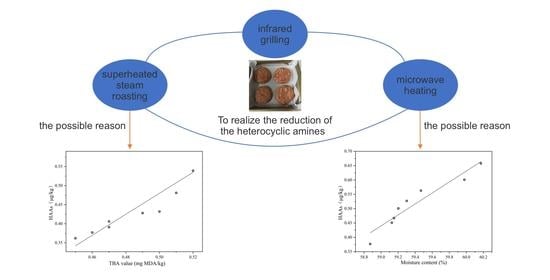
Reduction of the Heterocyclic Amines in Grilled Beef Patties through the Combination of Thermal Food Processing Techniques without Destroying the Grilling Quality Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Cooking of Patties by Different Thermal Processes
2.4. Quantification of HAs
2.5. Moisture Content Analysis
2.6. Determination of TBA Value
2.7. Color Analysis
2.8. Texture Profile Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Thermal Processes on the Production of HAs in Grilled Beef Patties
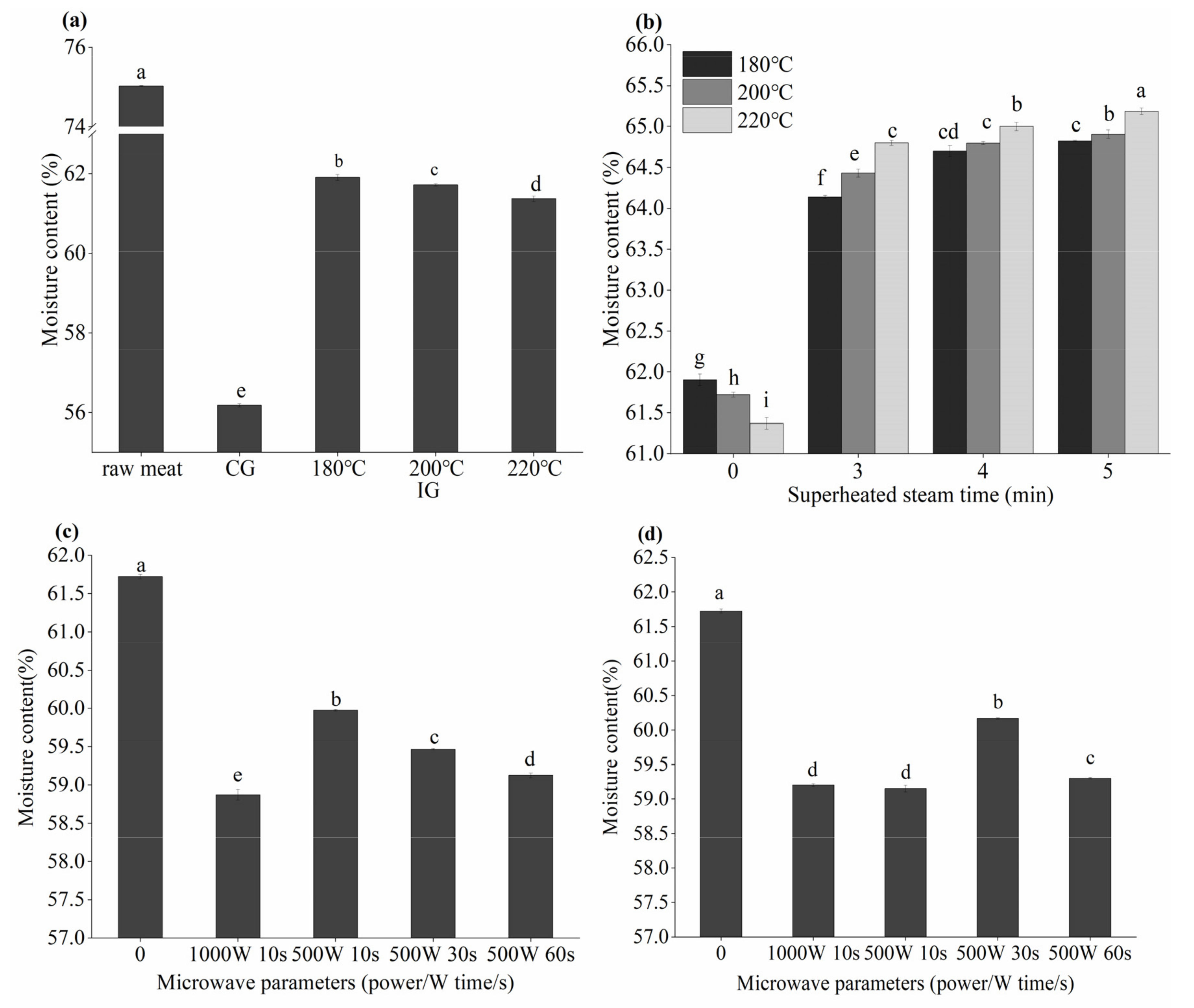
3.2. Effects of Different Thermal Processes on the Moisture Content in Grilled Beef Patties
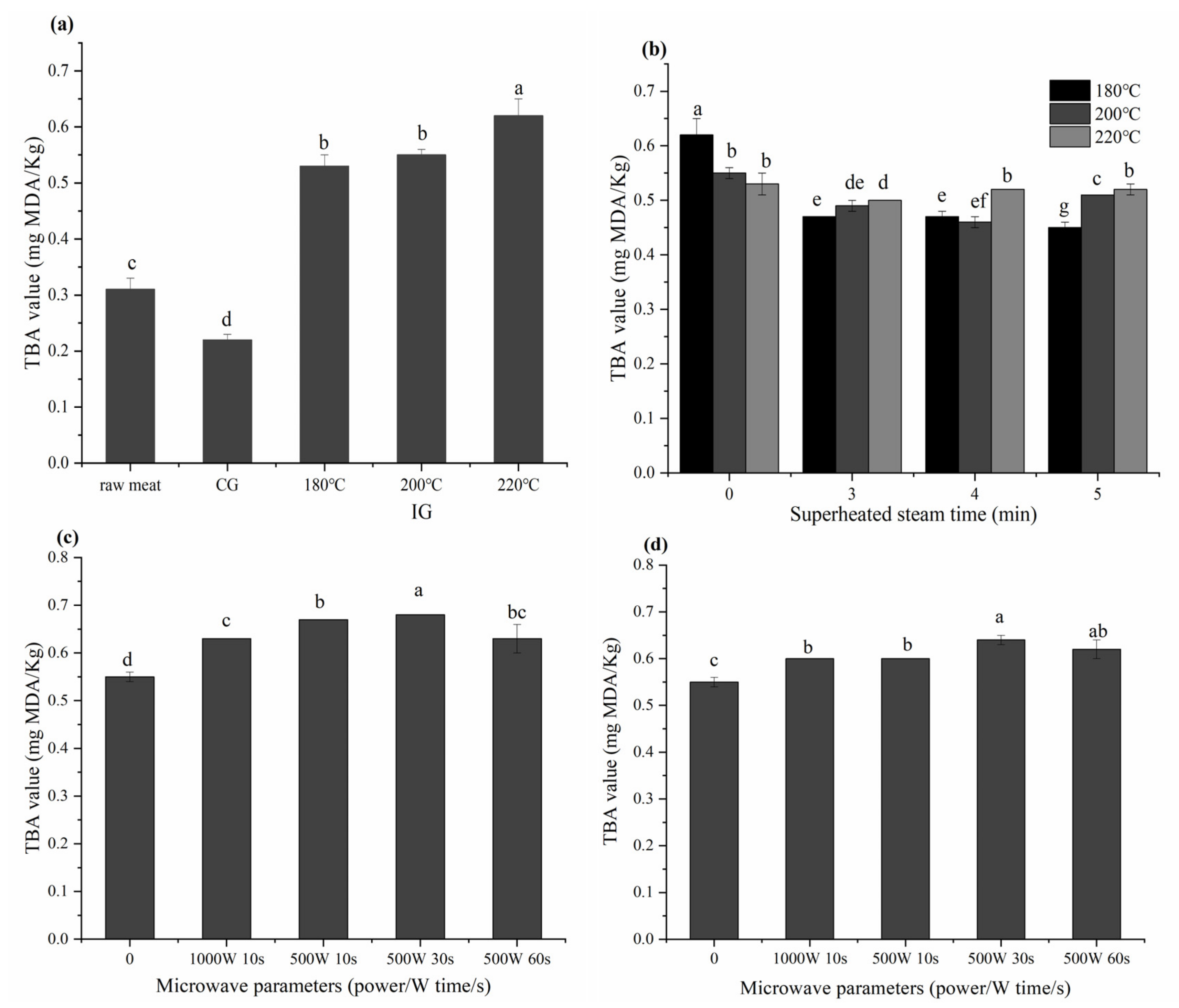
3.3. Effects of Different Thermal Processes on the TBA Value in Grilled Beef Patties
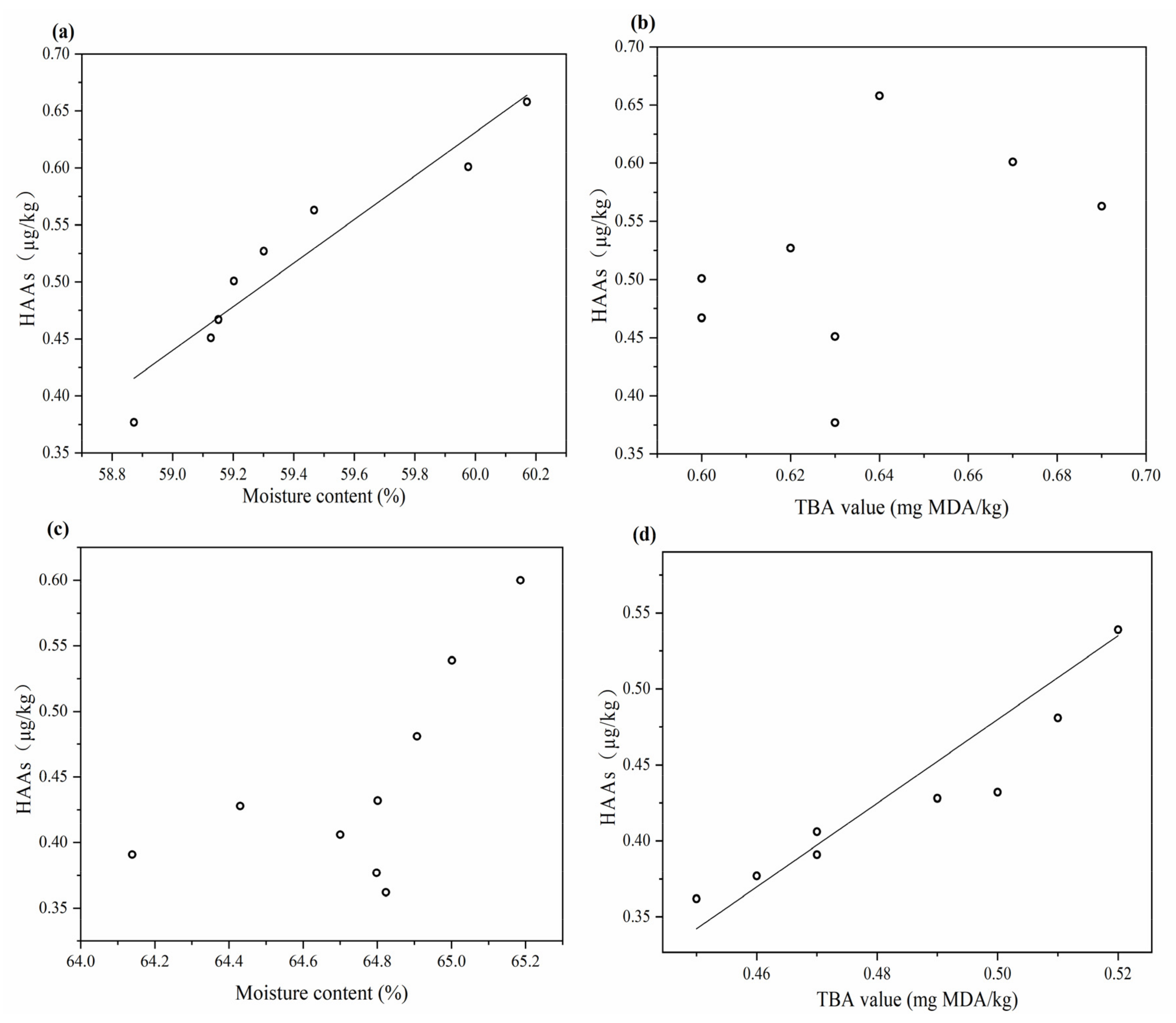
3.4. Correlation Analysis and Regression Analysis of HAs with TBA Value and Moisture Content of Grilled Beef Patties
3.5. Effects of Different Thermal Processes on Quality Characteristics in Grilled Beef Patties
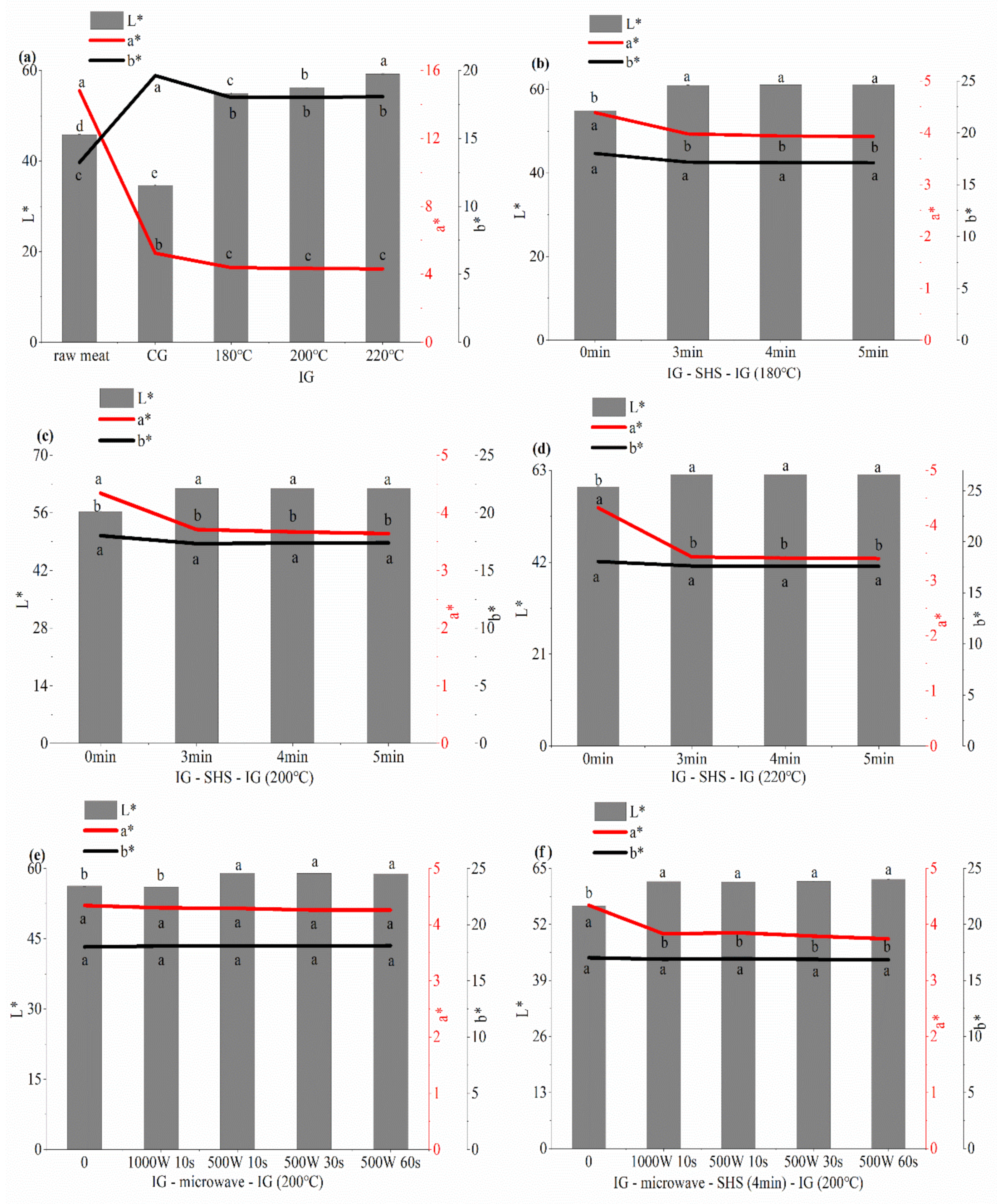
3.5.1. Effects of Different Thermal Processes on the Color of Grilled Beef Patties
3.5.2. Effects of Different Thermal Processes on the Texture of Grilled Beef Patties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A. Thermal processing food-related toxicants: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3579–3596. [Google Scholar] [CrossRef]
- Gibis, M.; Loeffler, M. Effect of Creatine and Glucose on Formation of Heterocyclic Amines in Grilled Chicken Breasts. Foods 2019, 8, 616. [Google Scholar] [CrossRef]
- Gibis, M.; Weiss, J. Antioxidant capacity and inhibitory effect of grape seed and rosemary extract in marinades on the formation of heterocyclic amines in fried beef patties. Food Chem. 2012, 134, 766–774. [Google Scholar] [CrossRef]
- Zeng, M.; He, Z.; Zheng, Z.; Qin, F.; Tao, G. Effect of six Chinese spices on heterocyclic amine profiles in roast beef patties by ultraperformance liquid chromatography– tandem mass spectrometry and principal component analysis. J. Agric. Food Chem. 2014, 62, 9908–9915. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.; Qin, F.; Chen, J.; Cao, D. Inhibitory profiles of spices against free and protein-bound heterocyclic amines of roast beef patties as revealed by ultraperformance liquid chromatography–tandem mass spectrometry and principal component analysis. Food Funct. 2017, 8, 3938–3950. [Google Scholar] [CrossRef] [PubMed]
- Viegas, O.; Novo, P.; Pinto, E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Effect of charcoal types and grilling conditions on formation of heterocyclic aromatic amines (HAs) and polycyclic aromatic hydrocarbons (PAHs) in grilled muscle foods. Food Chem. Toxicol. 2012, 50, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.K. Recent Trends and Developments in Infrared Heating in Food Processing. Crit. Rev. Food Sci. Nutr. 2012, 52, 737–760. [Google Scholar] [CrossRef] [PubMed]
- Haskaraca, G.; Demirok, E.; Kolsarici, N.; Oz, F.; Ozsarac, N. Effect of green tea extract and microwave pre-cooking on the formation of heterocyclic aromatic amines in fried chicken meat products. Food Res. Int. 2014, 63, 373–381. [Google Scholar] [CrossRef]
- Somjai, T.; Achariyaviriya, S.; Achariyaviriya, A.; Namsanguan, K. Strategy for longan drying in two-stage superheated steam and hot air. J. Food Eng. 2009, 95, 313–321. [Google Scholar] [CrossRef]
- Raza, A.; Shabbir, M.A.; Khan, M.I. Effect of Thermal Treatments on the Formation of Heterocyclic Aromatic Amines in Various Meats. J. Food Process. Preserv. 2015, 39, 376–383. [Google Scholar] [CrossRef]
- Omojola, A.B.; Ahmed, S.A.; Attoh-Kotoku, V. Effect of cooking methods on cholesterol, mineral composition and formation of total heterocyclic aromatic amines in Muscovy drake meat. J. Sci. Food Agric. 2015, 95, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Kaban, G.; Kaya, M. Effects of Cooking Techniques and Levels on the Formation of Heterocyclic Aromatic Amines in Chicken and Fish. J. Anim. Vet. Adv. 2010, 9, 1259–1264. [Google Scholar] [CrossRef][Green Version]
- Jinap, S.; Mohd-Mokhtar, M.S.; Farhadian, A. Effects of varying degrees of doneness on the formation of Heterocyclic Aromatic Amines in chicken and beef satay. Meat Sci. 2013, 94, 202–207. [Google Scholar] [CrossRef]
- Jung, K.H.; Shin, H.S. Influence of Microwave Pretreatment on the Formation of Heterocyclic Amines in Fried Beef Patties. Korean J. Food Sci. Anim. Resour. 2009, 29, 719–725. [Google Scholar] [CrossRef]
- Chiu, C.P.; Yang, D.Y.; Chen, B.H. Formation of heterocyclic amines in cooked chicken legs. J. Food Prot. 1998, 61, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Isleroglu, H.; Kemerli, T.; Ozdestan, O. Effect of oven cooking method on formation of heterocyclic amines and quality characteristics of chicken patties: Steam-assisted hybrid oven versus convection ovens. Poult. Sci. 2014, 93, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Suleman, R.; Hui, T.; Wang, Z.; Liu, H. Comparative analysis of charcoal grilling, infrared grilling and superheated steam roasting on the colour, textural quality and heterocyclic aromatic amines of lamb patties. Int. J. Food Sci. Technol. 2019, 55, 1057–1068. [Google Scholar] [CrossRef]
- Hai, D.; Huang, X.Q.; Song, L.J. Effects of different modified atmosphere treatments on lipid oxidation in spiced beef at different storage temperatures. Food Sci. Nutr. 2021, 9, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Felton, J.S.; Knize, M.G.; Hatch, F.T.; Tanga, M.J.; Colvin, M.E. Heterocyclic amine formation and the impact of structure on their mutagenicity. Cancer Lett. 1999, 143, 127–134. [Google Scholar] [CrossRef]
- Polak, T.; Dosler, D.; Zlender, B.; Gasperlin, L. Heterocyclic amines in aged and thermally treated pork longissimus dorsi muscle of normal and PSE quality. LWT Food Sci. Technol. 2009, 42, 504–513. [Google Scholar] [CrossRef]
- Suleman, R.; Wang, Z.; Aadil, R.M.; Hui, T.; Hopkins, D.L.; Zhang, D. Effect of cooking on the nutritive quality, sensory properties and safety of lamb meat: Current challenges and future prospects. Meat Sci. 2020, 167, 108172. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Roskilly, A.P. Quality and Energy Evaluation in Meat Cooking. Food Eng. Rev. 2016, 8, 435–447. [Google Scholar] [CrossRef]
- Oz, F.; Yuzer, M.O. The effects of different cooking methods on the formation of heterocyclic aromatic amines in turkey meat. J. Food Process. Preserv. 2017, 41, e13196. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Avramidis, S.; Weng, X.; Cai, Y.C.; Zhou, Y.D. Influence mechanism of radio frequency heating on moisture transfer and drying stress in larch boxed-heart square timber. Dry. Technol. 2019, 37, 1625–1632. [Google Scholar] [CrossRef]
- Bornez, R.; Linares, M.B.; Vergara, H. Microbial quality and lipid oxidation of Manchega breed suckling lamb meat: Effect of stunning method and modified atmosphere packaging. Meat Sci. 2009, 83, 383–389. [Google Scholar] [CrossRef]
- Meinert, L.; Andersen, L.T.; Bredie, W.L.P.; Bjergegaard, C.; Aaslyng, M.D. Chemical and sensory characterisation of pan-fried pork flavour: Interactions between raw meat quality, ageing and frying temperature. Meat Sci. 2007, 75, 229–242. [Google Scholar] [CrossRef]
- Kondjoyan, A.; Chevolleau, S.; Greve, E.; Gatellier, P.; Sante-Lhoutellier, V.; Bruel, S.; Touzet, C.; Portanguen, S.; Debrauwer, L. Formation of heterocyclic amines in slices of Longissimus thoracis beef muscle subjected to jets of superheated steam. Food Chem. 2010, 119, 19–26. [Google Scholar] [CrossRef]
- Wang, R.; Huang, F.; Zhang, L.; Liu, Q.; Zhang, C.; Zhang, H. Changes in the texture, microstructures, colour and volatile compounds of pork meat loins during superheated steam cooking. Int. J. Food Sci. Technol. 2019, 54, 2821–2830. [Google Scholar] [CrossRef]
- Rababah, T.M.; Feng, H.; Yang, W.; Al-Mahasneh, M.; Ereifej, K.; Al-u’datt, M. Effect of Grape Seed Extracts on Physicochemical and Sensory Properties of Goat Meat Cooked by Conventional Electric or Microwave Ovens. Food Sci. Technol. Res. 2012, 18, 325–332. [Google Scholar] [CrossRef]
- Weber, J.; Bochi, V.C.; Ribeiro, C.P.; Victorio, A.D.; Emanuelli, T. Effect of different cooking methods on the oxidation, proximate and fatty acid composition of silver catfish (Rhamdiaquelen) fillets. Food Chem. 2008, 106, 140–146. [Google Scholar] [CrossRef]
- Broncano, J.M.; Petron, M.J.; Parra, V.; Timon, M.L. Effect of different cooking methods on lipid oxidation and formation of free cholesterol oxidation products (COPs) in Latissimus dorsi muscle of Iberian pigs. Meat Sci. 2009, 83, 431–437. [Google Scholar] [CrossRef]
- Hernandez, P.; Navarro, J.L.; Toldra, F. Lipids of pork meat as affected by various cooking techniques. Food Sci. Technol. Int. 1999, 5, 501–508. [Google Scholar] [CrossRef]
- Persson, E.; Sjoholm, I.; Skog, K. Heat and mass transfer in chicken breasts—Effect on PhIP formation. Eur. Food Res. Technol. 2002, 214, 455–459. [Google Scholar]
- Persson, E.; Sjoholm, I.; Skog, K. Effect of high water-holding capacity on the formation of heterocyclic amines in fried beefburgers. J. Agric. Food Chem. 2003, 51, 4472–4477. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, S. Influence of honey containing marinades on heterocyclic aromatic amine formation and overall mutagenicity in fried pork chops. Food Sci. Biotechnol. 2004, 13, 651–656. [Google Scholar]
- Soladoye, O.P.; Shand, P.; Dugan, M.E.R.; Gariepy, C.; Aalhus, J.L.; Estevez, M.; Juarez, M. Influence of cooking methods and storage time on lipid and protein oxidation and heterocyclic aromatic amines production in bacon. Food Res. Int. 2017, 99, 660–669. [Google Scholar] [CrossRef]
- Zamora, R.; Alcon, E.; Hidalgo, F.J. Effect of lipid oxidation products on the formation of 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine (PhIP) in model systems. Food Chem. 2012, 135, 2569–2574. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. 2-Amino-1-methyl-6-phenylimidazo 4,5-b pyridine (PhIP) formation and fate: An example of the coordinate contribution of lipid oxidation and Maillard reaction to the production and elimination of processing-related food toxicants. RSC Adv. 2015, 5, 9709–9721. [Google Scholar] [CrossRef]
- Sanz Alaejos, M.; Afonso, A.M. Factors That Affect the Content of Heterocyclic Aromatic Amines in Foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 52–108. [Google Scholar] [CrossRef]
- Wu, X.G.; Zhang, Z.G.; He, Z.Y.; Wang, Z.J.; Qin, F.; Zeng, M.M.; Chen, J. Effect of Freeze-Thaw Cycles on the Oxidation of Protein and Fat and Its Relationship with the Formation of Heterocyclic Aromatic Amines and Advanced Glycation End Products in Raw Meat. Molecules 2021, 26, 1264. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Fredholm, L.; Bjerne, I.; Jagerstad, M. Influence of frying fat on the formation of heterocyclic amines in fried beefburgers and pan residues. Food Chem. Toxicol. 1995, 33, 993–1004. [Google Scholar] [CrossRef]
- Zamora, R.; Navarro, J.L.; Aguilar, I.; Hidalgo, F.J. Lipid-derived aldehyde degradation under thermal conditions. Food Chem. 2015, 174, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kondjoyan, A.; Chevolleau, S.; Portanguen, S.; Molina, J.; Ikonic, P.; Clerjon, S.; Debrauwer, L. Relation between crust development and heterocyclic aromatic amine formation when air-roasting a meat cylinder. Food Chem. 2016, 213, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, W.; Zhu, L.; Mao, L.; Zhang, Y. Recent advances in heterocyclic aromatic amines: An update on food safety and hazardous control from food processing to dietary intake. Compr. Rev. Food Sci. Food Saf. 2020, 19, 124–148. [Google Scholar] [CrossRef]
- Kaur, L.; Maudens, E.; Haisman, D.R.; Boland, M.J.; Singh, H. Microstructure and protein digestibility of beef: The effect of cooking conditions as used in stews and curries. LWT Food Sci. Technol. 2014, 55, 612–620. [Google Scholar] [CrossRef]
| Cooking Process | IG | SHS | IG | ||
|---|---|---|---|---|---|
| IG (8 min)-SHS (3 min)-IG (180 °C) | 180 °C | 8 min | 180 °C | 3 min | grill at 180 °C until the center temperature of the patties is 75 °C |
| IG (8 min)-SHS (4 min)-IG (180 °C) | 4 min | ||||
| IG (8 min)-SHS (5 min)-IG (180 °C) | 5 min | ||||
| IG (8 min)-SHS (3 min)-IG (200 °C) | 200 °C | 200 °C | 3 min | grill at 200 °C until the center temperature of the patties is 75 °C | |
| IG (8 min)-SHS (4 min)-IG (200 °C) | 4 min | ||||
| IG (8 min)-SHS (5 min)-IG (200 °C) | 5 min | ||||
| IG (8 min)-SHS (3 min)-IG (220 °C) | 220 °C | 220 °C | 3 min | grill at 220 °C until the center temperature of the patties is 75 °C | |
| IG (8 min)-SHS (4 min)-IG (220 °C) | 4 min | ||||
| IG (8 min)-SHS (5 min)-IG (220 °C) | 5 min | ||||
| Cooking Process | IG | Microwave Heating | IG | |
|---|---|---|---|---|
| IG (8 min)-microwave (1000 w 10 s)-IG (200 °C) | 200 °C, 8 min | 1000 W | 10 s | grill at 200 °C until the center temperature of the patties is 75 °C |
| IG (8 min)-microwave (500 w 10 s)-IG (200 °C) | 500 W | 10 s | ||
| IG (8 min)-microwave (500 w 30 s)-IG (200 °C) | 30 s | |||
| IG (8 min)-microwave (500 w 60 s)-IG (200 °C) | 60 s | |||
| Cooking Process | IG | Microwave Heating | SHS | IG | |
|---|---|---|---|---|---|
| IG (8 min)-microwave (1000 w 10 s)-SHS (4 min)-IG (200 °C) | 200 °C, 8 min | 1000 W | 10 s | 200 °C, 4 min | grill at 200 °C until the center temperature of the patties is 75 °C |
| IG (8 min)-microwave (500 w 10 s)-SHS (4 min)-IG (200 °C) | 500 W | 10 s | |||
| IG (8 min)-microwave (500 w 30 s)-SHS (4 min)-IG (200 °C) | 30 s | ||||
| IG (8 min)-microwave (500 w 60 s)-SHS (4 min)-IG (200 °C) | 60 s | ||||
| Cooking Process | Types of HAs (μg/kg) | Total HAs | ||||
|---|---|---|---|---|---|---|
| 8-MeIQX | Norharman | 4,8-DiMeIQX | Harman | PhIP | (μg/kg) | |
| CG | 0.320 ± 0.061 | 0.242 ± 0.040 | 0.118 ± 0.020 | 0.247 ± 0.014 | 0.166 ± 0.010 | 1.093 ± 0.090 |
| IG (180 °C) | ND | 0.355 ± 0.052 cd | ND | 0.088 ± 0.011 bc | ND | 0.443 ± 0.061 cd |
| IG (200 °C) | ND | 0.544 ± 0.013 aAx | ND | 0.136 ± 0.036 bcAx | ND | 0.679 ± 0.048 abAx |
| IG (220 °C) | ND | 0.501 ± 0.15 ab | ND | 0.235 ± 0.12 a | ND | 0.736 ± 0.27 a |
| IG (8 min)-SHS (3 min)-IG (180 °C) | ND | 0.297 ± 0.033 d | ND | 0.094 ± 0.016 bcA | ND | 0.261 ± 0.23 d |
| IG (8 min)-SHS (3 min)-IG (200 °C) | ND | 0.332 ± 0.028 cd | ND | 0.096 ± 0.021 bc | ND | 0.428 ± 0.042 cd |
| IG (8 min)-SHS (3 min)-IG (220 °C) | ND | 0.335 ± 0.072 cd | ND | 0.097 ± 0.036 bc | ND | 0.432 ± 0.11 cd |
| IG (8 min)-SHS (4 min)-IG (180 °C) | ND | 0.331 ± 0.039 cd | ND | 0.075 ± 0.003 c | ND | 0.406 ± 0.041 cd |
| IG (8 min)-SHS (4 min)-IG (200 °C) | ND | 0.300 ± 0.024 d | ND | 0.077 ± 0.03 c | ND | 0.377 ± 0.046 d |
| IG (8 min)-SHS (4 min)-IG (220 °C) | ND | 0.418 ± 0.020 bc | ND | 0.121 ± 0.021 bc | ND | 0.539 ± 0.041 bcd |
| IG (8 min)-SHS (5 min)-IG (180 °C) | ND | 0.269 ± 0.021 d | ND | 0.093 ± 0.015 bc | ND | 0.362 ± 0.036 d |
| IG (8 min)-SHS (5 min)-IG (200 °C) | ND | 0.364 ± 0.067 cd | ND | 0.117 ± 0.054 bc | ND | 0.481 ± 0.12 cd |
| IG (8 min)-SHS (5 min)-IG (220 °C) | ND | 0.422 ± 0.028 bc | ND | 0.178 ± 0.083 ab | ND | 0.600 ± 0.11 abc |
| IG (8 min)-microwave (1000 w 10 s)-IG (200 °C) | ND | 0.294 ± 0.032 C | ND | 0.083 ± 0.009 B | ND | 0.377 ± 0.039 C |
| IG (8 min)-microwave (500 w 10 s)-IG (200 °C) | ND | 0.462 ± 0.081 AB | ND | 0.139 ± 0.013 A | ND | 0.601 ± 0.092 AB |
| IG (8 min)-microwave (500 w 30 s)-IG (200 °C) | ND | 0.443 ± 0.051 B | ND | 0.119 ± 0.016 AB | ND | 0.563 ± 0.063 B |
| IG (8 min)-microwave (500 w 60 s)-IG (200 °C) | ND | 0.325 ± 0.020 C | ND | 0.126 ± 0.024 A | ND | 0.451 ± 0.042 C |
| IG (8 min)-microwave (1000 w 10 s)-SHS (4 min)-IG (200 °C) | ND | 0.387 ± 0.047 z | ND | 0.114 ± 0.027 y | ND | 0.501 ± 0.074 y |
| IG (8 min)-microwave (500 w 10 s)-SHS (4 min)-IG (200 °C) | ND | 0.383 ± 0.090 z | ND | 0.084 ± 0.003 y | ND | 0.467 ± 0.089 yz |
| IG (8 min)-microwave (500 w 30 s)-SHS (4 min)-IG (200 °C) | ND | 0.538 ± 0.004 y | ND | 0.120 ± 0.006 x | ND | 0.658 ± 0.010 x |
| IG (8 min)-microwave (500 w 60 s)-SHS (4 min)-IG (200 °C) | ND | 0.413 ± 0.048 z | ND | 0.114 ± 0.082 x | ND | 0.527 ± 0.054 y |
| Techniques | Types of HAs | TBA Value | Moisture Content |
|---|---|---|---|
| Microwave | Norharman | 0.408 | 0.936 ** |
| Harman | 0.541 | 0.651 | |
| SHS | Norharman | 0.934 ** | 0.624 |
| Harman | 0.754 * | 0.597 |
| Cooking Process | Parameter | |||||
|---|---|---|---|---|---|---|
| Hardness | Springiness | Cohesiveness | Gumminess | Chewiness | Resilience | |
| CG | 6766.43 ± 18.92 | 0.909 ± 0.02 | 0.832 ± 0.00 | 5629.67 ± 21.85 | 5117.37 ± 18.21 | 0.417 ± 0.01 |
| IG (180 °C) | 4682.62 ± 28.39 g | 0.81 ± 0.00 e | 0.91 ± 0.02 b | 4261.18 ± 21.46 d | 3451.56 ± 19.87 i | 0.37 ± 0.01 bc |
| IG (200 °C) | 4969.90 ± 28.37 dDz | 0.93 ± 0.02 dAz | 0.87 ± 0.04 cBx | 4323.81 ± 28.37 cDz | 4021.14 ± 20.81 eEz | 0.40 ± 0.01 abAx |
| IG (220 °C) | 5217.76 ± 34.42 b | 1.00 ± 0.01 bc | 0.96 ± 0.01 a | 5009.05 ± 15.37 a | 5009.05 ± 18.01 a | 0.40 ± 0.04 ab |
| IG (8 min)-SHS (3 min)-IG (180 °C) | 4781.28 ± 23.91 f | 0.93 ± 0.01 d | 0.83 ± 0.01 d | 3968.46 ± 13.24 f | 3690.67 ± 19.82 g | 0.35 ± 0.02 cd |
| IG (8 min)-SHS (3 min)-IG (200 °C) | 5001.43 ± 27.18 d | 0.99 ± 0.01 bc | 0.83 ± 0.00 d | 4151.19 ± 18.69 e | 4109.68 ± 29.18 d | 0.41 ± 0.00 a |
| IG (8 min)-SHS (3 min)-IG (220 °C) | 5481.71 ± 17.29 a | 1.03 ± 0.00 a | 0.80 ± 0.01 e | 4385.37 ± 13.90 b | 4516.92 ± 19.04 b | 0.43 ± 0.00 a |
| IG (8 min)-SHS (4 min)-IG (180 °C) | 4817.45 ± 27.19 f | 0.93 ± 0.02 d | 0.78 ± 0.02 ef | 3757.61 ± 28.19 h | 3494.57 ± 18.23 h | 0.34 ± 0.01 cd |
| IG (8 min)-SHS (4 min)-IG (200 °C) | 5061.81 ± 18.02 c | 0.98 ± 0.00 c | 0.77 ± 0.00 f | 3897.59 ± 13.91 g | 3819.64 ± 17.26 f | 0.40 ± 0.01 ab |
| IG (8 min)-SHS (4 min)-IG (220 °C) | 5497.82 ± 12.93 a | 1.01 ± 0.02 ab | 0.76 ± 0.01 f | 4178.29 ± 28.16 e | 4220.13 ± 21.16 c | 0.42 ± 0.01 a |
| IG (8 min)-SHS (5 min)-IG (180 °C) | 4908.26 ± 16.26 e | 0.95 ± 0.00 d | 0.73 ± 0.00 g | 3583.03 ± 28.19 j | 3403.88 ± 13.02 j | 0.33 ± 0.03 d |
| IG (8 min)-SHS (5 min)-IG (200 °C) | 5082.27 ± 18.01 c | 1.00 ± 0.03 bc | 0.73 ± 0.00 g | 3710.06 ± 18.21 i | 3710.06 ± 18.27 g | 0.42 ± 0.01 a |
| IG (8 min)-SHS (5 min)-IG (220 °C) | 5513.93 ± 17.91 a | 1.03 ± 0.01 a | 0.71 ± 0.01 g | 3914.89 ± 12.13 g | 4032.34 ± 16.23 e | 0.42 ± 0.00 a |
| IG (8 min)-microwave (1000 w 10 s)-IG (200 °C) | 5312.36 ± 23.19 BC | 0.87 ± 0.01 B | 0.95 ± 0.01 A | 5046.74 ± 13.87 B | 4390.67 ± 15.59 B | 0.39 ± 0.03 A |
| IG (8 min)-microwave (500 w 10 s)-IG (200 °C) | 5289.27 ± 9.25 C | 0.93 ± 0.00 A | 0.96 ± 0.00 A | 5077.70 ± 14.65 AB | 4722.26 ± 15.87 A | 0.40 ± 0.03 A |
| IG (8 min)-microwave (500 w 20 s)-IG (200 °C) | 5330.23 ± 10.23 B | 0.86 ± 0.00 B | 0.94 ± 0.03 A | 5010.42 ± 13.86 C | 4308.96 ± 24.54 C | 0.38 ± 0.01 A |
| IG (8 min)-microwave (500 w 30 s)-IG (200 °C) | 5429.26 ± 18.29 A | 0.83 ± 0.01 C | 0.94 ± 0.02 A | 5103.50 ± 12.87 A | 4235.91 ± 14.53 D | 0.41 ± 0.03 A |
| IG (8 min)-microwave (1000 w 10 s)-SHS (4 min)-IG (200 °C) | 5419.32 ± 17.28 y | 0.97 ± 0.01 y | 0.68 ± 0.01 z | 4446.74 ± 13.94 y | 4390.67 ± 23.98 y | 0.34 ± 0.01 y |
| IG (8 min)-microwave (500 w 10 s)-SHS (4 min)-IG (200 °C) | 5137.29 ± 16.39 z | 0.95 ± 0.00 z | 0.69 ± 0.01 z | 4577.70 ± 12.75 x | 4722.26 ± 34.72 x | 0.31 ± 0.02 z |
| IG (8 min)-microwave (500 w 20 s)-SHS (4 min)-IG (200 °C) | 5440.23 ± 12.38 y | 0.96 ± 0.00 yz | 0.72 ± 0.02 y | 4360.42 ± 17.92 z | 4308.96 ± 29.64 y | 0.34 ± 0.02 y |
| IG (8 min)-microwave (500 w 30 s)-SHS (4 min)-IG (200 °C) | 5567.29 ± 17.29 x | 1.01 ± 0.01 x | 0.82 ± 0.01 x | 4503.50 ± 18.46 x | 4235.91 ± 13.79 z | 0.35 ± 0.00 y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Dong, L.; Zhang, Y.; Yu, H.; Wang, S. Reduction of the Heterocyclic Amines in Grilled Beef Patties through the Combination of Thermal Food Processing Techniques without Destroying the Grilling Quality Characteristics. Foods 2021, 10, 1490. https://doi.org/10.3390/foods10071490
Wang W, Dong L, Zhang Y, Yu H, Wang S. Reduction of the Heterocyclic Amines in Grilled Beef Patties through the Combination of Thermal Food Processing Techniques without Destroying the Grilling Quality Characteristics. Foods. 2021; 10(7):1490. https://doi.org/10.3390/foods10071490
Chicago/Turabian StyleWang, Wei, Lu Dong, Yan Zhang, Huaning Yu, and Shuo Wang. 2021. "Reduction of the Heterocyclic Amines in Grilled Beef Patties through the Combination of Thermal Food Processing Techniques without Destroying the Grilling Quality Characteristics" Foods 10, no. 7: 1490. https://doi.org/10.3390/foods10071490
APA StyleWang, W., Dong, L., Zhang, Y., Yu, H., & Wang, S. (2021). Reduction of the Heterocyclic Amines in Grilled Beef Patties through the Combination of Thermal Food Processing Techniques without Destroying the Grilling Quality Characteristics. Foods, 10(7), 1490. https://doi.org/10.3390/foods10071490