric. Food Chem. 2016, 64, 2280–2288. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Cai, S.; Zhang, X.; Qian, Q.; Huang, Y.; Dai, F.; Zhang, G. Development of predictive models for total phenolics and free p-coumaric acid contents in barley grain by near-infrared spectroscopy. Food Chem. 2017, 227, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Shenk, J.S.; Westerhaus, M.O. Population Definition, Sample Selection, and Calibration Procedures for Near Infrared Reflectance Spectroscopy. Crop. Sci. 1991, 31, 469–474. [Google Scholar] [CrossRef]
- Li, Q.; Yu, X.; Gao, J.M. A novel method to determine total sugar of Goji berry using FT-NIR spectroscopy with effective wavelength selection. Int. J. Food Prop. 2017, 20, S478–S488. [Google Scholar] [CrossRef] [Green Version]
- Sohrabi, M.M.; Ahmadi, E.; Monavar, H.M. Nondestructive analysis of packaged grape tomatoes quality using PCA and PLS regression by means of fiber optic spectroscopy during storage. J. Food Meas. Charact. 2018, 12, 949–966. [Google Scholar] [CrossRef]





| Parameters | Min | Max | Mean | Standard Deviation |
|---|---|---|---|---|
| Vitamin C (Vitamin C g kg−1) | 0.13 | 0.65 | 0.31 | 0.14 |
| Ascorbic Acid (AA) (Ascorbic Acid g kg−1) | 0.02 | 0.48 | 0.20 | 0.12 |
| Dehydroascorbic Acid (DHAA) (DHAA g kg−1) | 0.04 | 0.18 | 0.11 | 0.04 |
| Antioxidant Activity (Trolox equivalent g kg−1) | 1.63 | 3.29 | 2.31 | 0.41 |
| Total Phenols (gallic-acid g kg−1) | 2.09 | 3.37 | 2.62 | 0.32 |
| Anthocyanin (Cyanidin mg cm−2) | 0.67 | 1.35 | 1.05 | 0.17 |
| Soluble Solid Content (SSC) (%) | 6.50 | 25.90 | 21.62 | 3.48 |
| Total Acidity (TA) (%) | 0.11 | 0.92 | 0.56 | 0.15 |
| Parameter | Pre-Treatment | No. Var | No. Sample | LVs | R2Cal | RMSEC | R2CV | RMSECV |
|---|---|---|---|---|---|---|---|---|
| Vitamin C | SM + MC | 121 | 92 | 10 | 0.79 | 0.05 | 0.69 | 0.07 |
| SM + 1st Dev + MC | 121 | 92 | 5 | 0.60 | 0.06 | 0.56 | 0.07 | |
| SM + 2nd Dev + MC | 121 | 92 | 5 | 0.84 | 0.05 | 0.76 | 0.07 | |
| AA | SM + MC | 121 | 92 | 13 | 0.89 | 0.03 | 0.64 | 0.05 |
| SM + 1st Dev + MC | 121 | 92 | 7 | 0.75 | 0.04 | 0.64 | 0.05 | |
| SM + 2nd Dev + MC | 121 | 92 | 5 | 0.75 | 0.04 | 0.69 | 0.05 | |
| DHAA | SM + MC | 121 | 92 | 3 | 0.31 | 0.03 | 0.31 | 0.03 |
| SM + 1st Dev + MC | 121 | 92 | 1 | 0.36 | 0.03 | 0.40 | 0.03 | |
| SM + 2nd Dev + MC | 121 | 92 | 1 | 0.39 | 0.03 | 0.42 | 0.03 | |
| Total Antioxidant | SM + MC | 121 | 97 | 4 | 0.21 | 0.32 | 0.22 | 0.35 |
| SM + 1st Dev + MC | 121 | 97 | 3 | 0.31 | 0.30 | 0.31 | 0.34 | |
| SM + Log + 1st Dev + MC | 121 | 97 | 3 | 0.39 | 0.25 | 0.37 | 0.29 | |
| Phenols | SM + MC | 121 | 97 | 2 | 0.37 | 0.26 | 0.40 | 0.27 |
| SM + 1st Dev + MC | 121 | 97 | 5 | 0.36 | 0.19 | 0.33 | 0.23 | |
| SM + Log + 2nd Dev + MC | 121 | 97 | 2 | 0.41 | 0.23 | 0.44 | 0.24 | |
| Anthocyanin | SM + 1st Dev + MC | 121 | 97 | 4 | 0.14 | 0.15 | 0.15 | 0.16 |
| SM + 2nd Dev + MC | 121 | 97 | 2 | 0.18 | 0.16 | 0.18 | 0.17 | |
| SM + Log + 2nd Dev + MC | 121 | 97 | 1 | 0.15 | 0.16 | 0.16 | 0.16 | |
| SSC | SM + 1st Dev + MC | 121 | 97 | 6 | 0.82 | 0.92 | 0.73 | 1.15 |
| SM + 2nd Dev + MC | 121 | 97 | 4 | 0.79 | 0.98 | 0.69 | 1.25 | |
| SM + Log + 1st Dev + MC | 121 | 97 | 6 | 0.85 | 0.96 | 0.75 | 1.2 | |
| TA | SM + 2nd Dev + MC | 121 | 97 | 4 | 0.43 | 0.06 | 0.43 | 0.09 |
| SM + Log + 1st Dev + MC | 121 | 97 | 5 | 0.52 | 0.06 | 0.48 | 0.07 | |
| SM + Log + 2nd Dev + MC | 121 | 97 | 4 | 0.55 | 0.06 | 0.51 | 0.07 |
| Parameter | Pre-Treatment | No. Var | No. Sample | LVs | R2Cal | RMSEC | R2CV | RMSECV |
|---|---|---|---|---|---|---|---|---|
| Vitamin C | SM + 1st Dev + MC | 161 | 95 | 11 | 0.70 | 0.05 | 0.67 | 0.06 |
| SM + Log + 1st Dev + MC | 161 | 95 | 17 | 0.65 | 0.03 | 0.64 | 0.06 | |
| SM + Log + 2nd Dev + MC | 161 | 95 | 10 | 0.78 | 0.04 | 0.70 | 0.06 | |
| AA | SM + 1st Dev + MC | 161 | 95 | 13 | 0.94 | 0.04 | 0.81 | 0.06 |
| SM + Log + 1st Dev + MC | 161 | 95 | 12 | 0.65 | 0.03 | 0.54 | 0.05 | |
| SM + Log + 2nd Dev + MC | 161 | 95 | 10 | 0.65 | 0.04 | 0.57 | 0.06 | |
| DHAA | SM + MC | 161 | 95 | 4 | 0.22 | 0.03 | 0.26 | 0.03 |
| SM + 2nd Dev + MC | 161 | 95 | 3 | 0.20 | 0.03 | 0.25 | 0.03 | |
| SM + Log + 1st Dev + MC | 161 | 95 | 3 | 0.21 | 0.03 | 0.26 | 0.03 | |
| Total Antioxidant | SM + MC | 161 | 97 | 11 | 0.21 | 0.27 | 0.22 | 0.34 |
| SM + Log + 1st Dev + MC | 161 | 97 | 3 | 0.22 | 0.32 | 0.27 | 0.35 | |
| SM + Log + 2nd Dev + MC | 161 | 97 | 2 | 0.27 | 0.33 | 0.34 | 0.34 | |
| Phenols | SM + 1st Dev + MC | 161 | 97 | 8 | 0.39 | 0.18 | 0.43 | 0.22 |
| SM + Log + 1st Dev + MC | 161 | 97 | 3 | 0.41 | 0.22 | 0.50 | 0.24 | |
| SM + Log + 2nd Dev + MC | 161 | 97 | 4 | 0.34 | 0.21 | 0.42 | 0.22 | |
| Anthocyanin | SM + MC | 161 | 97 | 1 | 0.04 | 0.16 | 0.05 | 0.17 |
| SM + Log + 1st Dev + MC | 161 | 97 | 1 | 0.05 | 0.16 | 0.06 | 0.17 | |
| SM + Log + 2nd Dev + MC | 161 | 97 | 1 | 0.06 | 0.16 | 0.07 | 0.17 | |
| SSC | SM + MC | 161 | 97 | 10 | 0.14 | 1.32 | 0.16 | 1.57 |
| SM + 1st Dev + MC | 161 | 97 | 7 | 0.19 | 1.38 | 0.22 | 1.59 | |
| SM + Log + 2nd Dev + MC | 161 | 97 | 8 | 0.15 | 1.38 | 0.16 | 1.76 | |
| TA | SM + 1st Dev + MC | 161 | 97 | 3 | 0.42 | 0.07 | 0.54 | 0.08 |
| SM + 2nd Dev + MC | 161 | 97 | 4 | 0.40 | 0.07 | 0.52 | 0.08 | |
| SM + Log + 1st Dev + MC | 161 | 97 | 7 | 0.34 | 0.07 | 0.40 | 0.08 |
| Parameter | Effective WaveLength Range (nm) | No. Sample | No. Variables | LVs | R2Cal | RMSEC | R2CV | RMSECV | R2pred | RMSEP |
|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin C | 1475–1495 1525–1545 1600–1650 | 85 | 18 | 12 | 0.86 | 0.03 | 0.84 | 0.04 | 0.96 | 0.04 |
| AA | 925–945 975–1120 1175–1270 1300–1320 1425–1445 1475–1495 1525–1545 1600–1620 | 85 | 72 | 11 | 0.95 | 0.02 | 0.91 | 0.04 | 0.97 | 0.04 |
| Total Phenols | 425–520 725–995 | 87 | 73 | 2 | 0.77 | 0.16 | 0.61 | 0.17 | 0.62 | 0.16 |
| SSC | 400–495 525–545 625–670 850–895 925–970 | 89 | 50 | 4 | 0.97 | 0.75 | 0.95 | 0.91 | 0.94 | 0.70 |
| TA | 400–445 600–620 775–795 825–895 | 87 | 31 | 3 | 0.88 | 0.04 | 0.87 | 0.04 | 0.84 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatchurrahman, D.; Nosrati, M.; Amodio, M.L.; Chaudhry, M.M.A.; de Chiara, M.L.V.; Mastrandrea, L.; Colelli, G. Comparison Performance of Visible-NIR and Near-Infrared Hyperspectral Imaging for Prediction of Nutritional Quality of Goji Berry (Lycium barbarum L.). Foods 2021, 10, 1676. https://doi.org/10.3390/foods10071676
Fatchurrahman D, Nosrati M, Amodio ML, Chaudhry MMA, de Chiara MLV, Mastrandrea L, Colelli G. Comparison Performance of Visible-NIR and Near-Infrared Hyperspectral Imaging for Prediction of Nutritional Quality of Goji Berry (Lycium barbarum L.). Foods. 2021; 10(7):1676. https://doi.org/10.3390/foods10071676
Chicago/Turabian StyleFatchurrahman, Danial, Mojtaba Nosrati, Maria Luisa Amodio, Muhammad Mudassir Arif Chaudhry, Maria Lucia Valeria de Chiara, Leonarda Mastrandrea, and Giancarlo Colelli. 2021. "Comparison Performance of Visible-NIR and Near-Infrared Hyperspectral Imaging for Prediction of Nutritional Quality of Goji Berry (Lycium barbarum L.)" Foods 10, no. 7: 1676. https://doi.org/10.3390/foods10071676
APA StyleFatchurrahman, D., Nosrati, M., Amodio, M. L., Chaudhry, M. M. A., de Chiara, M. L. V., Mastrandrea, L., & Colelli, G. (2021). Comparison Performance of Visible-NIR and Near-Infrared Hyperspectral Imaging for Prediction of Nutritional Quality of Goji Berry (Lycium barbarum L.). Foods, 10(7), 1676. https://doi.org/10.3390/foods10071676