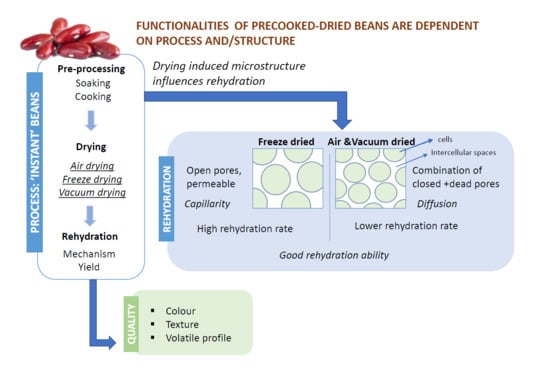
The Impact of Drying and Rehydration on the Structural Properties and Quality Attributes of Pre-Cooked Dried Beans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Dried Beans
2.2. Moisture Content
2.3. Rehydration
2.3.1. Empirical Models
2.3.2. Diffusion Model
2.4. Microstructure
2.5. Texture
2.6. Starch Retrogradation
2.7. Color
2.8. Analysis of Volatile Compounds
2.8.1. Headspace-Solid-Phase Micro-Extraction-Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS) Analysis
2.8.2. Data Processing and Multivariate Analysis
2.9. Statistical Analysis
3. Results
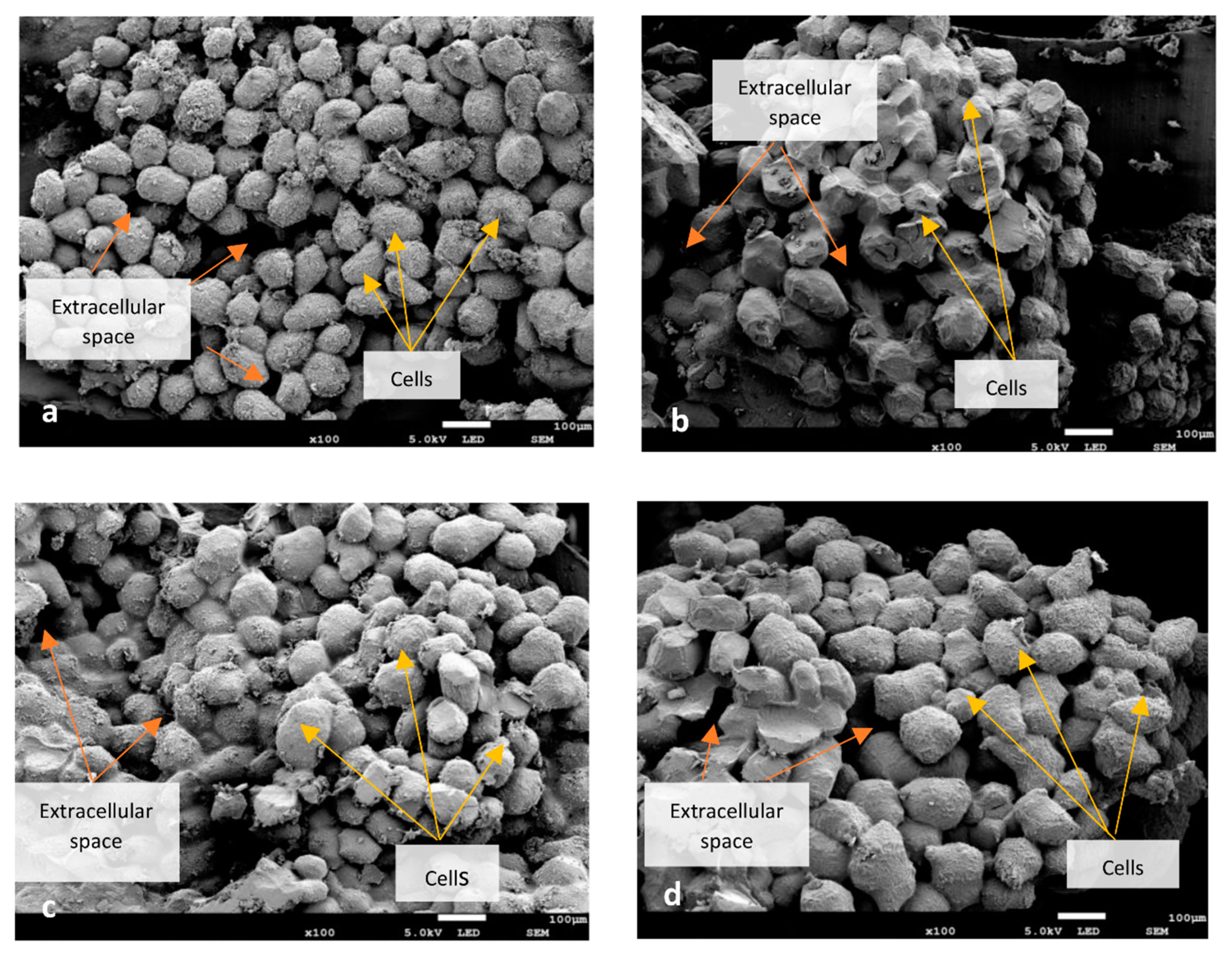
3.1. Microstructure of Dried Beans
3.2. Rehydration
3.2.1. Effect of Different Drying Processes on Rehydration
3.2.2. Modeling of Rehydration
Empirical Models
Diffusion Model
3.3. Microstructure of Rehydrated Beans
3.4. Starch Retrogradation
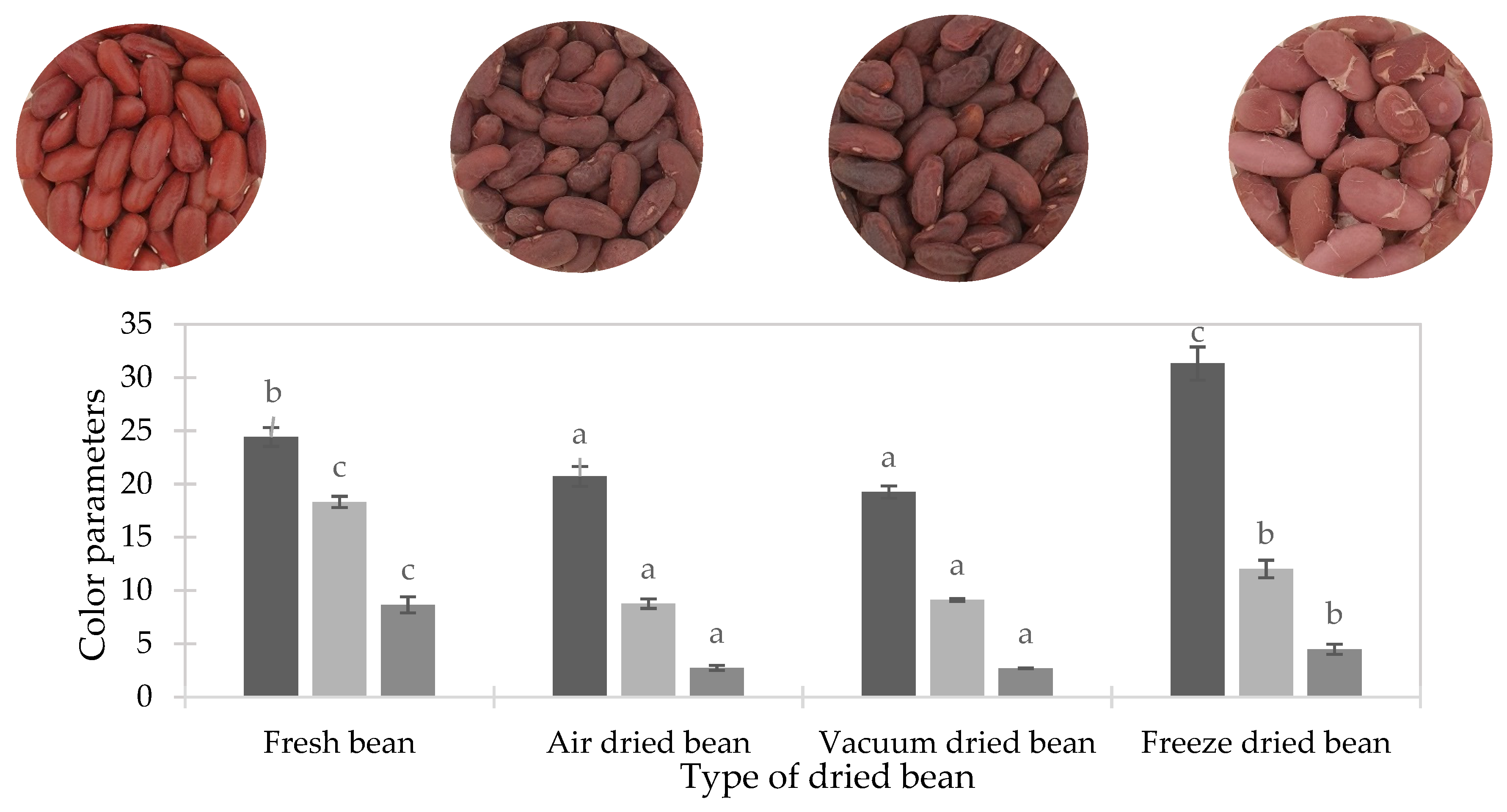
3.5. Color
3.6. Texture
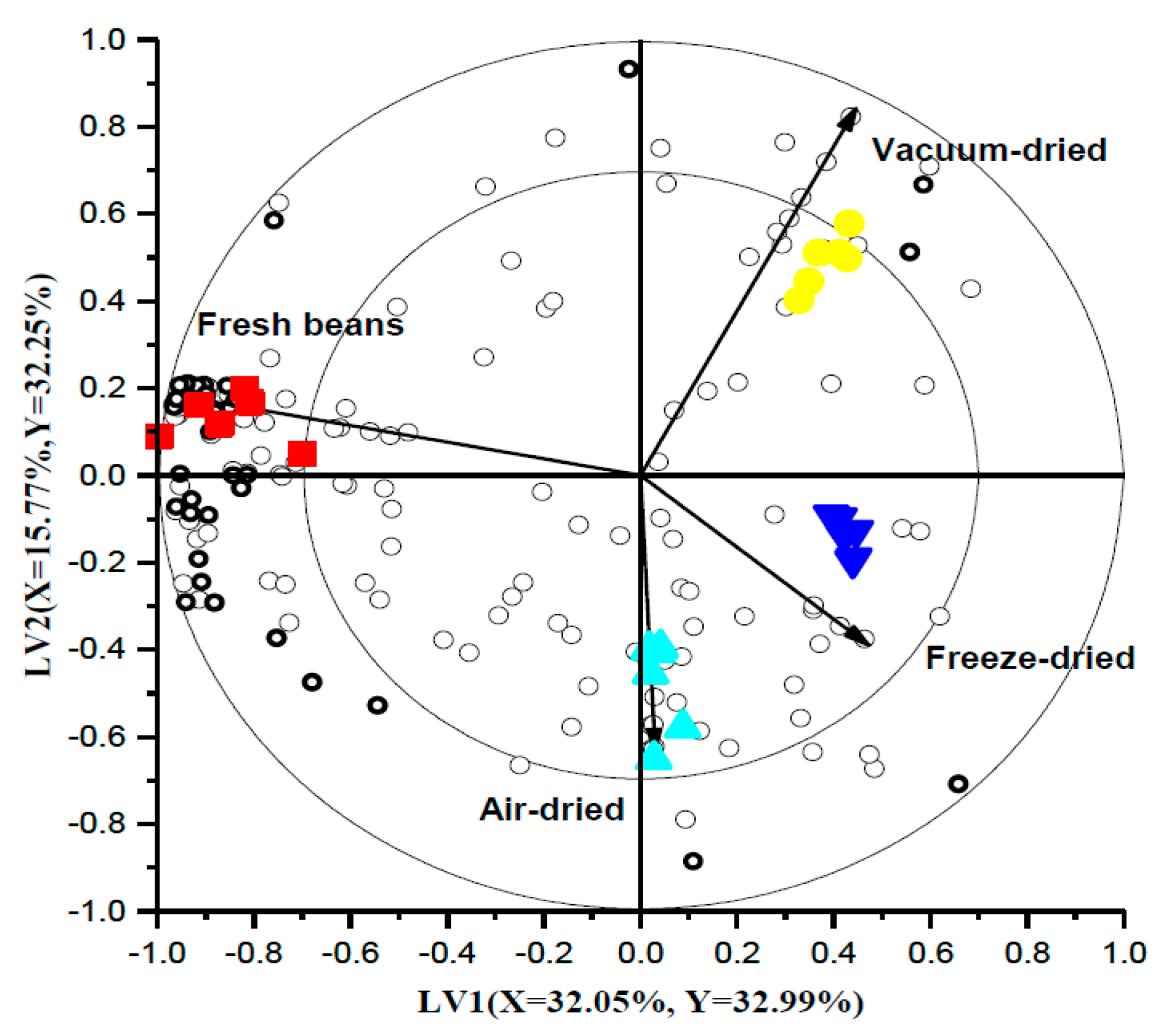
3.7. Untargeted Volatile Fingerprinting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ulloa, J.A.; Ibarra-Zavala, S.J.; Ramírez-Salas, S.P.; Rosas-Ulloa, P.; Ramírez-Ramírez, J.C.; Ulloa-Rangel, B.E. Chemical, physicochemical, nutritional, microbiological, sensory and rehydration characteristics of instant whole beans (Phaseolus vulgaris). Food Technol. Biotechnol. 2015, 53, 48–56. [Google Scholar] [CrossRef]
- Gonzalez, E.; Paredes-Lapez, O. Hard-to-cook phenomenon in common beans—A review. Crit. Rev. Food Sci. Nutr. 1993, 33, 227–286. [Google Scholar] [CrossRef]
- Njoroge, D.M.; Kinyanjui, P.K.; Makokha, A.O.; Christiaens, S.; Shpigelman, A.; Sila, D.N.; Hendrickx, M.E. Extraction and characterization of pectic polysaccharides from easy- and hard-to-cook common beans (Phaseolus vulgaris). Food Res. Int. 2014, 64, 314–322. [Google Scholar] [CrossRef]
- Aseete, P.; Katungi, E.; Bonabana-Wabbi, J.; Birachi, E.; Ugen, M.A. Consumer demand heterogeneity and valuation of value-added pulse products: A case of precooked beans in Uganda. Agric. Food Secur. 2018, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Del Valle, F.R.; Marco, E. Production of Quality Quick-Cooking Beans By a Cooking/Dehydration Process. J. Food Process. Preserv. 1988, 12, 83–93. [Google Scholar] [CrossRef]
- Zhao, B.; Chang, K.C. Evaluation of effects of soaking and precooking conditions on the quality of precooked dehydrated pea, lentil and chickpea products. J. Food Process. Preserv. 2008, 32, 517–532. [Google Scholar] [CrossRef]
- Su, H.L.; Chang, K.C. Dehydrated Precooked Pinto Bean Quality as Affected by Cultivar and Coating Biopolymers. J. Food Sci. 1995, 60, 1330–1332. [Google Scholar] [CrossRef]
- Cai, T.D.; Chang, K.C. Processing to improve quality of dehydrated precooked pinto beans. J. Food Sci. 1997, 62, 141–144. [Google Scholar] [CrossRef]
- Schoeninger, V.; Coelho, S.R.M.; Christ, D.; Sampaio, S.C. Processing parameter optimization for obtaining dry beans with reduced cooking time. LWT Food Sci. Technol. 2014, 56, 49–57. [Google Scholar] [CrossRef]
- Krokida, M.K.; Philippopoulos, C. Rehydration of dehydrated foods. Dry. Technol. 2005, 23, 799–830. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S. Microstructure and Its Relationship with Quality and Storage Stability of Dried Foods; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081007648. [Google Scholar]
- Prothon, F.; Ahrné, L.; Sjöholm, I. Mechanisms and Prevention of Plant Tissue Collapse during Dehydration: A Critical Review. Crit. Rev. Food Sci. Nutr. 2003, 43, 447–479. [Google Scholar] [CrossRef]
- Ondier, G.O.; Siebenmorgen, T.J.; Mauromoustakos, A. Low-temperature, low-relative humidity drying of rough rice. J. Food Eng. 2010, 100, 545–550. [Google Scholar] [CrossRef]
- Chigwedere, C.M.; Humerez Flores, J.N.; Panozzo, A.; Van Loey, A.M.; Hendrickx, M.E. Instability of common beans during storage causes hardening: The role of glass transition phenomena. Food Res. Int. 2019, 121, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Gowen, A.A.; Abu-Ghannam, N.; Frias, J.M.; Barat, J.M.; Andres, A.M.; Oliveira, J.C. Comparative study of quality changes occurring on dehydration and rehydration of cooked chickpeas (Cicer arietinum L.) subjected to combined microwave-convective and convective hot air dehydration. J. Food Sci. 2006, 71. [Google Scholar] [CrossRef]
- Benseddik, A.; Azzi, A.; Zidoune, M.N.; Khanniche, R.; Besombes, C. Empirical and diffusion models of rehydration process of differently dried pumpkin slices. J. Saudi Soc. Agric. Sci. 2019, 18, 401–410. [Google Scholar] [CrossRef]
- Parthasarathi, S.; Anandharamakrishnan, C. Modeling of shrinkage, rehydration and textural changes for food structural analysis: A review. J. Food Process. Eng. 2014, 37, 199–210. [Google Scholar] [CrossRef]
- Peleg, M. An Empirical Model for the Description of Moisture Sorption Curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- Srikiatden, J.; Roberts, J.S. Moisture loss kinetics of apple during convective hot air and isothermal drying. Int. J. Food Prop. 2005, 8, 493–512. [Google Scholar] [CrossRef] [Green Version]
- Jian, F.; Jayas, D.S. Characterization of isotherms and thin-layer drying of red kidney beans, part i: Choosing appropriate empirical and semitheoretical models. Dry. Technol. 2018, 36, 1696–1706. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 1st ed.; Oxford University Press: Oxford, UK, 1964; ISBN 9780198534112. [Google Scholar]
- Van Boekel, M.A.J.S. Kinetic modeling of food quality: A critical review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 144–158. [Google Scholar] [CrossRef]
- Gwala, S.; Wainana, I.; Pallares Pallares, A.; Kyomugasho, C.; Hendrickx, M.; Grauwet, T. Texture and interlinked post-process microstructures determine the in vitro starch digestibility of Bambara groundnuts with distinct hard-to-cook levels. Food Res. Int. 2019, 120, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Zhang, X.; Copeland, L.; Wang, S. Retrogradation enthalpy does not always reflect the retrogradation behavior of gelatinized starch. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.T.; Hai Dang, D.N.; Buvé, C.; Grauwet, T.; Van Loey, A.; Hu, X.; Hendrickx, M. Quality change during high pressure processing and thermal processing of cloudy apple juice. LWT Food Sci. Technol. 2017, 75, 85–92. [Google Scholar] [CrossRef]
- Chigwedere, C.M.; Tadele, W.W.; Yi, J.; Wibowo, S.; Kebede, B.T.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Insight into the evolution of flavor compounds during cooking of common beans utilizing a headspace untargeted fingerprinting approach. Food Chem. 2019, 275, 224–238. [Google Scholar] [CrossRef]
- Vervoort, L.; Grauwet, T.; Njoroge, D.M.; Van Der Plancken, I.; Matser, A.; Hendrickx, M.; Van Loey, A. Comparing thermal and high pressure processing of carrots at different processing intensities by headspace fingerprinting. Innov. Food Sci. Emerg. Technol. 2013, 18, 31–42. [Google Scholar] [CrossRef]
- Kebede, B.T.; Grauwet, T.; Tabilo-Munizaga, G.; Palmers, S.; Vervoort, L.; Hendrickx, M.; Van Loey, A. Headspace components that discriminate between thermal and high pressure high temperature treated green vegetables: Identification and linkage to possible process-induced chemical changes. Food Chem. 2013, 141, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Joardder, M.U.H.; Kumar, C.; Karim, M.A. Food structure: Its formation and relationships with other properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1190–1205. [Google Scholar] [CrossRef]
- Oikonomopoulou, V.P.; Krokida, M.K.; Karathanos, V.T. The influence of freeze drying conditions on microstructural changes of food products. Procedia Food Sci. 2011, 1, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Piergiovanni, A.R. Kinetic of water adsorption in common bean: Considerations on the suitability of peleg’s model for describing bean hydration. J. Food Process. Preserv. 2011, 35, 447–452. [Google Scholar] [CrossRef]
- Shafaei, S.M.; Masoumi, A.A.; Roshan, H. Analysis of water absorption of bean and chickpea during soaking using Peleg model. J. Saudi Soc. Agric. Sci. 2016, 15, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Johnny, S.; Razavi, S.M.A.; Khodaei, D. Hydration kinetics and physical properties of split chickpea as affected by soaking temperature and time. J. Food Sci. Technol. 2015, 52, 8377–8382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Quiroga, E.; Prosapio, V.; Fryer, P.J.; Norton, I.T.; Bakalis, S. Model discrimination for drying and rehydration kinetics of freeze-dried tomatoes. J. Food Process. Eng. 2020, 43, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ulloa, J.A.; Bonilla-Sánchez, C.R.; Ortíz-Jiménezc, M.A.; Rosas-Ulloa, P.; Ramírez-Ramírez, J.C.; Ulloa-Rangel, B.E. Rehydration properties of precooked whole beans (Phaseolus vulgaris) dehydrated at room temperature. CYTA J. Food 2013, 11, 94–99. [Google Scholar] [CrossRef]
- Resende, O.; Corrêa, P.C.; Jarén, C.; Moure, A.J. Bean moisture diffusivity and drying kinetics: A comparison of the liquid diffusion model when taking into account and neglecting grain shrinkage. Span. J. Agric. Res. 2007, 5, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Berk, Z. Food Process. Engineering and Technology; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780123736604. [Google Scholar]
- Marabi, A.; Saguy, I.S. Effect of porosity on rehydration of dry food particulates. J. Sci. Food Agric. 2004, 84, 1105–1110. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Azarnia, S.; Simpson, B.K. Volatile Flavor Profile of Saskatchewan Grown Pulses as Affected by Different Thermal Processing Treatments. Int. J. Food Prop. 2016, 19, 2251–2271. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.K.; Tripathi, J.; Gupta, S.; Variyar, P.S. Effect of cooking on aroma profile of red kidney beans (Phaseolus vulgaris) and correlation with sensory quality. Food Chem. 2017, 215, 401–409. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Dings, L.; Aprea, E.; Odake, S. Comparison of volatile flavour profiles of kidney beans and soybeans by GC-MS and PTR-MS. Food Sci. Technol. Res. 2005, 11, 63–70. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Roozen, J.P.; Posthumus, M.A. Instrumental and sensory evaluation of the flavour of dried French beans (Phaseolus vulgaris) influenced by storage conditions. J. Sci. Food Agric. 1995, 69, 393–401. [Google Scholar] [CrossRef]
- Kebede, B.T.; Grauwet, T.; Palmers, S.; Vervoort, L.; Carle, R.; Hendrickx, M.; Van Loey, A. Effect of high pressure high temperature processing on the volatile fraction of differently coloured carrots. Food Chem. 2014, 153, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Adamiec, J.; Rössner, J.; Velíšek, J.; Cejpek, K.; Šavel, J. Minor Strecker degradation products of phenylalanine and phenylglycine. Eur. Food Res. Technol. 2001, 212, 135–140. [Google Scholar] [CrossRef]
- Koutidou, M.; Grauwet, T.; Van Loey, A.; Acharya, P. Potential of different mechanical and thermal treatments to control off-flavour generation in broccoli puree. Food Chem. 2017, 217, 531–541. [Google Scholar] [CrossRef]
- Oomah, B.D.; Liang, L.S.Y.; Balasubramanian, P. Volatile compounds of dry beans (Phaseolus vulgaris L.). Plant Foods Hum. Nutr. 2007, 62, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Landaud, S.; Helinck, S.; Bonnarme, P. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 2008, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Van Ruth, S.M.; Dings, L.; Buhr, K.; Posthumus, M.A. In vitro and in vivo volatile flavour analysis of red kidney beans by proton transfer reaction-mass spectrometry. Food Res. Int. 2004, 37, 785–791. [Google Scholar] [CrossRef]
- Oikonomopoulou, V.P.; Krokida, M.K. Novel Aspects of Formation of Food Structure during Drying. Dry. Technol. 2013, 31, 990–1007. [Google Scholar] [CrossRef]



| Peleg Model | Weibull Model | Fick’s Diffusion Model | |||||
|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | Kref (min %−) | Ea (kJ/mol) | Kref (min) | Ea (kJ/mol) | Deff0 × 10−8 (m2/s) | Geometric Factor | |
| VD | 13.32 ± 1.76 | 0.020 ± 0.00 | 14.37 ± 1.31 | 4.52 ± 0.06 | 14.70 ± 1.31 | 6.533 ± 0.0766 | 6.53 ± 0.07 |
| AD | 4.36 ± 3.01 | 0.017 ± 0.00 | 6.90 ± 1.92 | 4.77 ± 0.11 | 8.99 ± 1.54 | 5.53 ± 0.0704 | 5.53 ± 0.07 |
| FD | 2.05 ± 1.58 | 0.002 ± 0.00 | 2.86 ± 1.18 | 0.61 ± 0.008 | 3.45 ± 1.59 | 6.093 ± 0.0912 | 6.09 ± 0.09 |
| Control | Air-Dried | Vacuum-Dried | Freeze-Dried | |
|---|---|---|---|---|
| L* | 24.42 ± 0.89 b | 20.72 ± 0.93 a | 19.24 ± 0.58 a | 31.32 ± 1.55 c |
| a* | 18.31 ± 0.53 c | 8.76 ± 0.45 a | 9.10 ± 0.13 a | 12.0 ± 0.83 b |
| b* | 8.65 ± 0.74 c | 2.73 ± 0.24 a | 2.70 ±0.02 a | 4.50 ±0.47 b |
| ΔE* | - | 11.85 ± 0.68 a | 12.14 ± 0.35 a | 10.26 ± 1.14 a |
| ΔEr* | - | 0.96 ± 0.05 a | 3.55 ± 1.04 ab | 2.20 ± 0.99 b |
| H (N) | 64.82 ± 11.40 a | 62.90 ± 12.86 a | 70.50 ± 13.50 a | 48.21 ± 9.74 b |
| TG (°C) | 79.74 ± 0.06 b | 57.57 ± 0.71 a | 64.76 ± 0.74 c | 58.33 ± 0.54 a |
| DR (%) | - | 44.79 ± 1.62 b | 28.89 ± 1.47 a | 27.04 ± 0.70 a |
| Y | - | 2.30 ± 0.013 a | 2.28 ± 0.043 a | 2.56 ± 0.001 b |
| RRM | - | 0.96 ± 0.008 a | 0.93 ± 0.029 a | 1.12 ± 0.001 b |
| VID | Compound | RI | Chemical Group |
|---|---|---|---|
| Freshly cooked beans | |||
| 0.994 | 2-Acetyl-3,5-dimethylfuran | 1589 | Furan compound |
| 0.993 | 1-[2-(1-Methylethylidene) cyclopropyl] ethanone | 1341 | Ketone |
| 0.992 | 2,4,4-Trimethyl-3-(3-methylbutyl) cyclohex-2-enone | 1760 | Ketone |
| 0.989 | 2-ethanol | 1719 | Alcohol |
| 0.987 | 4-methyl-3-penten-2-one | 1134 | Ketone |
| 0.979 | Tetramethylfuran | 1650 | Furan compound |
| 0.974 | 3-(2,4-Dinitrophenyl) propanoic acid | 1323 | Organic acid |
| 0.965 | Octyl ester cyclopentane carboxylic acid | 1453 | Ester |
| 0.956 | 1-Oxacyclododecan-2,8-dione | 1330 | Ketone |
| 0.950 | 5-methyl-5-(1-methylethyl)-3-Heptyne-2,6-dione | 1434 | Ketone |
| 0.943 | 5-Hepten-2-one | 1330 | Ketone |
| 0.942 | 2-Hydroxy-3,5-dimethylcyclopent-2-en-1-one | 1319 | Ketone |
| 0.905 | 4-Hydroxybutyric acid | 1657 | Organic acid |
| 0.903 | 2-methyl-1-propanol | 1093 | Alcohol |
| 0.899 | Methanethiol | 867 | Sulfur compound |
| 0.892 | Ethanol | 954 | Alcohol |
| 0.878 | 3-methylfuran | 932 | Furan compound |
| 0.866 | 1-(2,4,6-trimethylphenyl)- ethanone | 1870 | Ketone |
| 0.864 | 2,4-Dimethylfuran | 979 | Furan compound |
| 0.850 | 4-hydroxy-4-methyl-2-pentanone | 1373 | Ketone |
| 0.841 | 2-methyl-butanoic acid | 1685 | Organic acid |
| 0.839 | 2-Hexanol | 1231 | Alcohol |
| 0.826 | 2H-1-Benzopyran derivative (Edulan I) | 1496 | Benzopyran derivatives |
| 0.807 | Ethylhexyl benzoate | 1728 | Ester |
| Air-dried beans | |||
| 0.977 | n-Hexane | 859 | Alkane |
| 0.965 | Nonanal | 1399 | Aldehyde |
| 0.958 | o-Fluoroacetophenone | 1123 | Ketone |
| 0.900 | Methyl cyclopentane | 866 | Cycloalkane |
| 0.898 | Pentanoic acid | 1853 | Organic acid |
| 0.871 | 2-phenyl-ethanol | 1924 | Alcohol |
| 0.869 | 2-ethyl-3-methyl-butanal | 1063 | Aldehyde |
| 0.823 | 3-Methoxy-3-methylbutanol | 1447 | Alcohol |
| 0.767 | 4-Hydroxybenzyl alcohol | 1891 | Alcohol |
| Vacuum-dried beans | |||
| 0.951 | 4-ethyl-benzaldehyde | 1736 | Aldehyde |
| 0.945 | Limonene | 1203 | Terpene |
| 0.841 | 2,9-dimethyl-decane | 1033 | Alkane |
| 0.818 | 2,3,6,7-tetramethyl-octane | 1067 | Alkane |
| −0.816 | Dihydrobarrelene | 1132 | Alkene |
| Freeze-dried beans | |||
| 0.946 | Pentane | 855 | Alkane |
| 0.944 | 3,5-Octadien-2-one | 1535 | Ketone |
| 0.938 | 2-ethyl-1-hexanol | 1502 | Alcohol |
| 0.862 | 3,3-dimethyl-hexane | 1200 | Alkane |
| 0.807 | 1-Octanol | 1566 | Alcohol |
| 0.800 | Methyl Isobutyl Ketone | 1008 | Ketone |
| −0.942 | Acetic acid | 1469 | Organic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aravindakshan, S.; Nguyen, T.H.A.; Kyomugasho, C.; Buvé, C.; Dewettinck, K.; Van Loey, A.; Hendrickx, M.E. The Impact of Drying and Rehydration on the Structural Properties and Quality Attributes of Pre-Cooked Dried Beans. Foods 2021, 10, 1665. https://doi.org/10.3390/foods10071665
Aravindakshan S, Nguyen THA, Kyomugasho C, Buvé C, Dewettinck K, Van Loey A, Hendrickx ME. The Impact of Drying and Rehydration on the Structural Properties and Quality Attributes of Pre-Cooked Dried Beans. Foods. 2021; 10(7):1665. https://doi.org/10.3390/foods10071665
Chicago/Turabian StyleAravindakshan, Shruti, Thi Hoai An Nguyen, Clare Kyomugasho, Carolien Buvé, Koen Dewettinck, Ann Van Loey, and Marc E. Hendrickx. 2021. "The Impact of Drying and Rehydration on the Structural Properties and Quality Attributes of Pre-Cooked Dried Beans" Foods 10, no. 7: 1665. https://doi.org/10.3390/foods10071665
APA StyleAravindakshan, S., Nguyen, T. H. A., Kyomugasho, C., Buvé, C., Dewettinck, K., Van Loey, A., & Hendrickx, M. E. (2021). The Impact of Drying and Rehydration on the Structural Properties and Quality Attributes of Pre-Cooked Dried Beans. Foods, 10(7), 1665. https://doi.org/10.3390/foods10071665