Influence of the Production System (Intensive vs. Extensive) at Farm Level on Proximate Composition and Volatile Compounds of Portuguese Lamb Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Lamb Rearing and Feeding
2.2. Lamb Meat Samples
2.3. Analysis of Chemical Composition
2.4. Volatile Compounds Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Volatile Profile
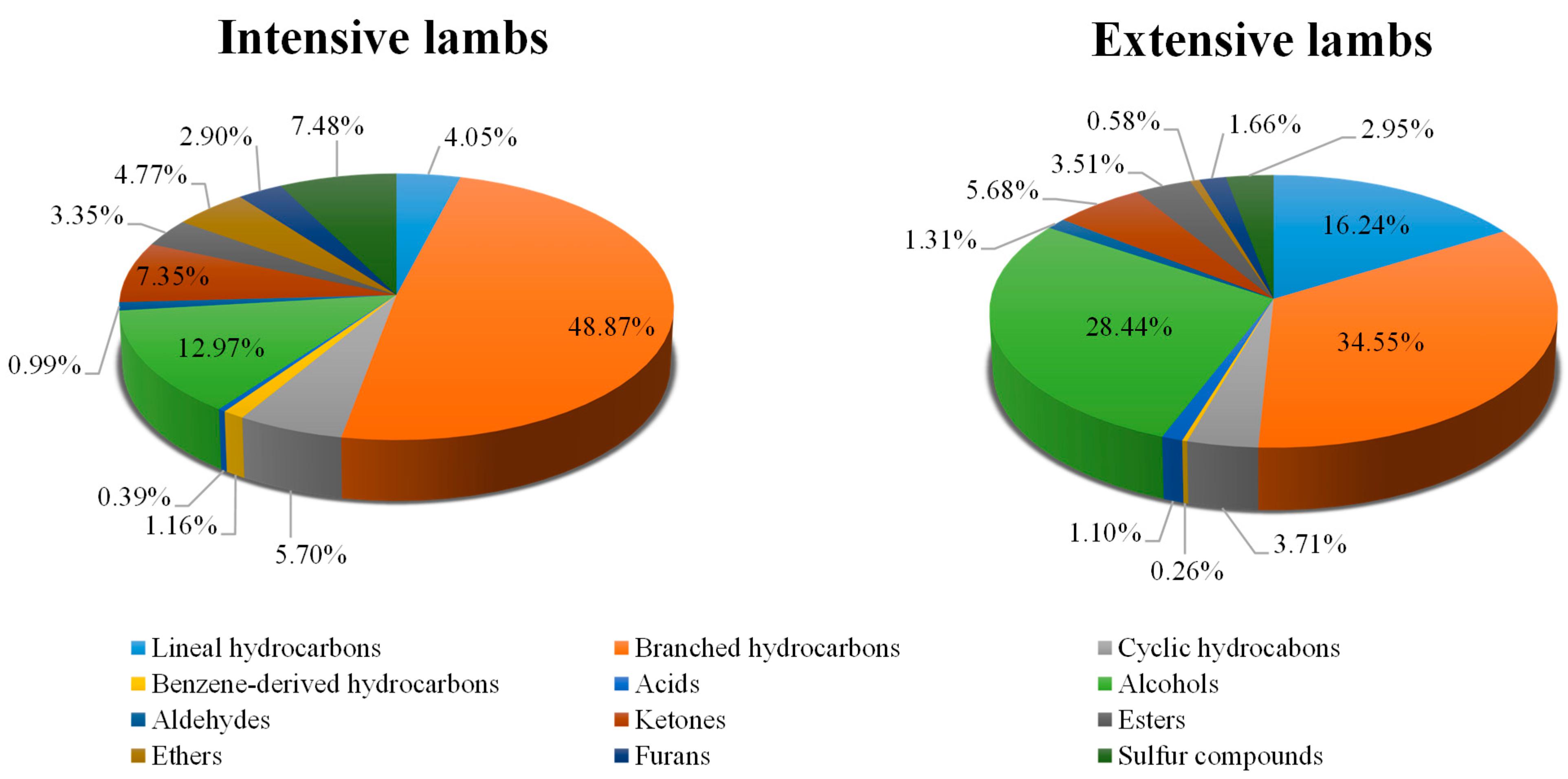
3.2.1. Hydrocarbons: Linear, Branched, Cyclic and Benzene-Derived
3.2.2. Acids
3.2.3. Alcohols
3.2.4. Aldehydes
3.2.5. Ketones
3.2.6. Esters, Ethers, Furans and Sulfur Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Font-i-Furnols, M.; Guerrero, L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef]
- Glitsch, K. Consumer perceptions of fresh meat quality: Cross-national comparison. Br. Food J. 2000, 102, 177–194. [Google Scholar] [CrossRef]
- Watkins, P.J.; Frank, D.; Singh, T.K.; Young, O.A.; Warner, R.D. Sheepmeat flavor and the effect of different feeding systems: A review. J. Agric. Food Chem. 2013, 61, 3561–3579. [Google Scholar] [CrossRef] [PubMed]
- Young, O.A.; Berdagué, J.L.; Viallon, C.; Rousset-Akrim, S.; Theriez, M. Fat-borne volatiles and sheepmeat odour. Meat Sci. 1997, 45, 183–200. [Google Scholar] [CrossRef]
- Almela, E.; Jordán, M.J.; Martínez, C.; Sotomayor, J.A.; Bedia, M.; Bañón, S. Ewe’s diet (pasture vs grain-based feed) affects volatile profile of cooked meat from light lamb. J. Agric. Food Chem. 2010, 58, 9641–9646. [Google Scholar] [CrossRef] [PubMed]
- Young, O.A.; Lane, G.A.; Priolo, A.; Fraser, K. Pastoral and species flavour in lambs raised on pasture, lucerne or maize. J. Sci. Food Agric. 2003, 83, 93–104. [Google Scholar] [CrossRef]
- Sañudo, C.; Alfonso, M.; Sanchez, A.; Berge, P.; Dransfield, E.; Zygoyiannis, D.; Stamataris, C.; Thorkelsson, G.; Valdimarsdottir, T.; Piasentier, E.; et al. Meat texture of lambs from different European production systems. Aust. J. Agric. Res. 2003, 54, 551–560. [Google Scholar] [CrossRef]
- De-Arriba, R.; Sánchez-Andrés, A. Production and Productivity in Eastern and Western European Sheep Farming: A Comparative Analysis. Available online: http://www.lrrd.org/lrrd26/4/arri26066.htm (accessed on 3 July 2020).
- Cadavez, V.A.P.; Popova, T.; Bermúdez, R.; Osoro, K.; Purriños, L.; Bodas, R.; Lorenzo, J.M.; Gonzales-Barron, U. Compositional attributes and fatty acid profile of lamb meat from Iberian local breeds. Small Rumin. Res. 2020, 193, 106244. [Google Scholar] [CrossRef]
- Ruano, Z.M.; Cortinhas, A.; Carolino, N.; Gomes, J.; Costa, M.; Mateus, T.L. Gastrointestinal parasites as a possible threat to an endangered autochthonous Portuguese sheep breed. J. Helminthol. 2019, 94, e103. [Google Scholar] [CrossRef]
- Mendelsohn, R. The challenge of conserving indigenous domesticated animals. Ecol. Econ. 2003, 45, 501–510. [Google Scholar] [CrossRef]
- Paim, T.d.P.; Da Silva, A.F.; Martins, R.F.S.; Borges, B.O.; Lima, P.d.M.T.; Cardoso, C.C.; Esteves, G.I.F.; Louvandini, H.; McManus, C. Performance, survivability and carcass traits of crossbred lambs from five paternal breeds with local hair breed Santa Inês ewes. Small Rumin. Res. 2013, 112, 28–34. [Google Scholar] [CrossRef][Green Version]
- Cruz, B.C.; Cerqueira, J.; Araújo, J.P.; Gonzales-Barron, U.; Cadavez, V. Study of growth performance of Churra-Galega-Bragançana and Bordaleira-de-Entre-Douro-e- Minho lamb breeds. In Proceedings of the XVIII Jornadas sobre Producción Animal, Zaragoza, Spain, 7–8 May 2019; pp. 66–68. [Google Scholar]
- Vasta, V.; Priolo, A. Ruminant fat volatiles as affected by diet. A review. Meat Sci. 2006, 73, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Priolo, A.; Micol, D.; Agabriel, J. Effects of grass feeding systems on ruminant meat colour and flavour. A review. Anim. Res. 2001, 50, 185–200. [Google Scholar] [CrossRef]
- Vasta, V.; Ratel, J.; Engel, E. Mass spectrometry analysis of volatile compounds in raw meat for the authentication of the feeding background of farm animals. J. Agric. Food Chem. 2007, 55, 4630–4639. [Google Scholar] [CrossRef]
- Sivadier, G.; Ratel, J.; Engel, E. Latency and persistence of diet volatile biomarkers in lamb fats. J. Agric. Food Chem. 2009, 57, 645–652. [Google Scholar] [CrossRef]
- Vasta, V.; D’Alessandro, A.G.; Priolo, A.; Petrotos, K.; Martemucci, G. Volatile compound profile of ewe’s milk and meat of their suckling lambs in relation to pasture vs. indoor feeding system. Small Rumin. Res. 2012, 105, 16–21. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Sebastiàn, I.; Viallon, C.; Berge, P. Analysis of the volatile fraction and the flavour characteristics of lamb: Relationships with the type of feeding. Sci. Aliment. 2003, 23, 497–511. [Google Scholar] [CrossRef]
- Suzuky, J.; Bailey, M.E. Direct sampling capillary GLC analysis of flavor volatiles from ovine fat. J. Agric. Food Chem. 1985, 33, 343–347. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Van Laar, H.; Demeyer, D. Rumen odd and branched chain fatty acids in relation to in vitro rumen volatile fatty acid productions and dietary characteristics of incubated substrates. J. Anim. Physiol. Anim. Nutr. 2004, 88, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Raes, K.; Balcaen, A.; Dirinck, P.; De Winne, A.; Claeys, E.; Demeyer, D.; De Smet, S. Meat quality, fatty acid composition and flavour analysis in belgian retail beef. Meat Sci. 2003, 65, 1237–1246. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G.; Dimauro, C.; Röhrle, F.; Priolo, A.; Monahan, F.J.; Moloney, A.P. The volatile profile of longissimus dorsi muscle of heifers fed pasture, pasture silage or cereal concentrate: Implication for dietary discrimination. Meat Sci. 2011, 87, 282–289. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization). Determination of moisture content, ISO 1442:1997 standard. In International Standards Meat and Meat Products; International Organization for Standardization: Genève, Switzerland, 1997. [Google Scholar]
- ISO (International Organization for Standardization). Determination of nitrogen content, ISO 937:1978 standard. In International Standards Meat and Meat Products; International Organization for Standardization: Genève, Switzerland, 1978. [Google Scholar]
- ISO (International Organization for Standardization). Determination of ash content, ISO 936:1998 standard. In International Standards Meat and Meat Products; International Organization for Standardization: Genève, Switzerland, 1998. [Google Scholar]
- AOCS. AOCS Official Procedure Am5-04. Rapid Determination of Oil/Fat Utilizing High Temperature Solvent Extraction; American Oil Chemists Society: Urbana, IL, USA, 2005. [Google Scholar]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of Volatile Compounds of Dry-Cured Meat Products Using HS-SPME-GC/MS Technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Popova, T.; Bermúdez Piedra, R.; Tolsdorf, A.; Geß, A.; Pires, J.; Domínguez, R.; Chiesa, F.; Brugiapaglia, A.; Viola, I.; et al. Fatty acid composition of lamb meat from Italian and German local breeds. Small Rumin. Res. 2021, 200, 106384. [Google Scholar] [CrossRef]
- Polidori, P.; Pucciarelli, S.; Cammertoni, N.; Polzonetti, V.; Vincenzetti, S. The effects of slaughter age on carcass and meat quality of Fabrianese lambs. Small Rumin. Res. 2017, 155, 12–15. [Google Scholar] [CrossRef]
- Cividini, A.; Levart, A.; Žgur, S.; Kompan, D. Fatty acid composition of lamb meat from the autochthonous Jezersko-Solčava breed reared in different production systems. Meat Sci. 2014, 97, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Velasco, S.; Cañeque, V.; Pérez, C.; Lauzurica, S.; Díaz, M.T.; Huidobro, F.; Manzanares, C.; González, J. Fatty acid composition of adipose depots of suckling lambs raised under different production systems. Meat Sci. 2001, 59, 325–333. [Google Scholar] [CrossRef]
- Gkarane, V.; Brunton, N.P.; Allen, P.; Gravador, R.S.; Claffey, N.A.; Diskin, M.G.; Fahey, A.G.; Farmer, L.J.; Moloney, A.P.; Alcalde, M.J.; et al. Effect of finishing diet and duration on the sensory quality and volatile profile of lamb meat. Food Res. Int. 2019, 115, 54–64. [Google Scholar] [CrossRef]
- Aurousseau, B.; Bauchart, D.; Faure, X.; Galot, A.L.; Prache, S.; Micol, D.; Priolo, A. Indoor fattening of lambs raised on pasture. Part 1: Influence of stall finishing duration on lipid classes and fatty acids in the longissimus thoracis muscle. Meat Sci. 2007, 76, 241–252. [Google Scholar] [CrossRef]
- Joy, M.; Ripoll, G.; Delfa, R. Effects of feeding system on carcass and non-carcass composition of Churra Tensina light lambs. Small Rumin. Res. 2008, 78, 123–133. [Google Scholar] [CrossRef]
- Wilches, D.; Rovira, J.; Jaime, I.; Palacios, C.; Lurueña-Martínez, M.A.; Vivar-Quintana, A.M.; Revilla, I. Evaluation of the effect of a maternal rearing system on the odour profile of meat from suckling lamb. Meat Sci. 2011, 88, 415–423. [Google Scholar] [CrossRef]
- Belhaj, K.; Mansouri, F.; Sindic, M.; Fauconnier, M.-L.; Boukharta, M.; Serghini Caid, H.; Elamrani, A. Effect of Rearing Season on Meat and Intramuscular Fat Quality of Beni-Guil Sheep. J. Food Qual. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Hajji, H.; Joy, M.; Ripoll, G.; Smeti, S.; Mekki, I.; Gahete, F.M.; Mahouachi, M.; Atti, N. Meat physicochemical properties, fatty acid profile, lipid oxidation and sensory characteristics from three North African lamb breeds, as influenced by concentrate or pasture finishing diets. J. Food Compos. Anal. 2016, 48, 102–110. [Google Scholar] [CrossRef]
- Majdoub-Mathlouthi, L.; Saïd, B.; Say, A.; Kraiem, K. Effect of concentrate level and slaughter body weight on growth performances, carcass traits and meat quality of Barbarine lambs fed oat hay based diet. Meat Sci. 2013, 93, 557–563. [Google Scholar] [CrossRef]
- Cañeque, V.; Velasco, S.; Díaz, M.; Pérez, C.; Huidobro, F.; Lauzurica, S.; Manzanares, C.; González, J. Effect of weaning age and slaughter weight on carcass and meat quality of Talaverana breed lambs raised at pasture. Anim. Sci. 2001, 73, 85–95. [Google Scholar] [CrossRef]
- Popova, T.; Gonzales-Barron, U.; Cadavez, V. A meta-analysis of the effect of pasture access on the lipid content and fatty acid composition of lamb meat. Food Res. Int. 2015, 77, 476–483. [Google Scholar] [CrossRef]
- Ekiz, B.; Demirel, G.; Yilmaz, A.; Ozcan, M.; Yalcintan, H.; Kocak, O.; Altinel, A. Slaughter characteristics, carcass quality and fatty acid composition of lambs under four different production systems. Small Rumin. Res. 2013, 114, 26–34. [Google Scholar] [CrossRef]
- Boughalmi, A.; Araba, A. Effect of feeding management from grass to concentrate feed on growth, carcass characteristics, meat quality and fatty acid profile of Timahdite lamb breed. Small Rumin. Res. 2016, 144, 158–163. [Google Scholar] [CrossRef]
- Nuernberg, K.; Nuernberg, G.; Ender, K.; Dannenberger, D.; Schabbel, W.; Grumbach, S.; Zupp, W.; Steinhart, H. Effect of grass vs. concentrate feeding on the fatty acid profile of different fat depots in lambs. Eur. J. Lipid Sci. Technol. 2005, 107, 737–745. [Google Scholar] [CrossRef]
- Ates, S.; Keles, G.; Demirci, U.; Dogan, S.; Kirbas, M.; Filley, S.J.; Parker, N.B. The effects of feeding system and breed on the performance and meat quality of weaned lambs. Small Rumin. Res. 2020, 192, 106225. [Google Scholar] [CrossRef]
- Ye, Y.; Schreurs, N.M.; Johnson, P.L.; Corner-Thomas, R.A.; Agnew, M.P.; Silcock, P.; Eyres, G.T.; Maclennan, G.; Realini, C.E. Carcass characteristics and meat quality of commercial lambs reared in different forage systems. Livest. Sci. 2020, 232, 103908. [Google Scholar] [CrossRef]
- Karabagias, I.K. Volatile profile of raw lamb meat stored at 4 ± 1 °C: The potential of specific aldehyde ratios as indicators of lamb meat quality. Foods 2018, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Meynier, A.; Novelli, E.; Chizzolini, R.; Zanardi, E.; Gandemer, G. Volatile compounds of commercial Milano salami. Meat Sci. 1999, 51, 175–183. [Google Scholar] [CrossRef]
- Sivadier, G.; Ratel, J.; Bouvier, F.; Engel, E. Authentication of meat products: Determination of animal feeding by parallel GC-MS analysis of three adipose tissues. J. Agric. Food Chem. 2008, 56, 9803–9812. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.J.; Fernández-García, E.; Mínguez-Mosquera, M.I.; Pérez-Gálvez, A. Description of volatile compounds generated by the degradation of carotenoids in paprika, tomato and marigold oleoresins. Food Chem. 2008, 106, 1145–1153. [Google Scholar] [CrossRef]
- Binnie, J.; Cape, J.N.; Mackie, N.; Leith, I.D. Exchange of organic solvents between the atmosphere and grass—The use of open top chambers. Sci. Total Environ. 2002, 285, 53–67. [Google Scholar] [CrossRef]
- Smith, K.E.C.; Jones, K.C. Particles and vegetation: Implications for the transfer of particle-bound organic contaminants to vegetation. Sci. Total Environ. 2000, 246, 207–236. [Google Scholar] [CrossRef]
- Franke, E.N. Recent advances in the chemistry of rancidity of fats. In Recent Advances in the Chemistry of Meat; The Royal Society of Chemistry: London, UK, 1984; pp. 87–118. [Google Scholar]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Insausti, K.; Beriain, M.; Gorraiz, C.; Purroy, A. Volatile compounds of raw beef from 5 local spanish cattle breeds stored under modified atmosphere. Sens. Nutr. Qual. Food 2002, 67, 1580–1589. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Ha, J.K.; Lindsay, R.C. Volatile alkylphenols and thiophenol in species-related characterizing flavors of red meats. J. Food Sci. 1991, 56, 1197–1202. [Google Scholar] [CrossRef]
- Erasmus, S.W.; Muller, M.; Hoffman, L.C. Authentic sheep meat in the European Union: Factors influencing and validating its unique meat quality. J. Sci. Food Agric. 2017, 97, 1979–1996. [Google Scholar] [CrossRef]
- Watkins, P.J.; Kearney, G.; Rose, G.; Allen, D.; Ball, A.J.; Pethick, D.W.; Warner, R.D. Effect of branched-chain fatty acids, 3-methylindole and 4-methylphenol on consumer sensory scores of grilled lamb meat. Meat Sci. 2014, 96, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Berdagué, J.J.; Antequera, T.; López-Bote, C.; Córdoba, J.J.; Ventanas, J. Volatile components of dry cured Iberian ham. Food Chem. 1991, 41, 23–32. [Google Scholar] [CrossRef]
- Barbieri, G.; Bolzoni, L.; Parolari, G.; Virgili, R.; Buttini, R.; Careri, M.; Mangia, A. Flavor compounds of dry-cured ham. J. Agric. Food Chem. 1992, 40, 2389–2394. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Elmore, J.S.; Cooper, S.L.; Enser, M.; Mottram, D.S.; Sinclair, L.A.; Wilkinson, R.G.; Wood, J.D. Dietary manipulation of fatty acid composition in lamb meat and its effect on the volatile aroma compounds of grilled lamb. Meat Sci. 2005, 69, 233–242. [Google Scholar] [CrossRef]
- Morand-Fehr, P.; Tran, G. La fraction lipidique des aliments et les corps gras utilisés en alimentation animale. Prod. Anim. 2001, 14, 285–302. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef]
- Lane, G.A.; Fraser, K. A comparison of phenol and indole flavour compounds in fat, and of phenols in urine of cattle fed pasture or grain. N. Zeal. J. Agric. Res. 1999, 42, 289–296. [Google Scholar] [CrossRef]
- Gravador, R.S.; Serra, A.; Luciano, G.; Pennisi, P.; Vasta, V.; Mele, M.; Pauselli, M.; Priolo, A. Volatiles in raw and cooked meat from lambs fed olive cake and linseed. Animal 2015, 9, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Coppa, M.; Martin, B.; Pradel, P.; Leotta, B.; Priolo, A.; Vasta, V. Milk volatile compounds to trace cows fed a hay-based diet or different grazing systems on upland pastures. J. Agric. Food Chem. 2011, 59, 4947–4954. [Google Scholar] [CrossRef] [PubMed]
- Selli, S.; Cayhan, G.G. Analysis of volatile compounds of wild gilthead sea bream (Sparus aurata) by simultaneous distillation-extraction (SDE) and GC-MS. Microchem. J. 2009, 93, 232–235. [Google Scholar] [CrossRef]
- Argemí-Armengol, I.; Villalba, D.; Tor, M.; Pérez-Santaescolástica, C.; Purriños, L.; Lorenzo, J.M.; Álvarez-Rodríguez, J. The extent to which genetics and lean grade affect fatty acid profiles and volatile compounds in organic pork. PeerJ 2019, 7, e7322. [Google Scholar] [CrossRef]
- Maggiolino, A.; Lorenzo, J.M.; Marino, R.; della Malva, A.; Centoducati, P.; De Palo, P. Foal meat volatile compounds: Effect of vacuum ageing on semimembranosus muscle. J. Sci. Food Agric. 2019, 99, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Van, H.; Hwang, I.; Jeong, D.; Touseef, A. Principle of Meat Aroma Flavors and Future Prospect. In Latest Research into Quality Control; Akyar, I., Ed.; IntechOpen: London, UK, 2012; pp. 145–176. [Google Scholar]
- Frankel, E.N. Lipid Oxidation, 2nd ed.; Oily Press: Dundee, Scotland, 1998. [Google Scholar]
- DeMan, J.H. Lipids. In Principles of Food Chemistry; Reinhold, V.N., Ed.; Springer: New York, NY, USA, 1990. [Google Scholar]
- Ortuño, J.; Serrano, R.; Bañón, S. Use of dietary rosemary diterpenes to inhibit rancid volatiles in lamb meat packed under protective atmosphere. Animal 2016, 10, 1391–1401. [Google Scholar] [CrossRef]
- Marco, A.; Navarro, J.L.; Flores, M. Quantitation of selected odor-active constituents in dry fermented sausages prepared with different curing salts. J. Agric. Food Chem. 2007, 55, 3058–3065. [Google Scholar] [CrossRef]
- Larick, D.K.; Tuener, B.E. Headspace volatiles and sensory characteristics of ground beef from forage- and grain-fed heifers. J. Food Sci. 1990, 55, 649–654. [Google Scholar] [CrossRef]
- Du, X. Determination of flavor substances in fermented pork by GC-MS. Meat Res. 2012, 26, 34–36. [Google Scholar]
- Elmore, J.S.S.; Mottram, D.S.D.S.; Enser, M.; Wood, J.D.J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Resconi, V.C.; Campo, M.M.; Montossi, F.; Ferreira, V.; Sañudo, C.; Escudero, A. Relationship between odour-active compounds and flavour perception in meat from lambs fed different diets. Meat Sci. 2010, 85, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, F.; Sink, J.D.; Dimick, P.S.; Mussinan, C.J.; Sanderson, A. Volatile flavor constituents of ovine adipose tissue. J. Agric. Food Chem. 1977, 25, 1230–1234. [Google Scholar] [CrossRef]
- Gkarane, V.; Brunton, N.P.; Harrison, S.M.; Gravador, R.S.; Allen, P.; Claffey, N.A.; Diskin, M.G.; Fahey, A.G.; Farmer, L.J.; Moloney, A.P.; et al. Volatile profile of grilled lamb as affected by castration and age at slaughter in two breeds. J. Food Sci. 2018, 83, 2466–2477. [Google Scholar] [CrossRef]
- Manner, W.; Maxwell, R.J.; Williams, J.E. Effects of dietary regimen and tissue site on bovine fatty acid profiles. J. Anim. Sci. 1984, 59, 109–121. [Google Scholar] [CrossRef]
- Gargouri, M.; Drouet, P.; Legoy, M.D. Synthesis of a novel macrolactone by lipase-catalyzed intra-esterification of hydroxy-fatty acid in organic media. J. Biotechnol. 2002, 92, 259–266. [Google Scholar] [CrossRef]
- Urbach, G. Effect of feed on flavor in dairy foods. J. Dairy Sci. 1990, 73, 3639–3650. [Google Scholar] [CrossRef]
- Krvavica, M.; Bradaš, M.; Rogošić, J.; Jug, T.; Vnučec, I.; Marušić Radovčić, N. Volatile aroma compounds of Lika lamb. MESO 2015, 3, 469–476. [Google Scholar]
- Osorio, M.T.; Zumalacárregui, J.M.; Cabeza, E.A.; Figueira, A.; Mateo, J. Effect of rearing system on some meat quality traits and volatile compounds of suckling lamb meat. Small Rumin. Res. 2008, 78, 1–12. [Google Scholar] [CrossRef]
- Petričević, S.; Marušić Radovčić, N.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- Vasta, V.; Jerónimo, E.; Brogna, D.M.R.; Dentinho, M.T.P.; Biondi, L.; Santos-Silva, J.; Priolo, A.; Bessa, R.J.B. The effect of grape seed extract or Cistus ladanifer L. on muscle volatile compounds of lambs fed dehydrated lucerne supplemented with oil. Food Chem. 2010, 119, 1339–1345. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Liu, M.; Zhao, X.; Luo, H. Effect of breed on the volatile compound precursors and odor profile attributes of lamb meat. Foods 2020, 9, 1178. [Google Scholar] [CrossRef]
- Del Bianco, S.; Natalello, A.; Luciano, G.; Valenti, B.; Monahan, F.; Gkarane, V.; Rapisarda, T.; Carpino, S.; Piasentier, E. Influence of dietary cardoon meal on volatile compounds and flavour in lamb meat. Meat Sci. 2020, 163, 108086. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. Gas chromatographic-olfactometric characterisation of headspace and mouthspace key aroma compounds in fresh and frozen lamb meat. Food Chem. 2011, 129, 1909–1918. [Google Scholar] [CrossRef]
- Fruet, A.P.B.; Trombetta, F.; Stefanello, F.S.; Speroni, C.S.; Donadel, J.Z.; De Souza, A.N.M.; Rosado Júnior, A.; Tonetto, C.J.; Wagner, R.; De Mello, A.; et al. Effects of feeding legume-grass pasture and different concentrate levels on fatty acid profile, volatile compounds, and off-flavor of the M. longissimus thoracis. Meat Sci. 2018, 140, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Oliveira, I.; Silva, J.A.; Martins, C.; Ventanas, J.; García, C. Implementation of multivariate techniques for the selection of volatile compounds as indicators of sensory quality of raw beef. J. Food Sci. Technol. 2015, 52, 3887–3898. [Google Scholar] [CrossRef] [PubMed]
- Kontou, S.; Tsipi, D.; Tzia, C. Stability of the dithiocarbamate pesticide maneb in tomato homogenates during cold storage and thermal processing. Food Addit. Contam. 2004, 21, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, G.; Kowaliszyn, B.; Martemucci, G.; Breeding, G.A.; Amendola, G. The effect of production system information on consumer expectation and acceptability of Leccese lamb meat. Ann. Food Sci. Technol. 2010, 11, 9–13. [Google Scholar]
| Intensive | Extensive | SEM | Sig. | |
|---|---|---|---|---|
| Moisture (%) | 78.00 | 75.91 | 0.290 | *** |
| Intramuscular fat (%) | 0.49 | 1.51 | 0.132 | *** |
| Protein (%) | 19.32 | 20.92 | 0.234 | *** |
| Ash (%) | 1.37 | 1.20 | 0.022 | *** |
| LRI | m/z | Intensive | Extensive | SEM | Sig. | |
|---|---|---|---|---|---|---|
| Linear hydrocarbons | ||||||
| Butane | 496 | 43 | 0.00 | 3.68 | 0.480 | *** |
| Pentane | 500 | 43 | 9.52 | 11.75 | 0.961 | ns |
| Heptane | 700 | 71 | 1.00 | 1.29 | 0.100 | ns |
| Octane | 800 | 85 | 0.00 | 8.49 | 0.884 | *** |
| 4-Octene, (E)- | 841 | 55 | 0.00 | 2.42 | 0.241 | *** |
| Decane | 1000 | 57 | 3.45 | 99.48 | 10.190 | *** |
| Undecane | 1100 | 57 | 5.53 | 0.27 | 0.633 | *** |
| 1-Undecene | 1129 | 83 | 0.68 | 0.48 | 0.057 | ns |
| Dodecane | 1200 | 57 | 3.33 | 1.30 | 0.263 | *** |
| Hexadecane | 1210 | 57 | 0.00 | 1.30 | 0.139 | *** |
| 1-Tetradecene | 1260 | 71 | 0.23 | 0.00 | 0.030 | *** |
| Tridecane | 1300 | 57 | 1.55 | 0.47 | 0.146 | *** |
| Tetradecane | 1400 | 57 | 0.87 | 0.00 | 0.101 | *** |
| Total linear hydrocarbons | 26.15 | 130.93 | 11.279 | *** | ||
| Branched hydrocarbons | ||||||
| Pentane, 2-methyl- | 541 | 71 | 0.51 | 2.45 | 0.199 | *** |
| Pentane, 3-methyl- | 550 | 56 | 1.18 | 29.79 | 2.839 | *** |
| Butane, 2,2,3,3-tetramethyl- | 656 | 57 | 0.00 | 10.92 | 1.208 | *** |
| Hexane, 2,2-dimethyl- | 656 | 57 | 17.00 | 0.00 | 2.111 | *** |
| Pentane, 2,3-dimethyl- | 675 | 56 | 0.66 | 0.00 | 0.091 | *** |
| Pentane, 2,3,4-trimethyl- | 759 | 71 | 21.00 | 0.09 | 2.375 | *** |
| Pentane, 2,3,3-trimethyl- | 767 | 70 | 46.63 | 0.19 | 5.061 | *** |
| Pentane, 3-ethyl- | 774 | 70 | 0.00 | 0.48 | 0.060 | *** |
| Hexane, 2,3-dimethyl- | 774 | 70 | 1.45 | 0.00 | 0.214 | *** |
| 1-Pentene, 3-ethyl-2-methyl- | 778 | 55 | 1.37 | 0.00 | 0.169 | *** |
| 3,4-Dimethyl-2-hexene | 778 | 83 | 1.38 | 0.00 | 0.193 | *** |
| 1-Pentene, 4,4-dimethyl- | 788 | 57 | 0.00 | 0.61 | 0.084 | *** |
| Butane, 2,2,3-trimethyl- | 789 | 85 | 0.56 | 0.00 | 0.067 | *** |
| Hexane, 2,2,5-trimethyl- | 806 | 57 | 18.43 | 0.37 | 2.071 | *** |
| Heptane, 3-methylene- | 820 | 70 | 5.10 | 0.00 | 0.692 | *** |
| Heptane, 3,4,5-trimethyl- | 850 | 85 | 0.00 | 8.92 | 0.894 | *** |
| Pentane, 2,3,3,4-tetramethyl- | 850 | 84 | 0.00 | 1.63 | 0.191 | *** |
| Heptane, 2,3-dimethyl- | 850 | 85 | 1.42 | 0.00 | 0.180 | *** |
| Heptane, 2,6-dimethyl- | 863 | 88 | 0.37 | 0.00 | 0.043 | *** |
| Heptane, 3-ethyl- | 917 | 57 | 1.50 | 0.00 | 0.166 | *** |
| Nonane, 3,7-dimethyl- | 925 | 57 | 1.08 | 0.00 | 0.129 | *** |
| Heptane, 2,2,4-trimethyl- | 933 | 57 | 1.99 | 1.20 | 0.168 | * |
| Heptane, 3,3,5-trimethyl- | 947 | 71 | 0.00 | 0.46 | 0.048 | *** |
| Octane, 3,3-dimethyl- | 947 | 71 | 1.95 | 0.00 | 0.218 | *** |
| Hexane, 2,3,4-trimethyl- | 948 | 57 | 0.00 | 0.43 | 0.046 | *** |
| Pentane, 2,2-dimethyl- | 948 | 57 | 1.29 | 0.00 | 0.141 | *** |
| 3-Ethyl-2-methyl-1-heptene | 996 | 84 | 0.89 | 0.00 | 0.100 | *** |
| Heptane, 3-ethyl-5-methylene- | 998 | 70 | 0.00 | 1.94 | 0.226 | *** |
| 2,3-Dimethyl-1-hexene | 1037 | 55 | 1.80 | 0.00 | 0.191 | *** |
| Pentane, 3,3-dimethyl- | 1046 | 71 | 3.01 | 0.00 | 0.349 | *** |
| 1-Hexene, 3-methyl- | 1062 | 70 | 3.04 | 0.00 | 0.386 | *** |
| (Z)-4-Methyl-2-hexene | 1072 | 98 | 0.89 | 0.00 | 0.096 | *** |
| 2,2,4,4-Tetramethyloctane | 1078 | 57 | 146.91 | 17.31 | 16.485 | *** |
| 1-Hexene, 5,5-dimethyl- | 1090 | 57 | 0.00 | 58.12 | 6.290 | *** |
| Nonane, 5-butyl- | 1097 | 127 | 1.45 | 0.00 | 0.172 | *** |
| Nonane, 5-(2-methylpropyl)- | 1097 | 71 | 7.01 | 0.00 | 0.868 | *** |
| Heptane, 2,3,4-trimethyl- | 1097 | 57 | 0.00 | 57.47 | 6.317 | *** |
| Dodecane, 2,6,10-trimethyl- | 1097 | 57 | 14.27 | 0.00 | 1.805 | *** |
| Heptane, 2,2-dimethyl- | 1101 | 57 | 0.93 | 0.00 | 0.141 | *** |
| Decane, 6-ethyl-2-methyl- | 1104 | 57 | 0.00 | 83.25 | 8.323 | *** |
| Heptane, 3,3,4-trimethyl- | 1135 | 71 | 0.00 | 0.50 | 0.060 | *** |
| Nonane, 2-methyl- | 1136 | 57 | 0.69 | 0.00 | 0.084 | *** |
| Hexane, 1-(hexyloxy)-3-methyl- | 1147 | 57 | 2.34 | 0.00 | 0.275 | *** |
| 2-Undecene, 9-methyl-, (Z)- | 1152 | 98 | 2.66 | 0.00 | 0.286 | *** |
| 4-Undecene, 5-methyl- | 1165 | 168 | 0.30 | 0.00 | 0.036 | *** |
| Pentane, 3,3-diethyl- | 1181 | 98 | 0.34 | 0.00 | 0.036 | *** |
| 2-Undecene, 3-methyl-, (Z)- | 1203 | 70 | 0.60 | 0.00 | 0.065 | *** |
| Octane, 2,4,6-trimethyl- | 1210 | 71 | 0.00 | 0.86 | 0.089 | *** |
| 5-Ethyl-1-nonene | 1224 | 83 | 0.32 | 0.00 | 0.039 | *** |
| 1-Decene, 2,4-dimethyl- | 1224 | 70 | 0.42 | 0.00 | 0.052 | *** |
| Hexane, 2-methyl-4-methylene- | 1227 | 71 | 0.00 | 0.42 | 0.046 | *** |
| Heptadecane, 8-methyl- | 1227 | 71 | 1.12 | 0.00 | 0.142 | *** |
| Undecane, 5-ethyl- | 1242 | 57 | 1.09 | 0.00 | 0.157 | *** |
| Dodecane, 2-methyl- | 1257 | 57 | 0.22 | 0.00 | 0.029 | *** |
| 1-Undecene, 8-methyl- | 1260 | 97 | 0.34 | 0.00 | 0.046 | *** |
| Tridecane, 3-methyl- | 1331 | 57 | 0.00 | 0.39 | 0.039 | *** |
| Heptane, 2,4-dimethyl- | 1349 | 71 | 0.00 | 0.35 | 0.036 | *** |
| 5,5-Dibutylnonane | 1358 | 71 | 0.00 | 0.36 | 0.037 | *** |
| Total branched hydrocarbons | 315.54 | 278.51 | 15.461 | ns | ||
| Cyclic hydrocarbons | ||||||
| Cyclopentane, 1,2-dimethyl-, cis- | 666 | 56 | 0.49 | 3.39 | 0.336 | *** |
| Cyclohexane, methyl- | 720 | 83 | 0.00 | 5.77 | 0.628 | *** |
| Bicyclo[3.2.0]hepta-2,6-diene | 810 | 91 | 15.94 | 12.09 | 0.788 | * |
| Cyclopentane, 1,2,3-trimethyl- | 820 | 56 | 0.62 | 0.00 | 0.083 | *** |
| Cyclooctane | 822 | 70 | 0.00 | 2.75 | 0.275 | *** |
| Cyclohexane, 1,3-dimethyl-, cis- | 840 | 97 | 2.02 | 0.00 | 0.228 | *** |
| Cyclohexane, 1,3-dimethyl- | 840 | 97 | 0.49 | 0.00 | 0.059 | *** |
| Cyclobutane, 1,1,2,3,3-pentamethyl- | 938 | 70 | 1.69 | 0.00 | 0.173 | *** |
| Cyclopropane, 1-methyl-2-pentyl- | 942 | 55 | 0.34 | 0.00 | 0.037 | *** |
| Bicyclo[3.1.1]hept-2-ene, 3,6,6-trimethyl- | 992 | 93 | 2.74 | 0.00 | 0.289 | *** |
| Cyclopentane, 1,2,3,4,5-pentamethyl- | 996 | 69 | 0.86 | 0.00 | 0.094 | *** |
| Cyclohexane, butylidene- | 1042 | 67 | 0.00 | 0.72 | 0.078 | *** |
| Cyclodecene, (Z)- | 1042 | 67 | 3.64 | 0.00 | 0.371 | *** |
| Cyclopropane | 1063 | 41 | 3.04 | 0.00 | 0.318 | *** |
| Cyclohexane, 1,2-diethyl-1-methyl- | 1075 | 125 | 0.52 | 0.00 | 0.057 | *** |
| Cyclopentane, pentyl- | 1084 | 68 | 1.87 | 0.00 | 0.193 | *** |
| D-Limonene | 1085 | 93 | 0.00 | 0.90 | 0.099 | *** |
| Cyclooctane, methyl- | 1129 | 55 | 0.00 | 0.74 | 0.115 | *** |
| Cyclopentane, 1-ethyl-1-methyl- | 1143 | 83 | 0.00 | 2.05 | 0.216 | *** |
| Butane, 2-cyclopropyl- | 1165 | 70 | 0.98 | 0.00 | 0.116 | *** |
| Cyclododecane | 1249 | 83 | 0.62 | 0.00 | 0.072 | *** |
| Heptylcyclohexane | 1322 | 82 | 0.95 | 0.78 | 0.094 | ns |
| Cyclopropane, 1,1,2,3-tetramethyl- | 1374 | 71 | 0.00 | 0.46 | 0.051 | *** |
| Cyclohexane, octyl- | 1444 | 82 | 0.00 | 0.24 | 0.024 | *** |
| Total cyclic hydrocarbons | 36.81 | 29.89 | 0.980 | *** | ||
| Benzene-derived hydrocarbons | ||||||
| Ethylbenzene | 928 | 91 | 0.82 | 0.00 | 0.087 | *** |
| Benzene, 1,3-dimethyl- | 937 | 106 | 2.54 | 1.58 | 0.177 | ** |
| Benzene, n-butyl- | 1118 | 91 | 0.91 | 0.00 | 0.097 | *** |
| Benzene, (1,1-dimethylethoxy)- | 1137 | 94 | 3.24 | 0.54 | 0.286 | *** |
| Total benzene-derived hydrocarbons | 7.50 | 2.12 | 0.590 | *** | ||
| TOTAL HYDROCARBONS | 386.00 | 441.44 | 18.278 | ns | ||
| LRI | m/z | Intensive | Extensive | SEM | Sig. | |
|---|---|---|---|---|---|---|
| Acids | ||||||
| Acetic acid | 696 | 60 | 0.05 | 0.45 | 0.044 | *** |
| 2-Propenoic acid | 709 | 55 | 0.00 | 3.64 | 0.393 | *** |
| Butanoic acid | 929 | 60 | 1.10 | 1.91 | 0.164 | * |
| Pentanoic acid | 1101 | 60 | 0.00 | 1.67 | 0.204 | *** |
| Hexanoic acid | 1102 | 60 | 1.33 | 0.00 | 0.158 | *** |
| Pentanoic acid, 2-methyl-, anhydride | 1157 | 99 | 0.04 | 0.95 | 0.106 | *** |
| Nonanoic acid | 1314 | 60 | 0.00 | 0.26 | 0.031 | *** |
| Total acids | 2.51 | 8.87 | 0.678 | *** | ||
| Alcohols | ||||||
| Glycidol | 499 | 44 | 2.10 | 90.02 | 12.230 | *** |
| 1-Propanol | 570 | 59 | 0.20 | 1.05 | 0.100 | *** |
| 1-Butanol | 709 | 56 | 2.01 | 29.94 | 3.053 | *** |
| 1-Butanol, 3-methyl- | 814 | 55 | 0.22 | 1.89 | 0.199 | *** |
| 1-Butanol, 2-methyl- | 818 | 57 | 0.00 | 4.70 | 0.506 | *** |
| 1-Pentanol | 855 | 55 | 0.00 | 33.61 | 3.542 | *** |
| Cyclobutanol, 2-ethyl- | 875 | 56 | 1.03 | 0.00 | 0.130 | *** |
| 2-Octen-1-ol, (Z)- | 875 | 67 | 0.72 | 0.00 | 0.098 | *** |
| 2,3-Butanediol, [S-(R*,R*)]- | 929 | 45 | 3.44 | 0.00 | 0.408 | *** |
| DL-2,3-Butanediol | 931 | 45 | 0.00 | 0.73 | 0.084 | *** |
| 1-Butanol, 3-methyl-, acetate | 952 | 55 | 0.05 | 1.37 | 0.306 | * |
| 1-Hexanol | 967 | 55 | 3.70 | 7.84 | 0.632 | *** |
| 1-Heptanol | 1062 | 70 | 4.66 | 5.29 | 0.449 | ns |
| 1-Octen-3-ol | 1068 | 57 | 39.65 | 33.11 | 3.525 | ns |
| Ethanol, pentamethyl- | 1079 | 59 | 0.00 | 0.72 | 0.074 | *** |
| 2,3,4-Trimethyl-1-pentanol | 1099 | 71 | 6.45 | 0.00 | 0.795 | *** |
| 1-Hexanol, 2-ethyl- | 1113 | 57 | 4.91 | 2.62 | 0.371 | *** |
| 1-Hexanol, 5-methyl-2-(1-methylethyl)- | 1128 | 71 | 0.94 | 0.00 | 0.109 | *** |
| 1-Undecanol | 1129 | 69 | 0.00 | 0.32 | 0.035 | *** |
| 4-Ethylcyclohexanol | 1130 | 81 | 0.24 | 0.41 | 0.052 | ns |
| Benzyl alcohol | 1145 | 108 | 0.27 | 0.00 | 0.030 | *** |
| 5-Methyl-1-heptanol | 1143 | 70 | 1.11 | 2.70 | 0.220 | *** |
| 1-Octanol | 1147 | 56 | 3.25 | 3.95 | 0.289 | ns |
| 2-Octen-1-ol, (E)- | 1148 | 57 | 1.62 | 1.94 | 0.203 | ns |
| 3-Octen-2-ol, (E)- | 1148 | 67 | 0.00 | 1.02 | 0.117 | *** |
| 3-Octen-1-ol, (Z)- | 1149 | 81 | 0.69 | 0.00 | 0.116 | ** |
| 1-Butanol, 2-methyl-, trifluoroacetate | 1152 | 70 | 3.35 | 0.00 | 0.361 | *** |
| 1,8-Octanediol | 1168 | 55 | 0.00 | 4.04 | 0.562 | *** |
| 6-Undecanol | 1183 | 55 | 0.00 | 0.78 | 0.093 | *** |
| 4-Methyl-5-decanol | 1184 | 83 | 0.42 | 0.00 | 0.066 | *** |
| 1-Butanol, 3,3-dimethyl- | 1189 | 56 | 0.00 | 0.37 | 0.038 | *** |
| 1,9-Nonanediol | 1224 | 55 | 0.00 | 0.20 | 0.021 | *** |
| 1-Nonanol | 1224 | 56 | 0.21 | 0.15 | 0.014 | * |
| 1-Butanol, 2-methyl-, propanoate | 1349 | 57 | 0.00 | 0.52 | 0.053 | *** |
| 2,4-Di-tert-butylphenol | 1456 | 191 | 2.52 | 0.00 | 0.353 | *** |
| Total alcohols | 83.76 | 229.30 | 16.597 | *** | ||
| LRI | m/z | Intensive | Extensive | SEM | Sig. | |
|---|---|---|---|---|---|---|
| Aldehydes | ||||||
| Propanal, 2-methyl- | 556 | 72 | 0.00 | 0.21 | 0.021 | *** |
| Butanal, 3-methyl- | 659 | 58 | 0.26 | 0.57 | 0.050 | *** |
| Butanal, 2-methyl- | 671 | 57 | 0.14 | 1.00 | 0.097 | *** |
| 2-Butenal | 841 | 70 | 0.39 | 0.00 | 0.047 | *** |
| Hexanal | 874 | 56 | 1.07 | 0.97 | 0.109 | ns |
| Heptanal | 987 | 70 | 0.27 | 0.95 | 0.100 | *** |
| Hexanal, 3-methyl- | 988 | 55 | 0.23 | 0.00 | 0.034 | *** |
| Hexanal, 3,3-dimethyl- | 1006 | 69 | 0.92 | 0.00 | 0.099 | *** |
| Octanal | 1084 | 84 | 2.30 | 0.00 | 0.276 | *** |
| Benzeneacetaldehyde | 1139 | 91 | 0.09 | 0.23 | 0.026 | ** |
| 2-Propenal | 1148 | 55 | 0.00 | 4.04 | 0.437 | *** |
| Nonanal | 1168 | 57 | 0.00 | 2.56 | 0.278 | *** |
| 2-Decenal, (E)- | 1298 | 83 | 0.40 | 0.00 | 0.039 | *** |
| 2-Decenal, (Z)- | 1299 | 70 | 0.32 | 0.00 | 0.040 | *** |
| Total aldehydes | 6.40 | 10.53 | 0.555 | *** | ||
| Ketones | ||||||
| 2,3-Butanedione | 589 | 86 | 15.54 | 0.00 | 1.711 | *** |
| 2-Butanone | 593 | 72 | 0.72 | 1.93 | 0.138 | *** |
| 2-Pentanone | 724 | 86 | 0.23 | 0.66 | 0.047 | *** |
| 3-Pentanone | 735 | 57 | 6.65 | 0.00 | 0.909 | *** |
| 2,3-Pentanedione | 739 | 100 | 0.00 | 0.79 | 0.097 | *** |
| 1,5-Heptadien-4-one, 3,3,6-trimethyl- | 779 | 83 | 0.00 | 0.99 | 0.115 | *** |
| Cyclobutanone, 2,2,3-trimethyl- | 815 | 70 | 0.00 | 2.90 | 0.306 | *** |
| 3-Heptanone | 973 | 57 | 0.32 | 2.32 | 0.258 | *** |
| 2-Heptanone | 980 | 58 | 1.68 | 8.67 | 0.851 | *** |
| Pyrolo[3,2-d]pyrimidin-2,4(1H,3H)-dione | 1057 | 151 | 9.83 | 8.21 | 0.456 | ns |
| 3-Ethylcyclopentanone | 1058 | 83 | 0.00 | 0.37 | 0.042 | *** |
| 4-Octanone, 5-hydroxy-2,7-dimethyl- | 1059 | 69 | 0.00 | 2.49 | 0.283 | *** |
| Butyrolactone | 1061 | 86 | 2.71 | 0.00 | 0.283 | *** |
| 4-Hexen-3-one, 5-methyl- | 1062 | 83 | 0.41 | 0.00 | 0.050 | *** |
| 3-Heptanone, 5-methyl- | 1069 | 99 | 3.28 | 0.00 | 0.395 | *** |
| 5-Hepten-2-one, 6-methyl- | 1073 | 68 | 0.74 | 0.49 | 0.059 | * |
| 2-Octanone | 1077 | 58 | 2.16 | 4.08 | 0.334 | * |
| 2(3H)-Furanone, dihydro-5-methyl- | 1095 | 56 | 0.00 | 8.53 | 1.108 | *** |
| 5-Hexen-3-one | 1151 | 98 | 0.90 | 1.24 | 0.076 | * |
| 3-Nonanone | 1155 | 72 | 0.61 | 0.57 | 0.050 | ns |
| 2-Nonanone | 1161 | 58 | 0.86 | 0.65 | 0.039 | ** |
| 2(3H)-Furanone, 5-ethyldihydro- | 1179 | 85 | 0.48 | 0.56 | 0.040 | ns |
| 2-Undecanone | 1310 | 58 | 0.36 | 0.00 | 0.038 | *** |
| 2(3H)-Furanone, dihydro-5-pentyl- | 1400 | 85 | 0.00 | 0.36 | 0.037 | *** |
| Total ketones | 47.48 | 45.81 | 10.626 | ns | ||
| LRI | m/z | Intensive | Extensive | SEM | Sig. | |
|---|---|---|---|---|---|---|
| Esters | ||||||
| Acetic acid, methyl ester | 537 | 74 | 0.18 | 0.46 | 0.044 | *** |
| Ethyl Acetate | 598 | 43 | 0.64 | 4.17 | 0.439 | *** |
| Formic acid, ethenyl ester | 708 | 43 | 0.00 | 11.24 | 1.193 | *** |
| Butanoic acid, ethyl ester | 856 | 70 | 1.51 | 0.00 | 0.158 | *** |
| Formic acid, heptyl ester | 1062 | 56 | 0.00 | 5.69 | 0.599 | *** |
| Sulfurous acid, 2-ethylhexyl nonyl ester | 1086 | 57 | 15.82 | 0.00 | 1.848 | *** |
| Formic acid, octyl ester | 1147 | 55 | 0.00 | 3.78 | 0.431 | *** |
| Propanoic acid, 2-methyl-, 2-propenyl ester | 1177 | 71 | 0.00 | 0.55 | 0.070 | *** |
| Butanoic acid, 2-propenyl ester | 1183 | 71 | 0.00 | 0.63 | 0.080 | *** |
| 2-Butenoic acid, 2-methyl-, 2-methylpropyl ester | 1183 | 83 | 0.31 | 0.45 | 0.039 | ns |
| 2-Propenoic acid, 2-methyl-, (tetrahydro-2-furanyl)methyl ester | 1297 | 71 | 1.00 | 0.00 | 0.103 | *** |
| Sulfurous acid, hexyl nonyl ester | 1298 | 85 | 1.70 | 0.00 | 0.182 | *** |
| Sulfurous acid, 2-ethylhexyl hexyl ester | 1331 | 85 | 0.00 | 0.45 | 0.049 | *** |
| Propanoic acid, 2-methyl-, 2-methylpropyl ester | 1384 | 71 | 0.00 | 0.38 | 0.046 | *** |
| Sulfurous acid, 2-ethylhexyl isohexyl ester | 1412 | 57 | 0.46 | 0.00 | 0.054 | *** |
| Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters | 1454 | 191 | 0.00 | 0.49 | 0.060 | *** |
| Total esters | 21.62 | 28.30 | 1.509 | * | ||
| Ethers | ||||||
| Ether, 3-butenyl pentyl | 1046 | 55 | 0.00 | 4.69 | 0.532 | *** |
| Ether, 2-ethylhexyl tert-butyl | 1090 | 57 | 28.67 | 0.00 | 3.068 | *** |
| Decyl heptyl ether | 1169 | 57 | 2.15 | 0.00 | 0.271 | *** |
| Total ethers | 30.83 | 4.69 | 2.849 | *** | ||
| Furans | ||||||
| Furan, 2-ethyl- | 706 | 81 | 0.90 | 4.75 | 0.468 | *** |
| Furan, 2,3-dihydro- | 806 | 70 | 0.00 | 1.76 | 0.228 | *** |
| 2-n-Butyl furan | 956 | 81 | 0.33 | 0.47 | 0.042 | ns |
| Furan, 2-pentyl- | 1054 | 81 | 17.49 | 6.41 | 1.331 | *** |
| Total furans | 18.73 | 13.38 | 1.059 | ** | ||
| Sulfur compounds | ||||||
| Dimethyl sulfide | 528 | 62 | 0.43 | 1.67 | 0.211 | ** |
| Carbon disulfide | 532 | 76 | 47.57 | 21.34 | 4.129 | *** |
| Dimethyl sulfone | 1090 | 79 | 0.30 | 0.81 | 0.091 | ** |
| Total sulfur compounds | 48.30 | 23.81 | 4.010 | ** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echegaray, N.; Domínguez, R.; Cadavez, V.A.P.; Bermúdez, R.; Purriños, L.; Gonzales-Barron, U.; Hoffman, E.; Lorenzo, J.M. Influence of the Production System (Intensive vs. Extensive) at Farm Level on Proximate Composition and Volatile Compounds of Portuguese Lamb Meat. Foods 2021, 10, 1450. https://doi.org/10.3390/foods10071450
Echegaray N, Domínguez R, Cadavez VAP, Bermúdez R, Purriños L, Gonzales-Barron U, Hoffman E, Lorenzo JM. Influence of the Production System (Intensive vs. Extensive) at Farm Level on Proximate Composition and Volatile Compounds of Portuguese Lamb Meat. Foods. 2021; 10(7):1450. https://doi.org/10.3390/foods10071450
Chicago/Turabian StyleEchegaray, Noemí, Rubén Domínguez, Vasco A. P. Cadavez, Roberto Bermúdez, Laura Purriños, Ursula Gonzales-Barron, Ettiene Hoffman, and José M. Lorenzo. 2021. "Influence of the Production System (Intensive vs. Extensive) at Farm Level on Proximate Composition and Volatile Compounds of Portuguese Lamb Meat" Foods 10, no. 7: 1450. https://doi.org/10.3390/foods10071450
APA StyleEchegaray, N., Domínguez, R., Cadavez, V. A. P., Bermúdez, R., Purriños, L., Gonzales-Barron, U., Hoffman, E., & Lorenzo, J. M. (2021). Influence of the Production System (Intensive vs. Extensive) at Farm Level on Proximate Composition and Volatile Compounds of Portuguese Lamb Meat. Foods, 10(7), 1450. https://doi.org/10.3390/foods10071450









