Microstructure and Physicochemical Properties of Light Ice Cream: Effects of Extruded Microparticulated Whey Proteins and Process Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
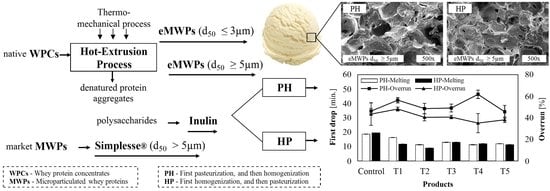
2.2. Experimental Design
2.3. eMWPs and Particle Size Distribution
2.4. Manufacturing of Ice Cream
2.5. Chemical Composition Analysis
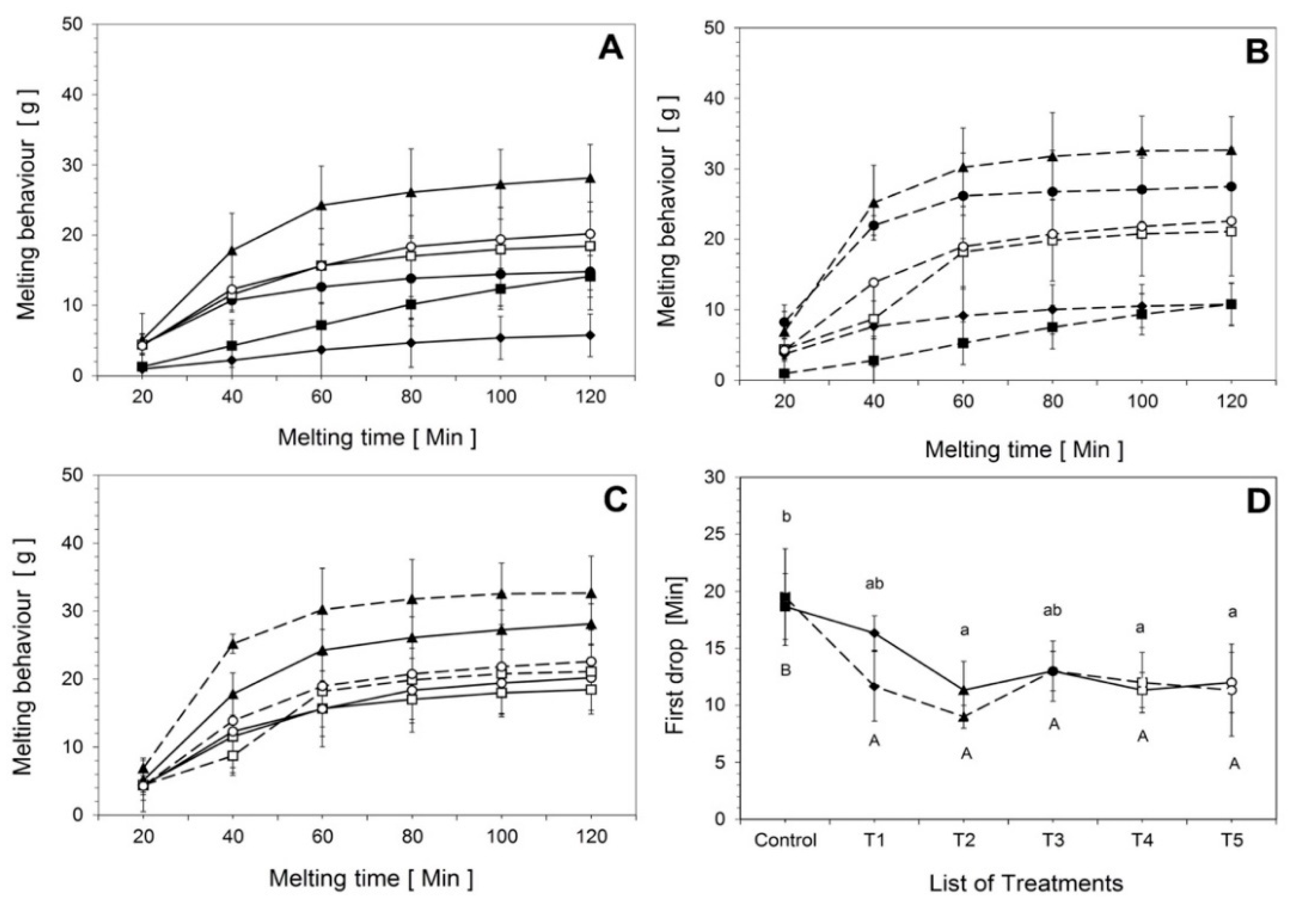
2.6. Melting Behavior
2.7. Overrun
2.8. Rheological Properties of Ice Cream
2.8.1. Texture analysis
2.8.2. Dynamic Viscosity Analysis
2.9. Microstructure Analysis of Ice Cream
2.10. Color Properties
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Fat Replacers on the Physicochemical Properties of Ice Cream
3.1.1. Chemical Composition of Ice Cream Mixes
3.1.2. Melting Behavior and Time until First Drop
3.1.3. Color of Ice Cream
3.2. Rheological Properties
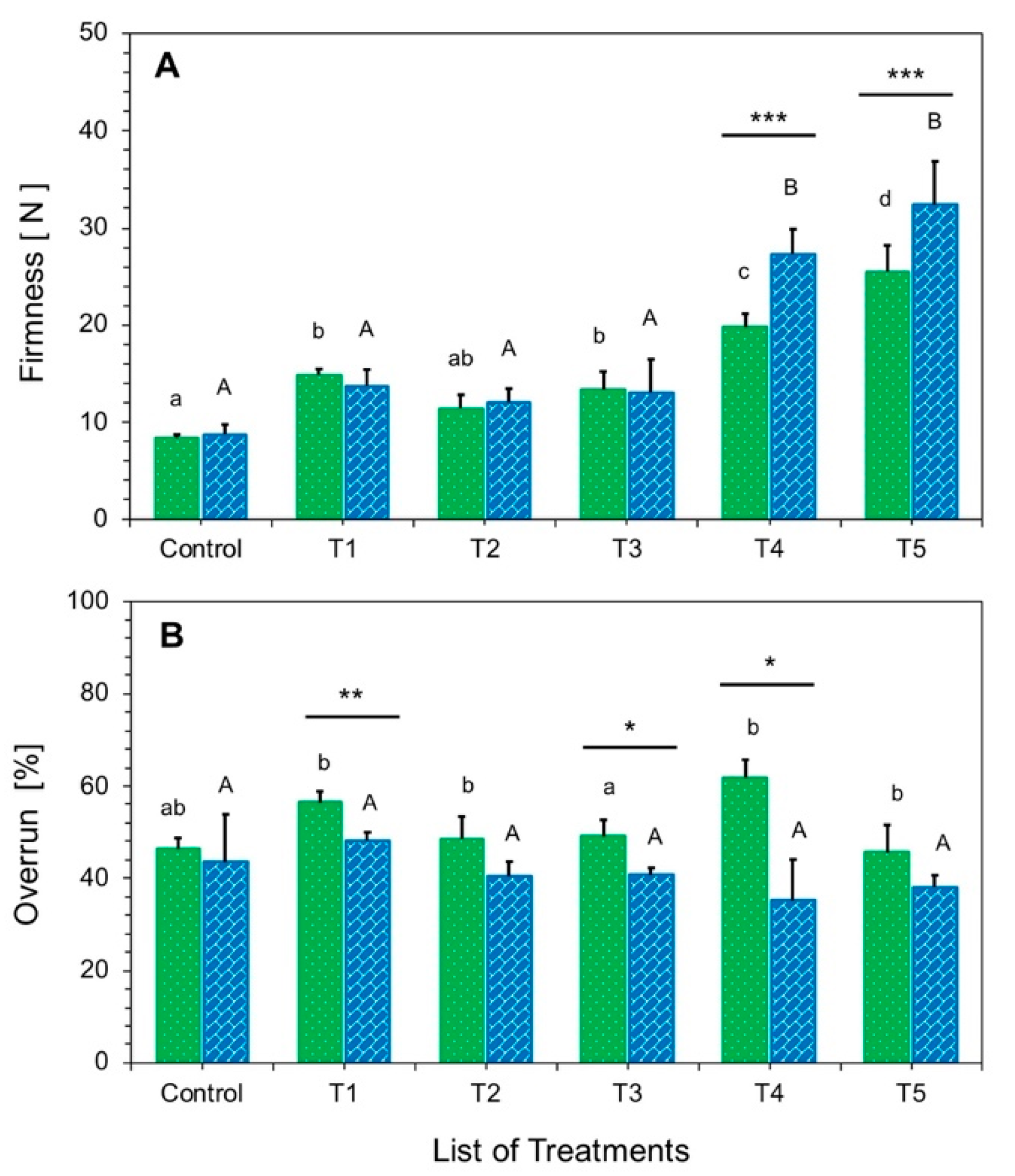
3.2.1. Firmness and Incorporated Air in Ice Cream
3.2.2. Viscosity of Ice Cream
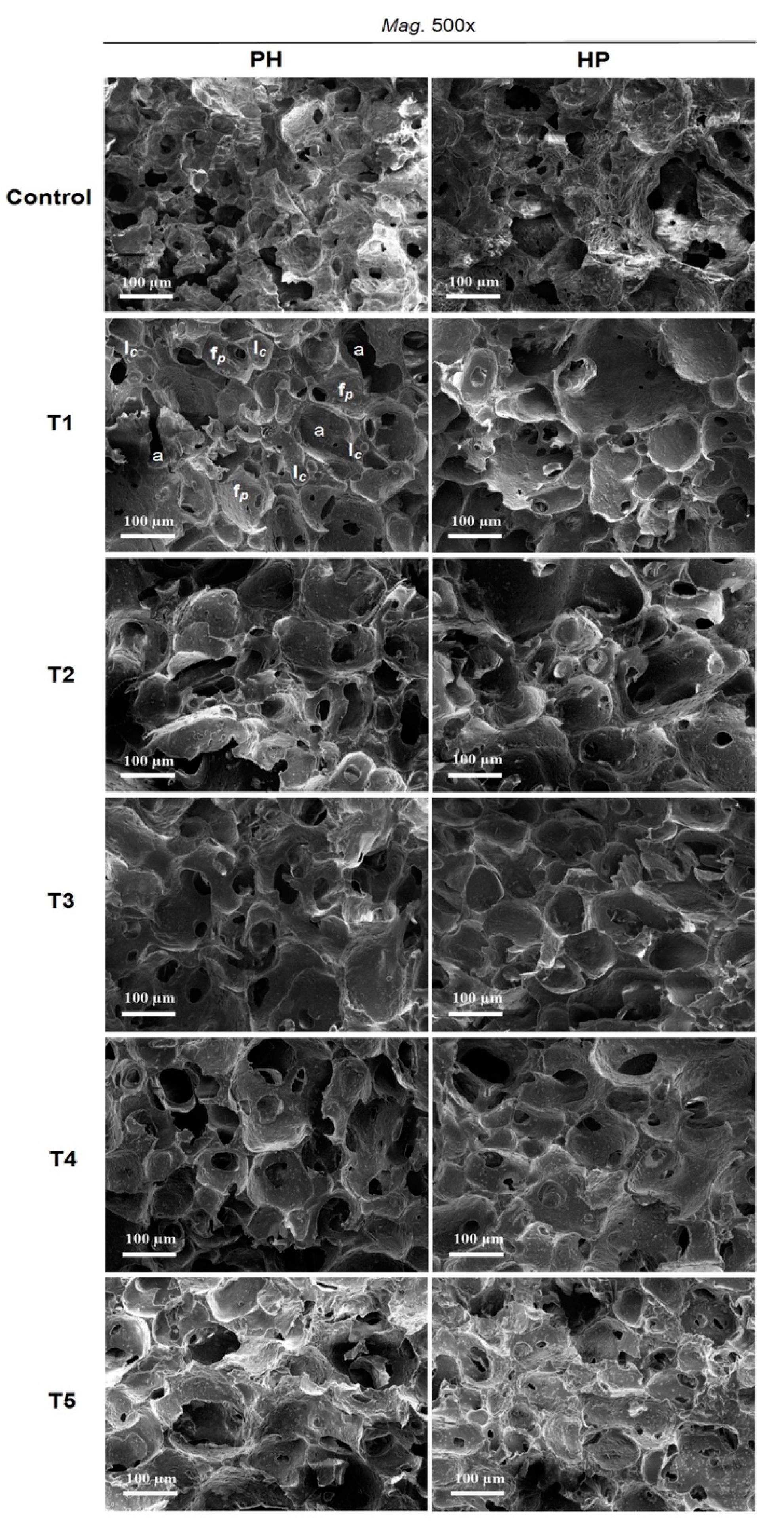
3.3. Effect of Fat Replacers on Microstructural Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quevedo, M.; Kulozik, U.; Karbstein, H.P.; Emin, M.A. Kinetics of Denaturation and Aggregation of Highly Concentrated β-Lactoglobulin under Defined Thermomechanical Treatment. J. Food Eng. 2020, 274, 109825. [Google Scholar] [CrossRef]
- Hossain, M.K.; Krah, A.; Hensel, O.; Diakité, M. Microparticulation of Whey Protein Concentrates Using the Hot Extrusion Process: The Influence of Protein Concentrations and Other Parameters. J. Food Process Technol. 2019, 10, 1–6. [Google Scholar]
- Wolz, M.; Kulozik, U. System Parameters in a High Moisture Extrusion Process for Microparticulation of Whey Proteins. J. Food Eng. 2017, 209, 12–17. [Google Scholar] [CrossRef]
- Yan, L.; Yu, D.; Liu, R.; Jia, Y.; Zhang, M.; Wu, T.; Sui, W. Microstructure and Meltdown Properties of Low-Fat Ice Cream: Effects of Microparticulated Soy Protein Hydrolysate/Xanthan Gum (Msph/Xg) Ratio and Freezing Time. J. Food Eng. 2021, 291, 110291. [Google Scholar] [CrossRef]
- Hossain, M.K.; Keidel, J.; Hensel, O.; Diakité, M. The Impact of Extruded Microparticulated Whey Proteins in Reduced-Fat, Plain-Type Stirred Yogurt: Characterization of Physicochemical and Sensory Properties. LWT 2020, 134, 109976. [Google Scholar] [CrossRef]
- Akalın, A.S.; Karagözlü, C.; Ünal, G. Rheological Properties of Reduced-Fat and Low-Fat Ice Cream Containing Whey Protein Isolate and Inulin. Eur. Food Res. Technol. 2007, 227, 889–895. [Google Scholar] [CrossRef]
- Goff, H.D.; Hartel, R.W. Ice Cream, 7th ed.; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Torres, I.C.; Mutaf, G.; Larsen, F.H.; Ipsen, R. Effect of Hydration of Microparticulated Whey Protein Ingredients on Their Gelling Behaviour in a Non-Fat Milk System. J. Food Eng. 2016, 184, 31–37. [Google Scholar] [CrossRef]
- Warren, M.M.; Hartel, R.W. Effects of Emulsifier, Overrun and Dasher Speed on Ice Cream Microstructure and Melting Prop-Erties. J. Food Sci. 2018, 83, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, C.F.; Silva, H.; Cavalcanti, R.; Esmerino, E.; Cappato, L.; Abud, Y.; Moraes, J.; Andrade, M.; Freitas, M.; Sant’Anna, C.; et al. Prebiotics Addition in Sheep Milk Ice Cream: A Rheological, Microstructural and Sensory Study. J. Funct. Foods 2017, 35, 564–573. [Google Scholar] [CrossRef]
- Tekin, E.; Sahin, S.; Sumnu, G. Physicochemical, Rheological, and Sensory Properties of Low-Fat Ice Cream Designed by Double Emulsions. Eur. J. Lipid Sci. Technol. 2017, 119, 1–9. [Google Scholar] [CrossRef]
- Rolon, M.L.; Bakke, A.J.; Coupland, J.N.; Hayes, J.E.; Roberts, R.F. Effect of Fat Content on the Physical Properties and Con-sumer Acceptability of Vanilla Ice Cream. J. Dairy Sci. 2017, 100, 5217–5227. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Jo, Y.-J.; Chun, J.-Y.; Hong, G.-P.; Davaatseren, M.; Choi, M.-J. Effect of Frozen Storage Temperature on the Quality of Premium Ice Cream. Food Sci. Anim. Resour. 2015, 35, 793–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.; Baer, R.; Acharya, M. Increasing the Protein Content of Ice Cream. J. Dairy Sci. 2006, 89, 1400–1406. [Google Scholar] [CrossRef]
- Güven, M.; Kalender, M.; Taşpinar, T. Effect of Using Different Kinds and Ratios of Vegetable Oils on Ice Cream Quality Char-acteristics. Foods 2018, 7, 104. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Sharma, H.K.; Kumar, N.; Kaur, M. The Effect of Inulin as a Fat Replacer on the Quality of Low-Fat Ice Cream. Int. J. Dairy Technol. 2015, 68, 374–380. [Google Scholar] [CrossRef]
- Akbari, M.; Eskandari, M.H.; Davoudi, Z. Application and Functions of Fat Replacers in Low-Fat Ice Cream: A review. Trends Food Sci. Technol. 2019, 86, 34–40. [Google Scholar] [CrossRef]
- Meyer, D.; Bayarri, S.; Tárrega, A.; Costell, E. Inulin as Texture Modifier in Dairy Products. Food Hydrocoll. 2011, 25, 1881–1890. [Google Scholar] [CrossRef]
- Cavender, G.A.; Kerr, W.L. Microfluidization of Full-Fat Ice Cream Mixes: Effects on Rheology and Microstructure. J. Food Process. Eng. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Cavender, G.A.; Kerr, W.L. Microfluidization of Full-Fat Ice Cream Mixes: Effects of Gum Stabilizer Choice on Physical and Sensory Changes. J. Food Process. Eng. 2011, 36, 29–35. [Google Scholar] [CrossRef]
- Monteagudo, S.M.; Kamat, S.; Patel, N.; Konuklar, G.; Rangavajla, N.; Balasubramaniam, V. Improvements in Emulsion Stability of Dairy Beverages Treated by High Pressure Homogenization: A Pilot-Scale Feasibility Study. J. Food Eng. 2017, 193, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Martínez, A.D.L.C.; Portales, R.E.D.; Martínez, J.D.P.; Ramírez, J.E.G.; Lara, A.D.V.; Enríquez, A.J.B.; Moscosa Santillán, M. Estimation of Ice Cream Mixture Viscosity during Batch Crystallization in a Scraped Surface Heat Exchang-er. Processes 2020, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Buyck, J.; Baer, R.; Choi, J. Effect of Storage Temperature on Quality of Light and Full-Fat Ice Cream. J. Dairy Sci. 2011, 94, 2213–2219. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; He, Y.; Feng, S. Measurement of Yogurt Internal Quality through Using Vis/Nir Spectroscopy. Food Res. Int. 2007, 40, 835–841. [Google Scholar] [CrossRef]
- Fiol, C.; Prado, D.; Romero, C.; Laburu, N.; Mora, M.; Alava, J.I. Introduction of a New Family of Ice Creams. Int. J. Gastron. Food Sci. 2017, 7, 5–10. [Google Scholar] [CrossRef]
- Limanowski, J.; Blankenburg, F. Integration of Visual and Proprioceptive Limb Position Information in Human Posterior Parietal, Premotor, and Extrastriate Cortex. J. Neurosci. 2016, 36, 2582–2589. [Google Scholar] [CrossRef] [Green Version]
- Labropoulos, A.E.; Lopez, A.; Palmer, J.K. Apparent Viscosity of Milk and Cultured Yogurt Thermally Treated by UHT and Vat Systems. J. Food Prot. 1981, 44, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Caillet, A.; Cogné, C.; Andrieu, J.; Laurent, P.; Rivoire, A. Characterization of Ice Cream Structure by Direct Optical Micros-Copy. Influence of freezing parameters. LWT-Food Sci. Technol. 2003, 36, 743–749. [Google Scholar] [CrossRef]
- Fischer, A.; Scholtes, B.; Niendorf, T. On the Influence of Surface Hardening Treatments on Microstructure Evolution and Residual Stress in Microalloyed Medium Carbon Steel. J. Mater. Eng. Perform. 2020, 29, 3040–3054. [Google Scholar] [CrossRef]
- Choi, M.J.; Shin, K.S. Studies on Physical and Sensory Properties of Premium Vanilla Ice Cream Distributed in Korean Mar-ket. Korean J. Food Sci. Anim. Resour. 2014, 34, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Donhowe, D.P.; Hartel, R.W.; Bradley, R.L., Jr. Determination of Ice Crystal Size Distributions in Frozen Desserts. J. Dairy Sci. 1991, 74, 3334–3344. [Google Scholar] [CrossRef]
- Muse, M.; Hartel, R. Ice Cream Structural Elements that Affect Melting Rate and Hardness. J. Dairy Sci. 2004, 87, 1–10. [Google Scholar] [CrossRef]
- Liou, B.-K.; Grün, I. Effect of Fat Level on the Perception of Five Flavor Chemicals in Ice Cream with or without Fat Mimetics by Using a Descriptive Test. J. Food Sci. 2007, 72, S595–S604. [Google Scholar] [CrossRef] [PubMed]
- Roland, A.M.; Phillips, L.G.; Boor, K.J. Effects of Fat Content on the Sensory Properties, Melting, Color, and Hardness of Ice Cream. J. Dairy Sci. 1999, 82, 32–38. [Google Scholar] [CrossRef]
- El-Nagar, G.; Clowes, G.; Tudoricǎ, C.M.; Kuri, V.; Brennan, C.S. Rheological Quality and Stability of Yog-Ice cream with Added inulin. Int. J. Dairy Technol. 2002, 55, 89–93. [Google Scholar] [CrossRef]
- Wildmoser, H.; Scheiwiller, J.; Windhab, E.J. Impact of Disperse Microstructure on Rheology and Quality Aspects of Ice Cream. LWT 2004, 37, 881–891. [Google Scholar] [CrossRef]
- Long, Z.; Zhao, M.; Zhao, Q.; Yang, B.; Liu, L. Effect of Homogenisation and Storage Time on Surface and Rheology Proper-Ties of Whipping Cream. Food Chem. 2012, 131, 748–753. [Google Scholar] [CrossRef]
- Lim, S.Y.; Swanson, B.G.; Ross, C.F.; Clark, S. High Hydrostatic Pressure Modification of Whey Protein Concentrate for im-Proved Body and Texture of Low-Fat Ice Cream. J. Dairy Sci. 2008, 91, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, F.; Xu, Z.; Sui, H.; Fang, Y.; Tang, X.; Shen, X. Characterization of Soybean Protein Adhesives Modified by Xanthan Gum. Coatings 2018, 8, 342. [Google Scholar] [CrossRef] [Green Version]
- Turan, S.; Kirkland, M.; Trusty, P.A.; Campbell, I. Interaction of Fat and Air in Ice Cream. Dairy Ind. Int. 1999, 64, 27–31. [Google Scholar]


| Major Ingredients | Treatments a | |||||
|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | T4 | T5 | |
| Fat f (%) | 12 | 6 | 6 | 6 | 6 | 6 |
| LSM b (g) | 445 | 639 | 639 | 639 | 517 | 515 |
| Cream (g) | 390 | 190 | 190 | 190 | 177 | 177 |
| SMP c (g) | 54 | – | – | – | 10 | 10 |
| Sugar (g) | 105 | 105 | 105 | 105 | 105 | 105 |
| eMWP d,f d50 < 3µm (g) | – | – | – | – | 185 | – |
| eMWP e,f d50 > 5 µm (g) | – | – | – | – | – | 187 |
| Inulin f (g) | – | 60 | – | 20 | – | – |
| Simplesse f (g) | – | – | 60 | 40 | – | – |
| Palsgaard (g) | 3 | 3 | 3 | 3 | 3 | 3 |
| Xanthan gum (g) | 1 | 1 | 1 | 1 | 1 | 1 |
| Vanilla flavour (g) | 2 | 2 | 2 | 2 | 2 | 2 |
| Treatments 4 | Components 1 (%) | |||||
|---|---|---|---|---|---|---|
| Dry Matter | Fat | Protein | ||||
| PH 2 | HP 3 | PH 2 | HP 3 | PH 2 | HP 3 | |
| Control | 35.57 ± 1.59 b | 35.91 ± 1.22 B | 12.56 ± 0.77 b | 13.04 ± 0.76 B | 6.04 ± 0.28 a | 6.15 ± 0.21 AB |
| T1 | 31.76 ± 0.35 a | 32.44 ± 0.15 A | 6.80 ± 0.67 a | 7.09 ± 0.30 A | 5.07 ± 0.11 a | 5.09 ± 0.12 A |
| T2 | 32.63 ± 0.76 a | 30.64 ± 0.29 A | 7.09 ± 0.30 a | 6.27 ± 0.51 A | 6.94 ± 0.66 ab | 6.43 ± 1.06 ABC |
| T3 | 31.79 ± 1.63 a | 31.75 ± 1.68 A | 6.73 ± 0.24 a | 6.05 ± 0.57 A | 5.75 ± 0.46 a | 5.62 ± 0.51 AB |
| T4 | 30.57 ± 1.97 a | 32.18 ± 2.01 A | 6.58 ± 0.86 a | 6.57 ± 0.85 A | 8.77 ± 0.97 b | 8.97 ± 0.88 C |
| T5 | 32.91 ± 2.46 a | 30.79 ± 2.54 A | 6.71 ± 0.44 a | 6.48 ± 0.52 A | 7.55 ± 1.38 ab | 8.12 ± 1.40 BC |
| Treatments 4 | Color properties 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| ∆E | L* | a * | b * | |||||
| PH 2 | HP 3 | PH 2 | HP 3 | PH 2 | HP 3 | PH 2 | HP 3 | |
| Control | 90.75 ± 0.49 a | 88.43 ± 1.51 A | 0.097 ± 0.09 a | 0.247 ± 0.12 A | 14.48 ± 0.47 a | 15.34 ± 0.84 A | – | – |
| T1 | 87.99 ± 1.44 ab | 87.72 ± 1.86 A | 0.077 ± 0.07 a | 0.100 ± 0.07 A | 10.69 ± 0.36 d | 10.66 ± 0.47 C | 4.77 ± 1.05 a | 5.47 ± 0.94 A |
| T2 | 84.43 ± 0.84 cd | 84.53 ± 1.68 AB | 0.197 ± 0.02 a | 0.153 ± 0.16 A | 13.38 ± 0.14 ab | 13.79 ± 0.79 AB | 6.46 ± 1.20 a | 4.65 ± 1.28 A |
| T3 | 86.94 ± 0.84 bc | 86.27 ± 1.45 AB | 0.177 ± 0.10 a | 0.127 ± 0.03 A | 11.40 ± 0.65 cd | 11.68 ± 0.79 BC | 5.02 ± 0.03 a | 4.61 ± 1.23 A |
| T4 | 84.55 ± 0.55 cd | 81.57 ± 1.20 B | 0.093 ± 0.04 a | 0.310 ± 0.20 A | 12.71 ± 0.36 bc | 14.01 ± 0.73 AB | 6.46 ± 0.75 a | 7.17 ± 2.17 A |
| T5 | 83.81 ± 0.46 d | 82.84 ± 0.30 B | 0.150 ± 0.17 a | 0.183 ± 0.07 A | 14.01 ± 0.36 ab | 13.62 ± 0.47 AB | 7.00 ± 0.78 a | 5.88 ± 1.83 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.K.; Petrov, M.; Hensel, O.; Diakité, M. Microstructure and Physicochemical Properties of Light Ice Cream: Effects of Extruded Microparticulated Whey Proteins and Process Design. Foods 2021, 10, 1433. https://doi.org/10.3390/foods10061433
Hossain MK, Petrov M, Hensel O, Diakité M. Microstructure and Physicochemical Properties of Light Ice Cream: Effects of Extruded Microparticulated Whey Proteins and Process Design. Foods. 2021; 10(6):1433. https://doi.org/10.3390/foods10061433
Chicago/Turabian StyleHossain, M Kamal, Miroslav Petrov, Oliver Hensel, and Mamadou Diakité. 2021. "Microstructure and Physicochemical Properties of Light Ice Cream: Effects of Extruded Microparticulated Whey Proteins and Process Design" Foods 10, no. 6: 1433. https://doi.org/10.3390/foods10061433
APA StyleHossain, M. K., Petrov, M., Hensel, O., & Diakité, M. (2021). Microstructure and Physicochemical Properties of Light Ice Cream: Effects of Extruded Microparticulated Whey Proteins and Process Design. Foods, 10(6), 1433. https://doi.org/10.3390/foods10061433