Hot-Melt-Extruded Active Films Prepared from EVOH/Trans-Cinnamaldehyde Blends Intended for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the EVOH-CIN Masterbatch
2.3. Characterization of the EVOH-CIN15 Masterbatch
2.4. Film Formation
2.5. Quantification of Cinnamaldehyde
2.6. Structural and Morphological Properties of Melt Processed EVOH-CIN Films
2.6.1. ATR-FTIR Analysis
2.6.2. Morphological Analysis
2.7. Thermal Characterization
2.7.1. Differential Scanning Calorimetry (DSC)
2.7.2. Thermogravimetric Analysis (TGA)
2.8. Functional Properties of the Films
2.8.1. Film Thickness, Transparency and Color Characterization
2.8.2. Mechanical Tests
2.9. Antioxidant Properties
2.10. Antimicrobial Activity of EVOH-CIN Films
2.10.1. Preparation of Fungal Cultures
2.10.2. Antifungal Activity of Trans-Cinnamaldehyde
2.10.3. Antifungal Activity of the Extruded Films
2.11. Statistical Analysis
3. Results
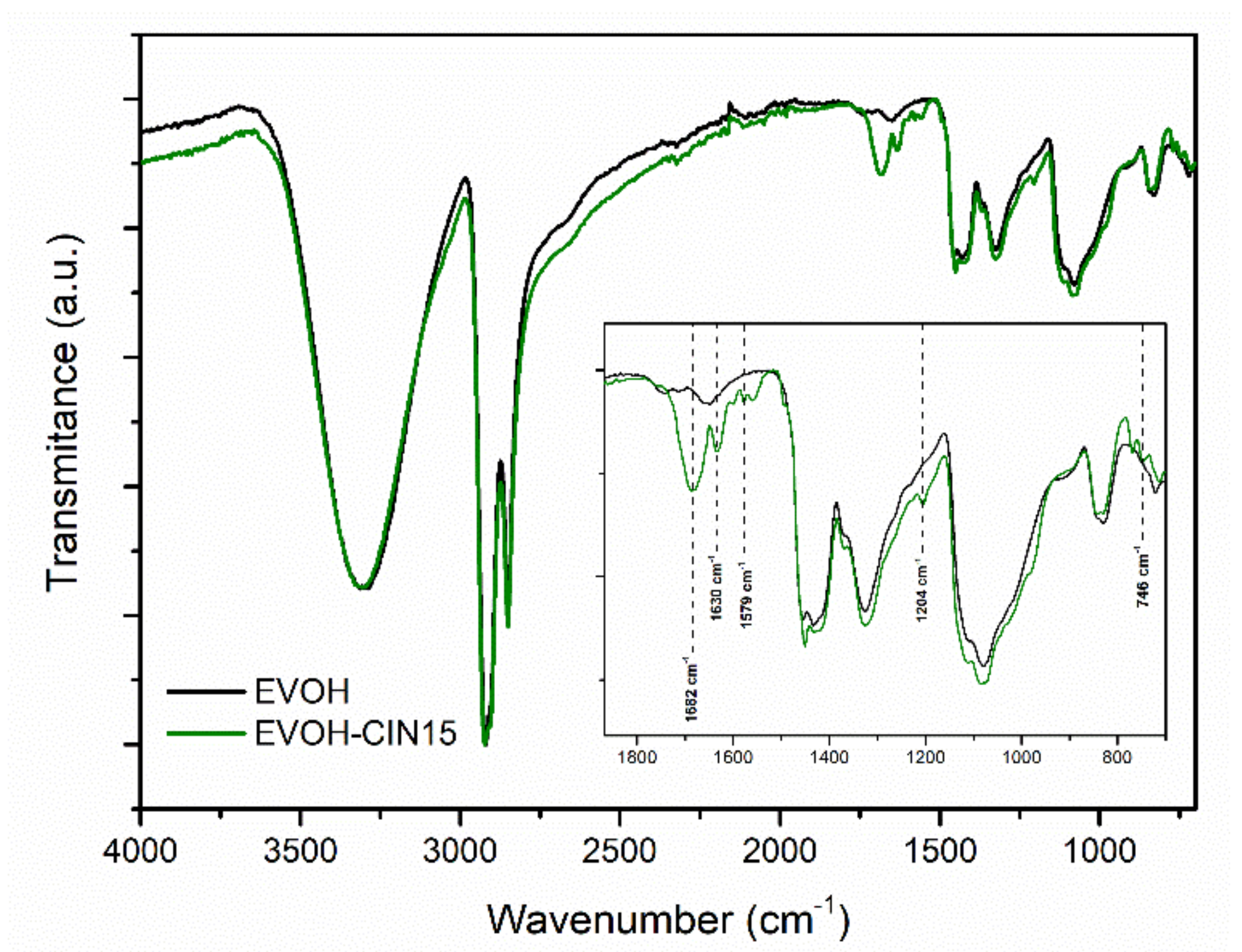
3.1. Characterization of the EVOH-CIN15 Masterbatch
3.2. Quantification of Cinnamaldehyde in EVOH Active Films after Melt-Processing
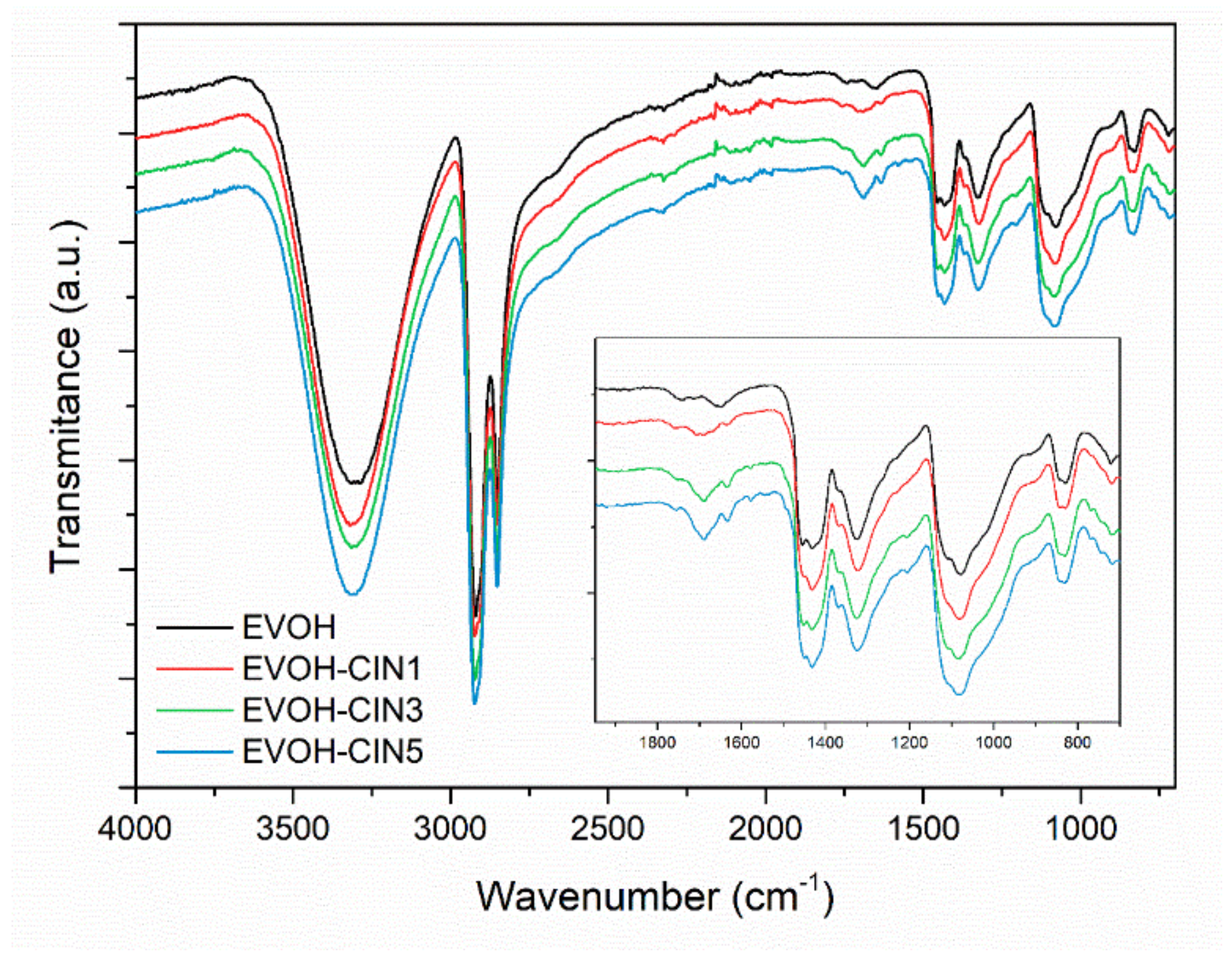
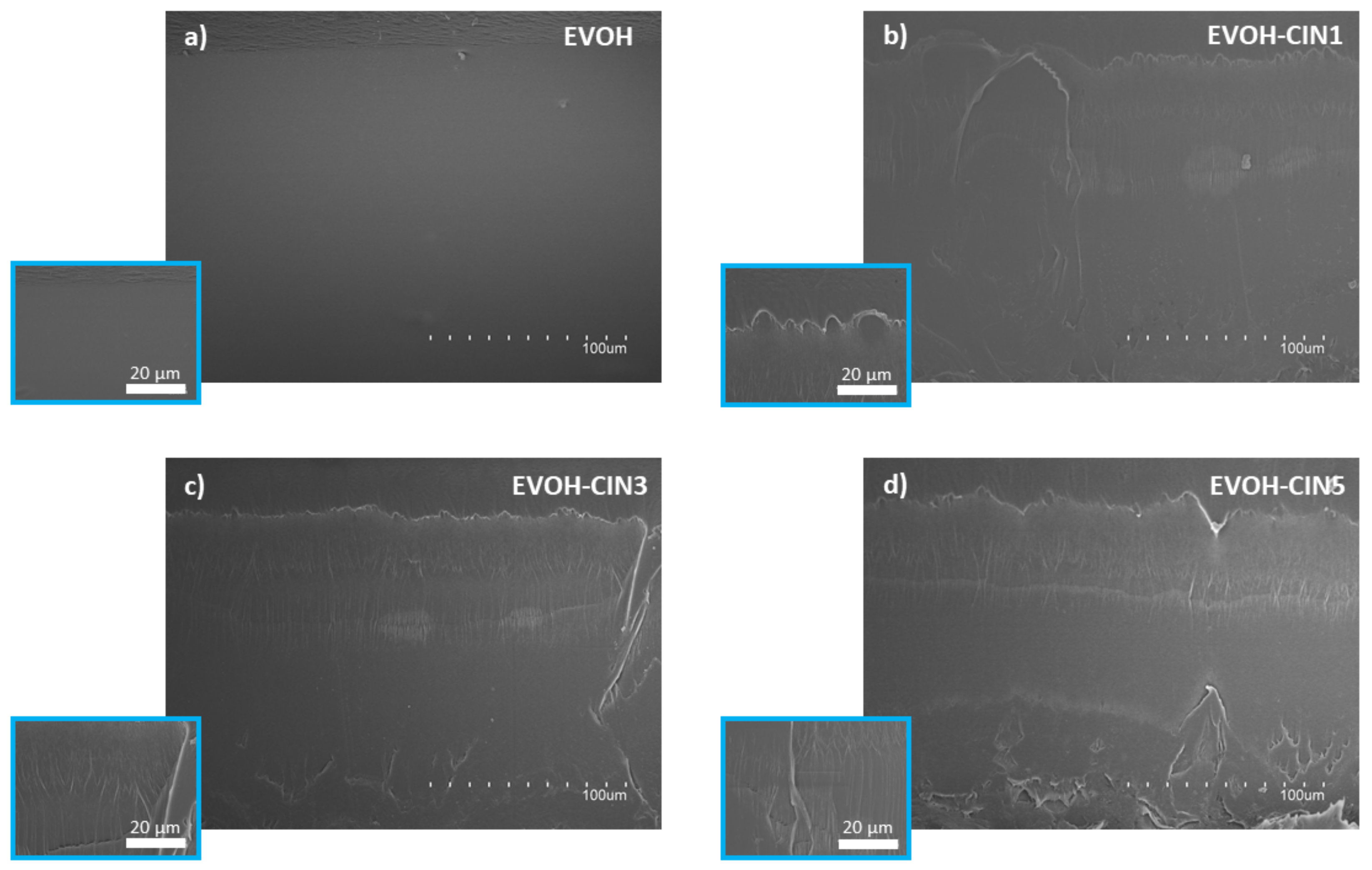
3.3. Structural and Morphological Properties of the Films
3.3.1. FTIR Analysis
3.3.2. SEM Analysis
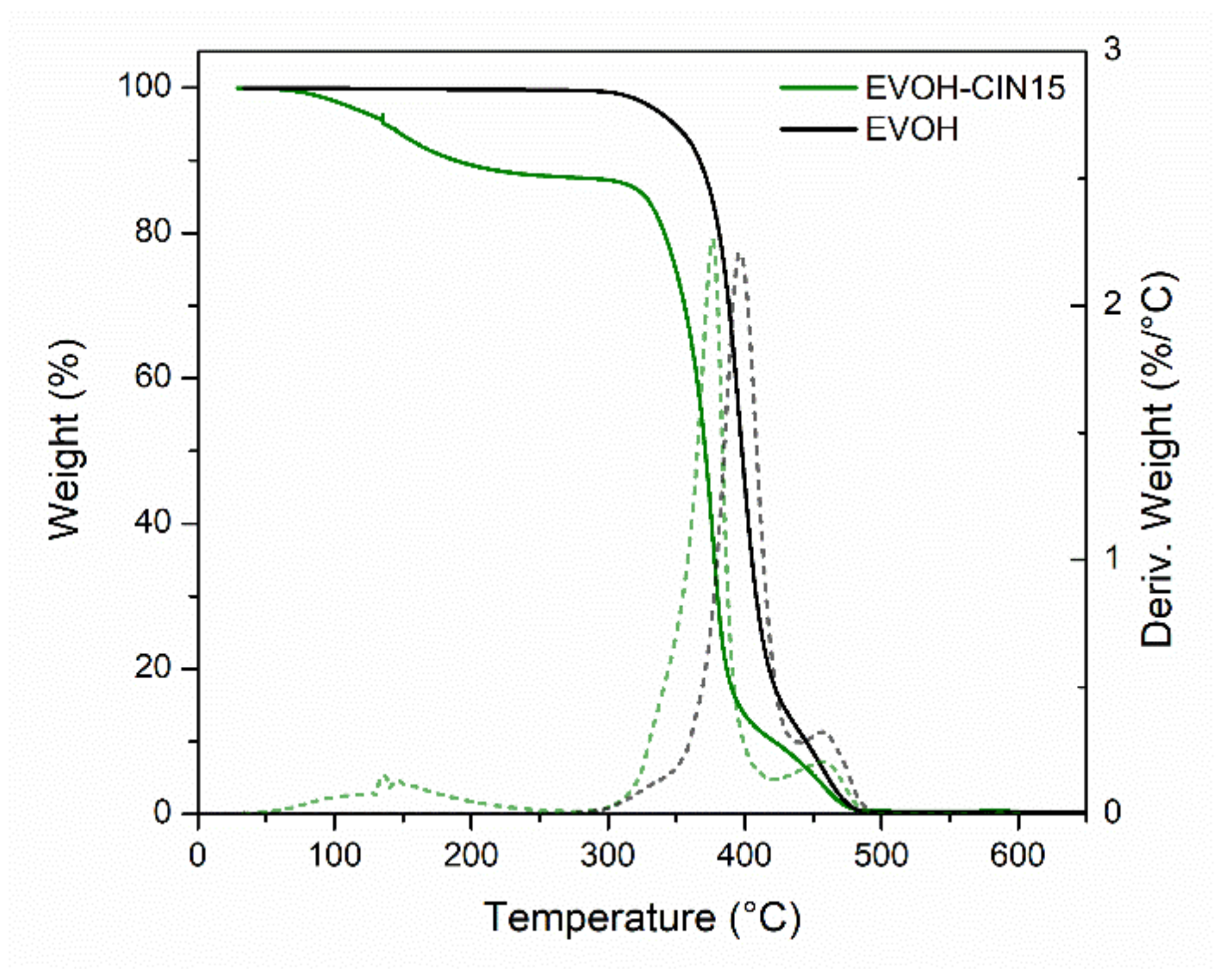
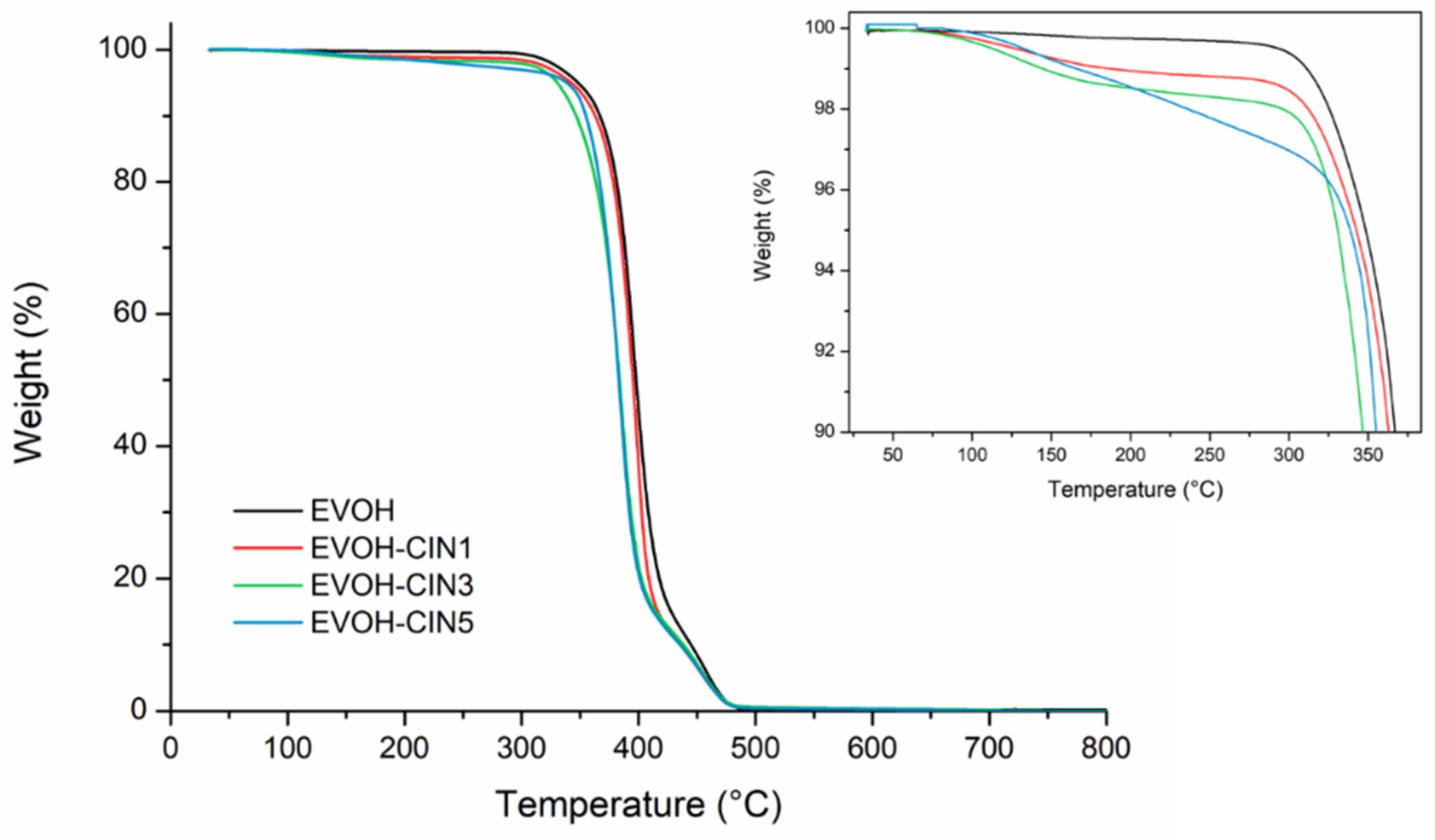
3.4. Film Thermal Properties
3.5. Functional Properties of the Film
3.5.1. Thickness and Optical Properties
3.5.2. Mechanical Properties
3.6. Antioxidant Properties
3.7. Antimicrobial Activity
3.7.1. Antifungal Activity of Cinnamaldehyde
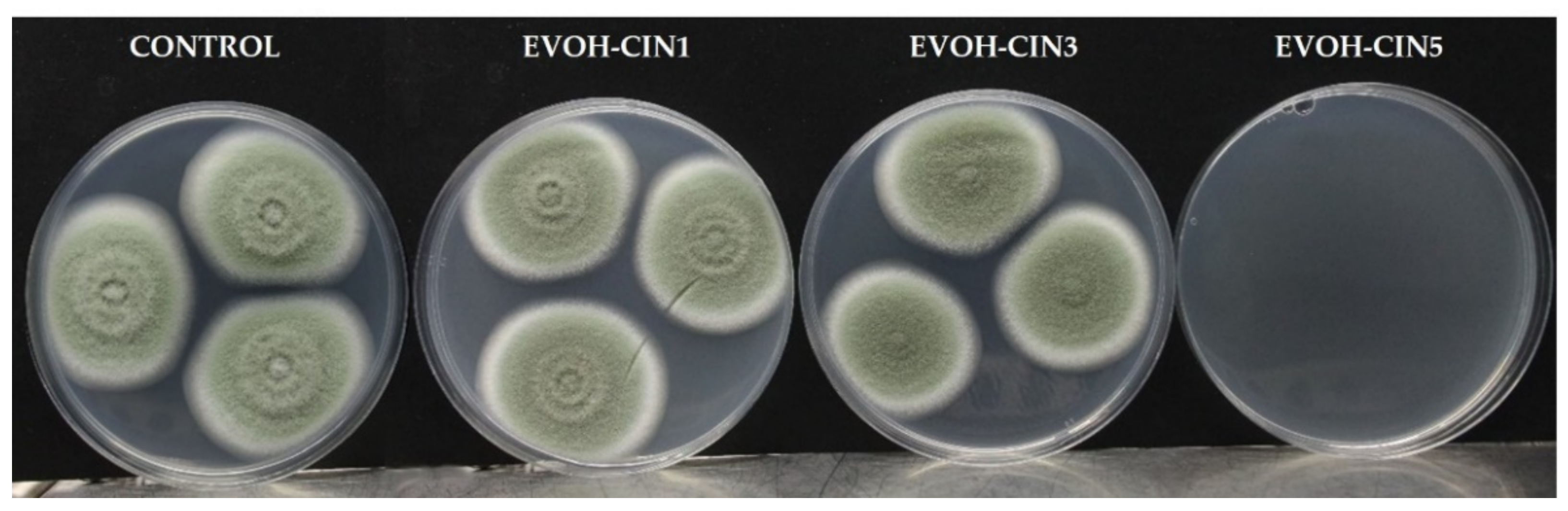
3.7.2. Antifungal Activity of Extruded Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- FUSIONS Estimates of European Food Waste Levels. FUSIONS Reducing Food Waste through Social Innovation. Food Use for Social Innovation by Optimising Waste Prevention Strategies (FUSIONS); IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016; ISBN 978-91-88319-01-2. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste; FAO: Rome, Italy, 2011. [Google Scholar]
- European Commission. Preparatory Study on Food Waste Across Eu 27; Report for the European Commission; European Commission: Brussels, Belgium, 2010; ISBN 9789279221385. [Google Scholar]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragón-Gutiérrez, A.; Rosa, E.; Gallur, M.; López, D.; Hernández-Muñoz, P.; Gavara, R. Melt-Processed Bioactive EVOH Films Incorporated with Ferulic Acid. Polymers 2020, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Heras-Mozos, R.; Muriel-Galet, V.; López-Carballo, G.; Catalá, R.; Hernandez-Munoz, P.; Gavara, R. Development and optimization of antifungal packaging for sliced pan loaf based on garlic as active agent and bread aroma as aroma corrector. Int. J. Food Microbiol. 2019, 290, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Lara Lledó, M.I. Antimicrobial Packaging System for Minimally Processed Fruit; Universitat Politècnica de València: Valencia, Spain, 2016. [Google Scholar]
- Balaguer, M.P.; Lopez-Carballo, G.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Antifungal properties of gliadin films incorporating cinnamaldehyde and application in active food packaging of bread and cheese spread foodstuffs. Int. J. Food Microbiol. 2013, 166, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Trinetta, V.; McDaniel, A.; Batziakas, K.G.; Yucel, U.; Nwadike, L.; Pliakoni, E. Antifungal Packaging Film to Maintain Quality and Control Postharvest Diseases in Strawberries. Antibiotics 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.; Smedsgaard, J.; Frisvad, J. Penicilliumexpansum: Consistent Production of Patulin, Chaetoglobosins, and Other Secondary Metabolites in Culture and Their Natural Occurrence in Fruit Products. J. Agric. Food Chem. 2004, 52, 2421–2428. [Google Scholar] [CrossRef]
- Tannous, J.; Keller, N.P.; Atoui, A.; El Khoury, A.; Lteif, R.; Oswald, I.; Puel, O. Secondary metabolism in Penicillium expansum: Emphasis on recent advances in patulin research. Crit. Rev. Food Sci. Nutr. 2017, 58, 2082–2098. [Google Scholar] [CrossRef]
- Pateiro, M.; Domínguez, R.; Bermúdez, R.; Munekata, P.E.; Zhang, W.; Gagaoua, M.; Lorenzo, J.M. Antioxidant active packaging systems to extend the shelf life of sliced cooked ham. Curr. Res. Food Sci. 2019, 1, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.M.; Sibaja, J.C.; Espitia, P.J.; Otoni, C.G. Antioxidant active packaging based on papaya edible films incorporated with Moringa oleifera and ascorbic acid for food preservation. Food Hydrocoll. 2020, 103, 105630. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Antimicrobial food packaging film based on the release of LAE from EVOH. Int. J. Food Microbiol. 2012, 157, 239–244. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of antimicrobial and antioxidant gelatin/curcumin composite films for active food packaging application. Colloids Surfaces B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Delampasona, M.; Catalan, C.A. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Srisa, A.; Harnkarnsujarit, N. Antifungal films from trans-cinnamaldehyde incorporated poly(lactic acid) and poly(butylene adipate-co-terephthalate) for bread packaging. Food Chem. 2020, 333, 127537. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Sánchez, C.; Batlle, R.; Nerin, C. Vapor-Phase Activities of Cinnamon, Thyme, and Oregano Essential Oils and Key Constituents against Foodborne Microorganisms. J. Agric. Food Chem. 2007, 55, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, L.; Wang, Y. Development of antimicrobial active film containing CINnamaldehyde and its application to snakehead (Ophiocephalus argus) fish. J. Food Process. Eng. 2017, 40, e12554. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.; Gutierrez, M.; Zavalla, E.; Romero, J.; Galotto, M.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. Part B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Fajardo, P.; Gartner, H.; Gómez-Estaca, J.; Gavara, R.; Almenar, E.; Hernandez-Munoz, P. Functional properties and antifungal activity of films based on gliadins containing cinnamaldehyde and natamycin. Int. J. Food Microbiol. 2014, 173, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, L.; Ma, Y.; Mcdonald, T.P.; Wang, Y. Development of active packaging film containing bioactive components encapsulated in β-cyclodextrin and its application. Food Hydrocoll. 2019, 90, 360–366. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Gavara, R.; Hernandez-Munoz, P. Reversible Covalent Immobilization of Cinnamaldehyde on Chitosan Films via Schiff Base Formation and Their Application in Active Food Packaging. Food Bioprocess Technol. 2014, 8, 526–538. [Google Scholar] [CrossRef] [Green Version]
- Lopes, F.A.; Soares, N.D.F.F.; Lopes, C.D.C.P.; Da Silva, W.A.; Júnior, J.C.B.; Medeiros, E.A.A. Conservation of Bakery Products Through Cinnamaldehyde Antimicrobial Films. Packag. Technol. Sci. 2013, 27, 293–302. [Google Scholar] [CrossRef]
- Nam, S.; Scanlon, M.; Han, J.; Izydorczyk, M. Extrusion of Pea Starch Containing Lysozyme and Determination of Antimicrobial Activity. J. Food Sci. 2007, 72, E477–E484. [Google Scholar] [CrossRef] [PubMed]
- Del Nobile, M.; Conte, A.; Buonocore, G.; Incoronato, A.; Massaro, A.; Panza, O. Active packaging by extrusion processing of recyclable and biodegradable polymers. J. Food Eng. 2009, 93, 1–6. [Google Scholar] [CrossRef]
- Lagarón, J.M.; Giménez, E.; Altava, B.; Del-Valle, V.; Gavara, R. Characterization of extruded ethylene-vinyl alcohol copol-ymer based barrier blends with interest in food packaging applications. In Macromolecular Symposia; WILEY-VCH Verlag: Weinheim, Germany, 2003. [Google Scholar]
- Gavara, R.; Catalá, R.; Carballo, G.L.; Cerisuelo, J.P.; Dominguez, I.; Muriel-Galet, V.; Hernandez-Muñoz, P. Use of EVOH for Food Packaging Applications. Ref. Modul. Food Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernandez-Munoz, P.; Gavara, R. Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Dominici, F.; Fortunati, E.; Giovanale, G.; Balestra, G.; Torre, L. Effect of gallic acid and umbelliferone on thermal, mechanical, antioxidant and antimicrobial properties of poly (vinyl alcohol-co-ethylene) films. Polym. Degrad. Stab. 2018, 152, 162–176. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Cejudo-Bastante, C.; Cran, M.J.; Heredia, F.J.; Bigger, S.W. Optical, structural, mechanical and thermal characterization of antioxidant ethylene vinyl alcohol copolymer films containing betalain-rich beetroot. Food Packag. Shelf Life 2020, 24, 100502. [Google Scholar] [CrossRef]
- Molinaro, S.; Romero, M.C.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and PLA optical purity on the characteristics of PLA-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Okada, Y.; Okada, M. Scavenging Effect of Water Soluble Proteins in Broad Beans on Free Radicals and Active Oxygen Species. J. Agric. Food Chem. 1998, 46, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Valdés García, A.; Juárez Serrano, N.; Beltrán Sanahuja, A.; Garrigós, M.C. Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds. Antioxidants 2020, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chaves, M.; Ruiz, C.; Cerrada, M.L.; Fernández-García, M. Novel glycopolymers containing aminosaccharide pendant groups by chemical modification of ethylene–vinyl alcohol copolymers. Polymer 2008, 49, 2801–2807. [Google Scholar] [CrossRef]
- Xu, D.; Lu, J.; Yan, S.; Xiao, R. Aminated EVOH nanofiber membranes for Cr(vi) adsorption from aqueous solution. RSC Adv. 2018, 8, 742–751. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-Q.; Kong, D.-X.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crop. Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Barrera, J.E.; Rodríguez, J.A.; Perilla, J.E.; Algecira, N.A. A study of poly (vinyl alcohol) thermal degradation by thermogravimetry and differential thermogravimetry. Ing. Investig. 2007, 27, 100–105. [Google Scholar]
- Boonruang, K.; Kerddonfag, N.; Chinsirikul, W.; Mitcham, E.J.; Chonhenchob, V. Antifungal effect of poly(lactic acid) films containing thymol and R-(-)-carvone against anthracnose pathogens isolated from avocado and citrus. Food Control. 2017, 78, 85–93. [Google Scholar] [CrossRef]
- Galotto, M.J.; Valenzuela, X.; Rodríguez, F.; Bruna, J.; Guarda, A. Evaluation of the Effectiveness of a New Antimicrobial Active Packaging for Fresh Atlantic Salmon (Salmo Salar L.) Shelf Life. Packag. Technol. Sci. 2011, 25, 363–372. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Wen, Q.-B.; Yang, X.-Q.; Xu, M.-S.; Yin, S.-W. Wettability, surface microstructure and mechanical properties of films based on phosphorus oxychloride-treated zein. J. Sci. Food Agric. 2011, 91, 1222–1229. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, J.; Xue, J. Characterization of antimicrobial poly(lactic acid)/poly(trimethylene carbonate) films with cinnamaldehyde. J. Mater. Sci. 2015, 50, 1150–1158. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Lagarón, J.M.; Gimenez, E.; Cava, D.; Hernández-Muñoz, P.; Yamamoto, T.; Gavara, R. Morphological Alterations Induced by Temperature and Humidity in Ethylene−Vinyl Alcohol Copolymers. Macromolecules 2003, 36, 9467–9476. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Ruseckaite, R.A.; Vázquez, A. Kinetic analysis of thermal degradation in poly (ethylene–vinyl al-cohol) copolymers. J. Appl. Polym. Sci. 2003, 90, 3157–3163. [Google Scholar] [CrossRef]
- Introzzi, L.; Fuentes-Alventosa, J.M.; Cozzolino, C.A.; Trabattoni, S.; Tavazzi, S.; Bianchi, C.L.; Schiraldi, A.; Piergiovanni, L.; Farris, S. “Wetting Enhancer” Pullulan Coating for Antifog Packaging Applications. ACS Appl. Mater. Interfaces 2012, 4, 3692–3700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; La Torre, A.I.R.-D. Antimicrobial, Optical and Mechanical Properties of Chitosan–Starch Films with Natural Extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Promkatkaew, M.; Suramitr, S.; Karpkird, T.; Wanichwecharungruang, S.; Ehara, M.; Hannongbua, S. Photophysical properties and photochemistry of substituted cinnamates and cinnamic acids for UVB blocking: Effect of hydroxy, nitro, and fluoro substitutions at ortho, meta, and para positions. Photochem. Photobiol. Sci. 2014, 13, 583–594. [Google Scholar] [CrossRef]
- Iahnke, A.O.E.S.; Costa, T.M.H.; Rios, A.D.O.; Flôres, S.H. Antioxidant films based on gelatin capsules and minimally processed beet root (Beta vulgaris L. var. Conditiva) residues. J. Appl. Polym. Sci. 2015, 133. [Google Scholar] [CrossRef]
- Suryanti, V.; Wibowo, F.R.; Khotijah, S.; Andalucki, N. Antioxidant Activities of Cinnamaldehyde Derivatives. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Surakarta, Indonesia, 4–5 September 2017; p. 012077. [Google Scholar]
- Naveena, B.; Muthukumar, M.; Sen, A.; Kumar, Y.P.; Kiran, M. Use of Cinnamaldehyde as a Potential Antioxidant in Ground Spent Hen Meat. J. Food Process. Preserv. 2013, 38, 1911–1917. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sharma, A.K.; Pandey, A.K. Medicinal attributes of major phenylpropanoids present in cinnamon. BMC Complement. Altern. Med. 2016, 16, 156. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Q.; Duan, X.; Li, L.; Tao, N. Cinnamaldehyde Exerts Its Antifungal Activity by Disrupting the Cell Wall Integrity of Geotrichum citri-aurantii. Front. Microbiol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, K.; Yang, H.; Yuan, Y.; Yue, T. Antifungal mechanism of cinnamaldehyde and citral combination against Penicillium expansum based on FT-IR fingerprint, plasma membrane, oxidative stress and volatile profile. RSC Adv. 2018, 8, 5806–5815. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M. Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacte-ria in Animal Feeds and Human Foods. J. Agric. Food Chem. 2017, 6, 10406–10423. [Google Scholar] [CrossRef] [PubMed]
- Neri, F.; Mari, M.; Brigati, S. Control of Penicillium expansum by plant volatile compounds. Plant Pathol. 2006, 55, 100–105. [Google Scholar] [CrossRef]
- Tunç, S.; Chollet, E.; Chalier, P.; Preziosi-Belloy, L.; Gontard, N. Combined effect of volatile antimicrobial agents on the growth of Penicillium notatum. Int. J. Food Microbiol. 2007, 113, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Ouyang, Q.; Tao, N. Effect of applying cinnamaldehyde incorporated in wax on green mould decay in citrus fruits. J. Sci. Food Agric. 2018, 98, 527–533. [Google Scholar] [CrossRef] [PubMed]
| Reference | Neat EVOH (g) | EVOH-CIN15 (g) | Cinnamaldehyde (wt.%) |
|---|---|---|---|
| EVOH | 14 | 0 | Not detected |
| EVOH-CIN1 | 13.07 | 0.93 | 0.58 ± 0.02 |
| EVOH-CIN3 | 11.20 | 2.80 | 1.72 ± 0.03 |
| EVOH-CIN5 | 9.30 | 4.70 | 3.12 ± 0.08 |
| First Heating Scan | Cooling Scan | Second Heating Scan | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Formulation | Tg (°C) | Tm (°C) | ∆Hm (J/g) | χc (%) | Tg (°C) | Tc (°C) | ∆Hc (J/g) | Tg (°C) | Tm (°C) | ∆Hm (J/g) | χc (%) |
| EVOH | - | 166 | 79.7 | 37 | 58 | 142 | 71.2 | 54 | 166 | 83.5 | 38 |
| EVOH-CIN1 | 41 | 165 | 69.6 | 32 | 46 | 142 | 63.0 | 49 | 163 | 74.0 | 34 |
| EVOH-CIN3 | 44 | 163 | 62.9 | 30 | 46 | 141 | 63.3 | 48 | 162 | 67.1 | 32 |
| EVOH-CIN5 | 43 | 157 | 60.9 | 29 | 46 | 134 | 53.3 | 48 | 157 | 62.2 | 30 |
| Formulation | ΔW30–300 °C (%) | T5% (°C) | TmaxI (°C) | TmaxII (°C) |
|---|---|---|---|---|
| EVOH | 0.2 | 348 | 397 | 457 |
| EVOH-CIN1 | 1.3 | 342 | 398 | 454 |
| EVOH-CIN3 | 2.1 | 330 | 386 | 453 |
| EVOH-CIN5 | 3.0 | 338 | 384 | 454 |
| Formulation | Thickness (µm) | L | a* | b* | ΔE | YI | Transparency (A600/t) |
|---|---|---|---|---|---|---|---|
| EVOH | 181 ± 3 | 99.1 ± 0.2 | −0.2 ± 0.1 | 0.5 ± 0.1 | - | 0.6 ± 0.1 | 509 ± 4 |
| EVOH-CIN1 | 193 ± 9 | 98.1 ± 0.1 | −1.1 ± 0.1 | 4.2 ± 0.2 | 3.9 ± 0.3 | 6.1 ± 0.3 | 464 ± 11 |
| EVOH-CIN3 | 202 ± 5 | 98.4 ± 0.0 | −1.6 ± 0.1 | 6.0 ± 0.5 | 5.7 ± 0.5 | 8.6 ± 0.7 | 441± 2 |
| EVOH-CIN5 | 204 ± 6 | 97.7 ± 0.3 | −2.8 ± 0.6 | 10.8 ± 2.7 | 10.7 ± 2.8 | 15.2 ± 3.7 | 404 ± 8 |
| Formulation | Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| EVOH | 3.2 ± 0.0 a | 62 ± 1 a | 33 ± 3 a |
| EVOH-CIN1 | 3.3 ± 0.1 a,b | 65 ± 4 b | 31 ± 4 a |
| EVOH-CIN3 | 3.3 ± 0.1 b | 66 ± 1 b | 41 ± 14 b |
| EVOH-CIN5 | 3.3 ± 0.1 b | 69 ± 2 c | 67 ± 4 c |
| Inhibition of P. expansum (%) | ||||
|---|---|---|---|---|
| Time (days) | 3 | 5 | 7 | 10 |
| EVOH-CIN1 | 9.1 ± 3.9 a | 3.5 ± 2.1 a | 2.8 ± 1.6 a | 3.6 ± 2.7 a |
| EVOH-CIN3 | 25.2 ± 8.2 b | 15.1 ± 4.7 b | 7.1 ± 2.9 b | 4.7 ± 3.6 a |
| EVOH-CIN5 | 100 c | 100 c | 100 c | 100 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragón-Gutiérrez, A.; Heras-Mozos, R.; Gallur, M.; López, D.; Gavara, R.; Hernández-Muñoz, P. Hot-Melt-Extruded Active Films Prepared from EVOH/Trans-Cinnamaldehyde Blends Intended for Food Packaging Applications. Foods 2021, 10, 1591. https://doi.org/10.3390/foods10071591
Aragón-Gutiérrez A, Heras-Mozos R, Gallur M, López D, Gavara R, Hernández-Muñoz P. Hot-Melt-Extruded Active Films Prepared from EVOH/Trans-Cinnamaldehyde Blends Intended for Food Packaging Applications. Foods. 2021; 10(7):1591. https://doi.org/10.3390/foods10071591
Chicago/Turabian StyleAragón-Gutiérrez, Alejandro, Raquel Heras-Mozos, Miriam Gallur, Daniel López, Rafael Gavara, and Pilar Hernández-Muñoz. 2021. "Hot-Melt-Extruded Active Films Prepared from EVOH/Trans-Cinnamaldehyde Blends Intended for Food Packaging Applications" Foods 10, no. 7: 1591. https://doi.org/10.3390/foods10071591
APA StyleAragón-Gutiérrez, A., Heras-Mozos, R., Gallur, M., López, D., Gavara, R., & Hernández-Muñoz, P. (2021). Hot-Melt-Extruded Active Films Prepared from EVOH/Trans-Cinnamaldehyde Blends Intended for Food Packaging Applications. Foods, 10(7), 1591. https://doi.org/10.3390/foods10071591










