Influence of Lactobacillus paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol
- Take one aluminum foil envelope per day (20 min before dinner or sleeping) with clean drinking water.
- Store the probiotic or placebo in the fridge at 4–6 °C.
- Record the number of aluminum foil envelopes taken each day in the study diary.
- Avoid eating or drinking yoghurt, fermented food, dietary supplements (i.e., vitamins, minerals, nutraceuticals, herbal preparations, probiotics, prebiotics or fish oils).
2.3. Outcome Measures
2.4. Biochemical Measurements
2.5. Gut Permeability
2.6. Inflammation
2.7. Fecal Analysis
2.8. Statistical Analysis
3. Results
3.1. The Effect of L. paracasei HII01 Supplementation on Blood Biochemical Parameters
3.2. The Effect of L. paracasei HII01 Supplementation on the Level of ZO-1 and Inflammation Parameters
3.3. The Effect of L. paracasei HII01 Supplementation on the Level of SCFAs
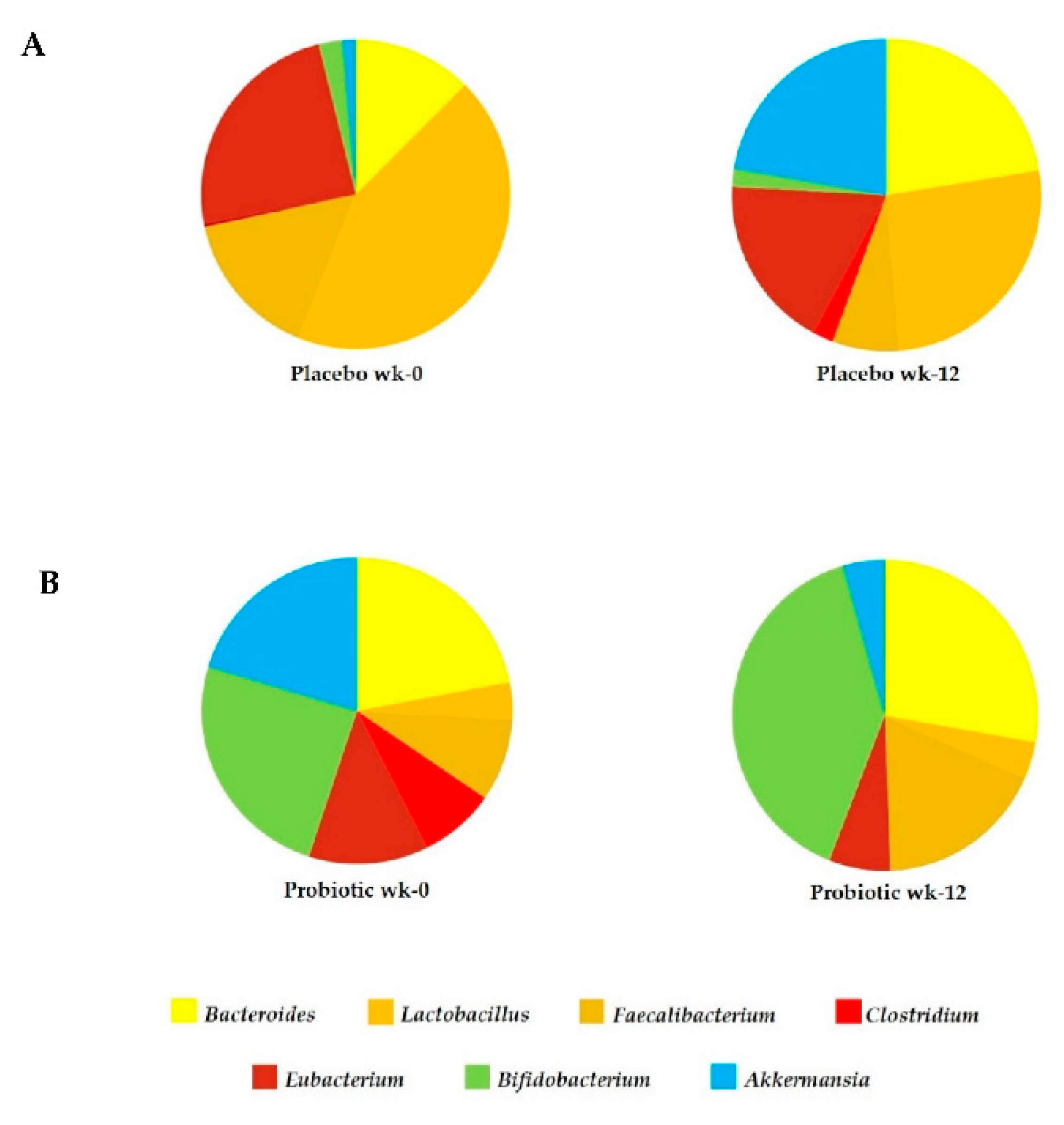
3.4. The Effect of L. paracasei HII01 Supplementation on Microbial Diversity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; IDF: Brussels, Belgium, 2019. [Google Scholar]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pr. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Gomes, A.C.; Bueno, A.A.; Machado de Souza, R.G.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 17, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.M.; Saad, M.J.A. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediat. Inflamm. 2013, 2013, 986734. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.J.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, 851–859. [Google Scholar] [CrossRef]
- Tonucci, L.B.; Olbrich Dos Santos, K.M.; Licursi de Oliveira, L.; Machado Rocha Ribeiro, S.; Stampini Duarte Martino, H. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ansari, M.G.A.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multistrain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [PubMed]
- Wanchai, K.; Yasom, S.; Tunapong, W.; Chunchai, T.; Eaimworawuthikul, S.; Thiennimitr, P.; Chaiyasut, C.; Pongchaidecha, A.; Chatsudthipong, V.; Chattipakorn, S.; et al. Probiotic Lactobacillus paracasei HII01 protects rats against obese-insulin resistance-induced kidney injury and impaired renal organic anion transporter 3 function. Clin. Sci. 2018, 132, 1545–1563. [Google Scholar] [CrossRef]
- Thiennimitr, P.; Yasom, S.; Tunapong, W.; Chunchai, T.; Wanchai, K.; Pongchaidecha, A.; Lungkaphin, A.; Sirilun, S.; Chaiyasut, C.; Chattipakorn, N.; et al. Lactobacillus paracasei HII01, xylooligosaccharides, and synbiotics reduce gut disturbance in obese rats. Nutrition 2018, 54, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.; Mizgier, M.L.; Morales, P.; Rios, I.; Carrasco-Pozo, C.; Diaz, E.; Brunser, O.; Gotteland, M. Effect of the Synbiotic (B. animalis spp. lactis Bb12 + Oligofructose) in Obese Subjects. A Randomized, Double-Blind, Controlled Clinical Trial. J. Food Nutr. Res. 2014, 2, 491–498. [Google Scholar] [CrossRef][Green Version]
- Classification of Diabetes Mellitus 2019; WHO: Geneva, Switzerland, 2019; pp. 1–36.
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 36. [Google Scholar] [CrossRef]
- Qian, J.; Cai, M.; Gao, J.; Tang, S.; Xu, L.; Critchley, J.A. Trends in smoking and quitting in China from 1993 to 2003: National Health Service Survey data. Bull. World Health Organ. 2010, 88, 769–776. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration. Alcohol Use Facts & Resources. Available online: https://www.samhsa.gov/sites/default/files/alcohol-use-facts-resources-fact-sheet.pdf. (accessed on 18 May 2021).
- Khat-udomkiri, N.; Toejing, P.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Antihyperglycemic effect of rice husk derived xylooligosaccharides in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rat mod. Food Sci. Nutr. 2019, 8, 428–444. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Gheshlaghi, Z.B.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran. J. Public Health 2015, 44, 228237. [Google Scholar]
- Moroti, C.; Souza Magri, L.F.; Costa, M.R.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29–36. [Google Scholar] [CrossRef]
- Khalili, L.; Alipoor, B.; Jafarabadi, M.A.; Faraji, I.; Sani, M.A. The effects of Lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-a levels in patients with type 2 diabetes mellitus: A randomized controlled trial. Iran. Biomed. J. 2018, 23, 68–77. [Google Scholar] [CrossRef]
- Horvath, A.; Leber, B.; Feldbacher, N.; Tripolt, N.; Rainer, F.; Blesl, A.; Trieb, M.; Marsche, G.; Sourij, H.; Stadlbauer, V. Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality of life in diabesity: A randomized, double-blind, placebo-controlled pilot study. Eur. J. Nutr. 2020, 59, 2969–2983. [Google Scholar] [CrossRef] [PubMed]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef]
- Cândido, T.L.N.; Bressan, J.; Alfenas, R.C.G. Dysbiosis and metabolic endotoxemia induced by high-fat diet. Nutr. Hosp. 2018, 35, 1432–1440. [Google Scholar]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.P.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Xu, J.; Liang, R.; Zhang, W.; Tian, K.; Li, J.; Chen, X.; Yu, T.; Chen, Q. Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J. Diabetes 2020, 12, 224–236. [Google Scholar] [CrossRef]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; Schaik, W.; Young, V.B.; Kuijpera, E.J. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019, 83, e00007-19. [Google Scholar] [CrossRef]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468–1475. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Fishman, G.; Yahav, D.; Goldberg, E.; Stein, G.Y.; Zvi, H.B.; Barsheshet, A.; Bishara, J. Predicting Clostridium difficile infection in diabetic patients and the effect of metformin therapy: A retrospective, case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1201–1205. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Allin, K.H.; Nielsen, T.; Pedersen, O. Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2015, 172, 167–177. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, K.S.H. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2006, 23, 87–104. [Google Scholar] [CrossRef]
- Toejing, P.; Khat-Udomkiri, N.; Intakhad, J.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Putative Mechanisms Responsible for the Antihyperglycemic Action of Lactobacillus paracasei HII01 in Experimental Type 2 Diabetic Rats. Nutrients 2020, 10, 3015. [Google Scholar] [CrossRef]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Medina, M.; Vintiñi, E.; Alvarez, S. Stimulation of respiratory immunity by oral administration of Lactococcus lactis. Can. J. Microbiol. 2008, 54, 630–638. [Google Scholar] [CrossRef]
- Bodera, P.; Chcialowski, A. Immunomodulatory effect of probiotic bacteria. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat. Inflamm. 2014, 2014, 162021. [Google Scholar] [CrossRef]
- Kim, C.H. Microbiota or short-chain fatty acids: Which regulates diabetes? Cell Mol. Immunol. 2018, 15, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Chen, G.; Xu, H.; Ge, R.; Lin, J. Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med. Hypotheses 2010, 74, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Q.; Ren, Y.; Ruan, Z. Effect of probiotic Lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PLoS ONE 2017, 12, e0178868. [Google Scholar] [CrossRef]
- Kasinska, M.A.; Drzewoski, J. Effectiveness of probiotics in type 2 diabetes: A meta-analysis. Pol. Arch. Intern. Med. 2015, 125, 803–813. [Google Scholar] [CrossRef]

| Characteristics | Placebo (n = 18) | Probiotic (n = 18) | p-Value |
|---|---|---|---|
| Age (year) | 61.78 ± 7.73 | 63.50 ± 5.94 | 0.459 a |
| Sex | 0.228 b | ||
| Male | 2 (11.11) | 6 (33.33) | |
| Female | 16 (88.89) | 12 (66.67) | |
| Education | 1.000 b | ||
| No education | 2 (11.11) | 3 (16.67) | |
| Primary | 14 (77.78) | 13 (72.22) | |
| Secondary | 2 (11.11) | 2 (11.11) | |
| Smokers | 0.486 b | ||
| No | 18 (100) | 16 (88.89) | |
| Yes | 0 (0) | 2 (11.11) | |
| Alcoholic | 1.000 b | ||
| No | 17 (94.44) | 18 (100) | |
| Yes | 1 (5.56) | 0 (0) | |
| BMI (kg/m2) | 23.05 ± 2.60 | 23.22 ± 2.72 | 0.852 a |
| Parameters | Placebo (n = 18) | Probiotic (n = 18) | ||||
|---|---|---|---|---|---|---|
| Wk-0 | Wk-12 | p-Value | Wk-0 | Wk-12 | p-Value | |
| FBG (mg/dL) | 139.29 ± 49.77 | 149.12 ± 47.42 | 0.208 a | 129.18 ± 34.52 | 109.35 ± 15.56 | 0.005 a |
| HbA1c (%) | 6.64 ± 1.44 | 6.73 ± 1.29 | 0.837 a | 7.05 ± 1.85 | 6.46 ± 1.49 | 0.145 a |
| Adiponectin (ng/mL) | 27.70 ± 7.26 | 24.03 ± 12.52 | 0.465 b | 23.40 ± 8.75 | 30.73 ± 18.40 | 0.763 b |
| Leptin (ng/mL) | 15.56 ± 13.56 | 26.46 ± 8.49 | 0.095 b | 15.74 ± 9.51 | 14.60 ± 8.70 | 0.345 b |
| TG (mg/dL) | 147.78 ± 71.32 | 159.50 ± 67.87 | 0.581 a | 153.27 ± 55.46 | 144.53 ± 58.68 | 0.560 a |
| Cholesterol (mg/dL) | 190.83 ± 43.22 | 184.94 ± 46.65 | 0.314 a | 188.59 ± 34.21 | 182.88 ± 30.38 | 0.453 a |
| LDL (mg/dL) | 102.11 ± 32.60 | 90.61 ± 16.06 | 0.106 a | 109.24 ± 38.44 | 81.47 ± 33.53 | <0.00 a |
| HDL (mg/dL) | 62.87 ± 9.83 | 68.67 ± 13.99 | 0.145 a | 62.06 ± 14.84 | 72.06 ± 21.34 | 0.026 a |
| Comparison to Placebo at Week-12 | |||
|---|---|---|---|
| Probiotic Group | |||
| Parameters | Coef. | 95% Confidence Interval | p-Value |
| FBG (mg/dL) | −37.16 | (−60.35, −13.97) | 0.004 |
| HbA1c (%) | −1.50 | (−4.62, 1.62) | 0.252 |
| Adiponectin (ng/mL) | 6.03 | (22.64, 10.59) | 0.371 |
| Leptin (ng/mL) | −7.44 | (−26.02, 11.15) | 0.292 |
| TG (mg/dL) | −45.35 | (−119.26, 28.55) | 0.211 |
| Cholesterol (mg/dL) | −17.35 | (−40.33, 5.63) | 0.128 |
| LDL (mg/dL) | −18.54 | (−34.63, −2.44) | 0.0026 |
| HDL (mg/dL) | 14.53 | (−0.29, 28.77) | 0.046 |
| Parameters | Placebo (n = 18) | Probiotic (n = 18) | ||||
|---|---|---|---|---|---|---|
| Wk-0 | Wk-12 | p-Value | Wk-0 | Wk-12 | p-Value | |
| ZO-1 (ng/mL) | 1.80 ± 0.86 | 1.74 ± 0.77 | 0.455 a | 1.61 ± 0.53 | 1.45 ± 0.63 | 0.204 a |
| LPS (pg/mL) | 72.38 ± 40.67 | 69.36 ± 24.91 | 0.638 b | 92.05 ± 35.95 | 51.07 ± 20.26 | 0.002 b |
| TNF-α (ng/mL) | 11.75 ± 3.01 | 9.62 ± 1.37 | 0.252 a | 11.91 ± 0.72 | 6.16 ± 0.90 | 0.009 a |
| IL-1β (ng/mL) | 7.26 ± 2.49 | 6.17 ± 1.42 | 0.345 b | 8.21 ± 3.52 | 5.46 ± 3.24 | 0.109 b |
| IL-6 (ng/mL) | 11.84 ± 1.78 | 10.76 ± 4.91 | 0.549 a | 11.01 ± 1.91 | 7.85 ± 2.92 | 0.033 a |
| IL-10 (ng/mL) | 1.27 ± 0.15 | 6.04 ± 5.43 | 0.068 b | 1.12 ± 0.05 | 10.58 ± 0.1 | 0.180 b |
| IgA (ng/mL) | 660.68 ± 262.60 | 679.65 ± 338.66 | 0.867 a | 526.24 ± 249.61 | 707.02 ± 265.37 | 0.027 a |
| hsCRP (mg/L) | 0.0160 ± 0.0069 | 0.0158 ± 0.0059 | 0.950 a | 0.0143 ± 0.0024 | 0.0124 ± 0.0026 | 0.032 a |
| Comparison to Placebo at Week-12 | |||
|---|---|---|---|
| Probiotic | |||
| Parameters | Coef. | 95% Confidence Interval | p-Value |
| ZO-1 (ng/mL) | −0.30 | (−0.69, −0.09) | 0.124 |
| LPS (pg/mL) | −26.46 | (−46.55, −6.37) | 0.020 |
| TNF-α (ng/mL) | −3.16 | (−5.50, −0.82) | 0.023 |
| IL-1β (ng/mL) | −2.13 | (−5.72, 1.46) | 0.125 |
| IL-6 (ng/mL) | −4.12 | (−5.68, −2.56) | 0.001 |
| IL-10 (ng/mL) | 5.61 | (−87.25, 98.47) | 0.060 |
| IgA (ng/mL) | 257.62 | (663.69, −148.45) | 0.188 |
| hsCRP (mg/L) | −0.004 | (−0.008, −0.001) | 0.026 |
| Parameters | Placebo (n = 18) | Probiotic (n = 18) | ||||
|---|---|---|---|---|---|---|
| Wk-0 | Wk-12 | p-Value | Wk-0 | Wk-12 | p-Value | |
| Lactic acid (μmol/g) | 22.60 ± 19.57 | 40.73 ± 25.80 | 0.068 b | 20.46 ± 16.08 | 51.00 ± 27.96 | 0.028 b |
| Propionic acid (μmol/g) | 42.83 ± 17.91 | 211.93 ± 30.28 | 0.109 b | 49.86 ± 94.36 | 307.64 ± 104.55 | 0.012 b |
| Acetic acid (μmol/g) | 359.40 ± 75.80 | 405.18 ± 71.08 | 0.217 a | 343.84 ± 46.56 | 512.54 ± 79.57 | 0.003 a |
| Butyric acid (μmol/g) | 51.46 ± 13.68 | 162.45 ± 57.14 | 0.109 b | 48.70 ± 29.76 | 181.80 ± 147.48 | 0.012 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toejing, P.; Khampithum, N.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Influence of Lactobacillus paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial. Foods 2021, 10, 1455. https://doi.org/10.3390/foods10071455
Toejing P, Khampithum N, Sirilun S, Chaiyasut C, Lailerd N. Influence of Lactobacillus paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial. Foods. 2021; 10(7):1455. https://doi.org/10.3390/foods10071455
Chicago/Turabian StyleToejing, Parichart, Nanticha Khampithum, Sasithorn Sirilun, Chaiyavat Chaiyasut, and Narissara Lailerd. 2021. "Influence of Lactobacillus paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial" Foods 10, no. 7: 1455. https://doi.org/10.3390/foods10071455
APA StyleToejing, P., Khampithum, N., Sirilun, S., Chaiyasut, C., & Lailerd, N. (2021). Influence of Lactobacillus paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial. Foods, 10(7), 1455. https://doi.org/10.3390/foods10071455







