A Systematic Review of Listeria Species and Listeria monocytogenes Prevalence, Persistence, and Diversity throughout the Fresh Produce Supply Chain
Abstract
1. Introduction
2. Materials and Methods
2.1. Definitions and Scope
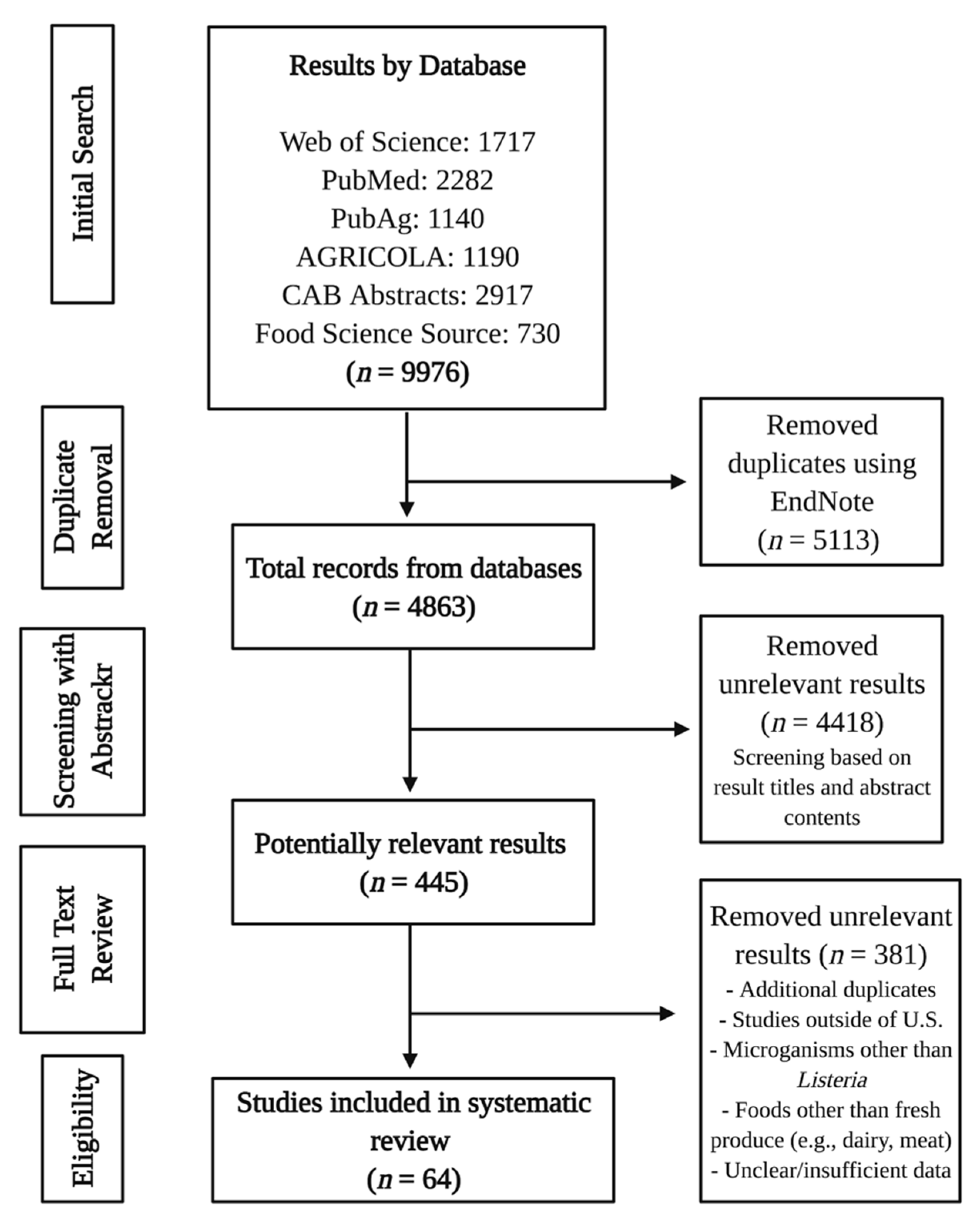
2.2. Literature Search
2.3. Study Selection
2.4. Data Extraction and Synthesis
3. Results
3.1. Included Study Characteristics
3.2. Natural Environment and Outdoor Production
3.2.1. Northeast Studies
3.2.2. Midwest Studies
3.2.3. Plains Studies
3.2.4. Pacific West Studies
3.2.5. Multiregional Studies
3.3. Packinghouse, Indoor Production, and Processing
3.3.1. Northeast Studies
3.3.2. Southern/Southeastern Studies
3.3.3. Pacific West Studies
3.3.4. Multiregional Studies
3.3.5. Other Relevant Studies
3.4. Transportation and Distribution
3.5. Retail
3.5.1. Northeast Studies
3.5.2. Midwest Studies
3.5.3. Pacific West Studies
3.5.4. Multiregional Studies
3.5.5. Other Relevant Studies
3.6. Farmers’ Market
3.6.1. Northeast Studies
3.6.2. Southeast Studies
3.6.3. Multiregional Studies
3.7. Restaurant
3.8. Domestic
4. Discussion
4.1. Natural Environments and Outdoor Production
4.2. Packinghouse, Indoor Production, and Processing
4.3. Transportation and Distribution
4.4. Retail
4.5. Farmers’ Market
4.6. Restaurant
4.7. Domestic
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orsi, R.H.; Wiedmann, M. Characteristics and distribution of Listeria spp., including Listeria species newly described since 2009. Appl. Microbiol. Biotechnol. 2016, 100, 5273–5287. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Listeria (Listeriosis). Available online: https://www.cdc.gov/listeria/index.html (accessed on 6 January 2019).
- Archer, D.L. The evolution of FDA’s policy on Listeria monocytogenes in ready-to-eat foods in the United States. Curr. Opin. Food Sci. 2018, 20, 64–68. [Google Scholar] [CrossRef]
- U.S. Food Drug Administration (FDA). The Bad Bug Book, Foodborne Pathogenic Microorganisms and Natural Toxins Handbook. Available online: https://www.fda.gov/food/foodborne-pathogens/bad-bug-book-second-edition (accessed on 8 January 2020).
- Farber, J.M.; Peterkin, P.I. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef] [PubMed]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Listeria Outbreaks. Available online: https://www.cdc.gov/listeria/outbreaks/index.html (accessed on 25 April 2020).
- Welshimer, H.J. Survival of Listeria monocytogenes in soil. J. Bacteriol. 1960, 80, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Dowe, M.J.; Jackson, E.D.; Mori, J.G.; Bell, C.R. Listeria monocytogenes survival in soil and incidence in agricultural soils. J. Food Prot. 1997, 60, 1201–1207. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S.D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review-Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Marik, C.M.; Zuchel, J.; Schaffner, D.W.; Strawn, L.K. Growth and Survival of Listeria monocytogenes on Intact Fruit and Vegetable Surfaces during Postharvest Handling: A Systematic Literature Review. J. Food Prot. 2020, 83, 108–128. [Google Scholar] [CrossRef]
- Cosgrove, S.; Cronquist, A.; Wright, G.; Ghosh, T.; Vogt, R.; Teitell, P.; Gelfius, A.; Spires, C.; Duvernoy, T.; Merriweather, S.; et al. Multistate Outbreak of Listeriosis Associated With Jensen Farms Cantaloupe-United States, August–September 2011 (Reprinted from MMWR, volume 60, pp. 1357–1358, 2011). JAMA 2011, 306, 2321. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms, Colorado (Final Update). Available online: https://www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/index.html (accessed on 22 April 2020).
- Gaul, L.K.; Farag, N.H.; Shim, T.; Kingsley, M.A.; Silk, B.J.; Hyytia-Trees, E. Hospital-acquired listeriosis outbreak caused by contaminated diced celery—Texas, 2010. Clin. Infect. Dis. 2013, 56, 20–26. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Listeriosis Linked to Commercially Produced, Prepackaged Caramel Apples Made from Bidart Bros. Apples (Final Update). Available online: https://www.cdc.gov/listeria/outbreaks/caramel-apples-12-14/index.html (accessed on 22 April 2020).
- Chen, Y.; Burall, L.S.; Luo, Y.; Timme, R.; Melka, D.; Muruvanda, T.; Payne, J.; Wang, C.; Kastanis, G.; Maounounen-Laasri, A.; et al. Listeria monocytogenes in stone fruits linked to a multistate outbreak: Enumeration of cells and whole-genome sequencing. Appl. Environ. Microbiol. 2016, 82, 7030–7040. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Outbreak of Listeria Infections Linked to Enoki Mushrooms. Available online: https://www.cdc.gov/listeria/outbreaks/enoki-mushrooms-03-20/index.html (accessed on 22 April 2020).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Listeriosis Linked to Frozen Vegetables (Final Update). Available online: https://www.cdc.gov/listeria/outbreaks/frozen-vegetables-05-16/index.html (accessed on 22 April 2020).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Listeriosis Linked to Packaged Salads Produced at Springfield, Ohio Dole Processing Facility (Final Update). Available online: https://www.cdc.gov/listeria/outbreaks/bagged-salads-01-16/index.html (accessed on 22 April 2020).
- U.S. Food and Drug Administration (FDA). FDA Investigated Listeria monocytogenes in Sprouts from Wholesome Soy Products, Inc. Available online: http://wayback.archive-it.org/7993/20171114154907/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/Outbreaks/ucm422562.htm (accessed on 22 April 2020).
- Centers for Disease Control and Prevention (CDC). National Outbreak Reporting System (NORS). Available online: www.cdc.gov/norsdashboard (accessed on 22 April 2020).
- Garner, D.; Kathariou, S. Fresh produce–associated listeriosis outbreaks, sources of concern, teachable moments, and insights. J. Food Prot. 2016, 79, 337–344. [Google Scholar] [CrossRef]
- Ho, J.L.; Shands, K.N.; Friedland, G.; Eckind, P.; Fraser, D.W. An outbreak of type-4B Listeria monocytogenes infection involving patients from 8 Boston hospitals. Arch. Intern. Med. 1986, 146, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Chapin, T.K.; Nightingale, K.K.; Worobo, R.W.; Wiedmann, M.; Strawn, L.K. Geographical and Meteorological Factors Associated with Isolation of Listeria Species in New York State Produce Production and Natural Environments. J. Food Prot. 2014, 77, 1919–1928. [Google Scholar] [CrossRef]
- Wallace, B.C.; Small, K.; Brodley, C.E.; Lau, J.; Trikalinos, T.A. Deploying an interactive machine learning system in an evidence-based practice center: Abstrackr. In Proceedings of the 2nd ACM SIGHIT international health informatics symposium, Miami, FL, USA, 28–30 January 2012; pp. 819–824. [Google Scholar]
- Ahlstrom, C.A.; Manuel, C.S.; Den Bakker, H.C.; Wiedmann, M.; Nightingale, K.K. Molecular ecology of Listeria spp., Salmonella, Escherichia coli O157:H7 and non-O157 Shiga toxin-producing E. coli in pristine natural environments in Northern Colorado. J. Appl. Microbiol. 2018, 124, 511–521. [Google Scholar] [CrossRef]
- Brinton, W.F.; Storms, P.; Blewett, T.C. Occurrence and Levels of Fecal Indicators and Pathogenic Bacteria in Market-Ready Recycled Organic Matter Composts. J. Food Prot. 2009, 72, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Heringa, S.; Kim, J.; Jiang, X.P. Analyzing Indicator Microorganisms, Antibiotic Resistant Escherichia coli, and Regrowth Potential of Foodborne Pathogens in Various Organic Fertilizers. Foodborne Pathog. Dis. 2013, 10, 520–527. [Google Scholar] [CrossRef]
- Cooley, M.B.; Quinones, B.; Oryang, D.; Mandrell, R.E.; Gorski, L. Prevalence of Shiga-toxin producing Escherichia coli, Salmonella enterica, and Listeria monocytogenes at public access watershed sites in a California Central Coast agricultural region. Front. Cell. Infect. Microbiol. 2014, 4, 13. [Google Scholar] [CrossRef]
- Cooley, M.B.; Carychao, D.; Gorski, L. Optimized Co-extraction and Quantification of DNA From Enteric Pathogens in Surface Water Samples Near Produce Fields in California. Front. Microbiol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Gorski, L.; Walker, S.; Liang, A.S.; Nguyen, K.M.; Govoni, J.; Carychao, D.; Cooley, M.B.; Mandrell, R.E. Comparison of Subtypes of Listeria monocytogenes Isolates from Naturally Contaminated Watershed Samples with and without a Selective Secondary Enrichment. PLoS ONE 2014, 9, e92467. [Google Scholar] [CrossRef]
- Tian, P.; Yang, D.; Shan, L.; Wang, D.P.; Li, Q.Q.; Gorski, L.; Lee, B.G.; Quinones, B.; Cooley, M.B. Concurrent Detection of Human Norovirus and Bacterial Pathogens in Water Samples from an Agricultural Region in Central California Coast. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Grewal, S.K.; Rajeev, S.; Sreevatsan, S.; Michel, F.C. Persistence of Mycobacterium avium subsp. paratuberculosis and other zoonotic pathogens during simulated composting, manure packing, and liquid storage of dairy manure. Appl. Environ. Microbiol. 2006, 72, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.Y.; Yin, H.B.; Ottesen, A.; Bolten, S.; Patel, J.; Rideout, S.; Nou, X.W. Microbiomes in Ground Water and Alternative Irrigation Water, and Spinach Microbiomes Impacted by Irrigation with Different Types of Water. Phytobiomes J. 2019, 3, 137–147. [Google Scholar] [CrossRef]
- Liao, C.H.; Honeycutt, C.W.; Griffin, T.S.; Jemison, J.M. Occurrence of gastrointestinal pathogens in soil of potato field treated with liquid dairy manure. J. Food Agric. Environ. 2003, 1, 224–228. [Google Scholar]
- Pang, H.; McEgan, R.; Mishra, A.; Micallef, S.A.; Pradhan, A.K. Identifying and modeling meteorological risk factors associated with pre-harvest contamination of Listeria species in a mixed produce and dairy farm. Food Res. Int. 2017, 102, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Prazak, A.M.; Murano, E.A.; Mercado, I.; Acuff, G.R. Prevalence of Listeria monocytogenes during production and postharvest processing of cabbage. J. Food Prot. 2002, 65, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Allard, S.M.; Callahan, M.T.; Bui, A.; Ferelli, A.M.C.; Chopyk, J.; Chattopadhyay, S.; Mongodin, E.F.; Micallef, S.A.; Sapkota, A.R. Creek to Table: Tracking fecal indicator bacteria, bacterial pathogens, and total bacterial communities from irrigation water to kale and radish crops. Sci. Total Environ. 2019, 666, 461–471. [Google Scholar] [CrossRef]
- Weller, D.; Wiedmann, M.; Strawn, L.K. Spatial and Temporal Factors Associated with an Increased Prevalence of Listeria monocytogenes in Spinach Fields in New York State. Appl. Environ. Microbiol. 2015, 81, 6059–6069. [Google Scholar] [CrossRef]
- Sauders, B.D.; Durak, M.Z.; Fortes, E.; Windham, K.; Schukken, Y.; Lembo, A.J.; Akey, B.; Nightingale, K.K.; Wiedmann, M. Molecular characterization of Listeria monocytogenes from natural and urban environments. J. Food Prot. 2006, 69, 93–105. [Google Scholar] [CrossRef]
- Sauders, B.D.; Overdevest, J.; Fortes, E.; Windham, K.; Schukken, Y.; Lembo, A.; Wiedmann, M. Diversity of Listeria Species in Urban and Natural Environments. Appl. Environ. Microbiol. 2012, 78, 4420–4433. [Google Scholar] [CrossRef]
- Weller, D.; Brassill, N.; Rock, C.; Ivanek, R.; Mudrak, E.; Roof, S.; Ganda, E.; Wiedmann, M. Complex Interactions between Weather, and Microbial and Physicochemical Water Quality Impact the Likelihood of Detecting Foodborne Pathogens in Agricultural Water. Front. Microbiol. 2020, 11, 20. [Google Scholar] [CrossRef]
- Zhu, L.; Torres, M.; Betancourt, W.Q.; Sharma, M.; Micallef, S.A.; Gerba, C.; Sapkota, A.R.; Sapkota, A.; Parveen, S.; Hashem, F.; et al. Incidence of fecal indicator and pathogenic bacteria in reclaimed and return flow waters in Arizona, USA. Environ. Res. 2019, 170, 122–127. [Google Scholar] [CrossRef]
- Sharma, M.; Handy, E.T.; East, C.L.; Kim, S.O.Y.; Jiang, C.S.; Callahan, M.T.; Allard, S.M.; Micallef, S.; Craighead, S.; Anderson-Coughlin, B.; et al. Prevalence of Salmonella and Listeria monocytogenes in non-traditional irrigation waters in the Mid-Atlantic United States is affected by water type, season, and recovery method. PLoS ONE 2020, 15, e229365. [Google Scholar] [CrossRef]
- Sheng, L.N.; Shen, X.Y.; Benedict, C.; Su, Y.; Tsai, H.C.; Schacht, E.; Kruger, C.E.; Drennan, M.; Zhu, M.J. Microbial Safety of Dairy Manure Fertilizer Application in Raspberry Production. Front. Microbiol. 2019, 10, 2276. [Google Scholar] [CrossRef]
- Strawn, L.K.; Fortes, E.D.; Bihn, E.A.; Nightingale, K.K.; Grohn, Y.T.; Worobo, R.W.; Wiedmann, M.; Bergholz, P.W. Landscape and Meteorological Factors Affecting Prevalence of Three Food-Borne Pathogens in Fruit and Vegetable Farms. Appl. Environ. Microbiol. 2013, 79, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Strawn, L.K.; Grohn, Y.T.; Warchocki, S.; Worobo, R.W.; Bihn, E.A.; Wiedmann, M. Risk Factors Associated with Salmonella and Listeria monocytogenes Contamination of Produce Fields. Appl. Environ. Microbiol. 2013, 79, 7618–7627. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.; Wiedmann, M.; Strawn, L.K. Irrigation Is Significantly Associated with an Increased Prevalence of Listeria monocytogenes in Produce Production Environments in New York State. J. Food Prot. 2015, 78, 1132–1141. [Google Scholar] [CrossRef]
- Viswanath, P.; Murugesan, L.; Knabel, S.J.; Verghese, B.; Chikthimmah, N.; LaBorde, L.F. Incidence of Listeria monocytogenes and Listeria spp. in a Small-Scale Mushroom Production Facility. J. Food Prot. 2013, 76, 608–615. [Google Scholar] [CrossRef]
- Xu, A.X.; Pahl, D.M.; Buchanan, R.L.; Micallef, S.A. Comparing the Microbiological Status of Pre- and Postharvest Produce from Small Organic Production. J. Food Prot. 2015, 78, 1072–1080. [Google Scholar] [CrossRef]
- Johnston, L.M.; Jaykus, L.A.; Moll, D.; Martinez, M.C.; Anciso, J.; Mora, B.; Moe, C.L. A field study of the microbiological quality of fresh produce. J. Food Prot. 2005, 68, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Q.; Chung, T.; Chen, Y.; Macarisin, D.; LaBorde, L.; Kovac, J. The occurrence of Listeria monocytogenes is associated with built environment microbiota in three tree fruit processing facilities. Microbiome 2019, 7, 18. [Google Scholar] [CrossRef]
- Johnston, L.M.; Jaykus, L.A.; Moll, D.; Anciso, J.; Mora, B.; Moe, C.L. A field study of the microbiological quality of fresh produce of domestic and Mexican origin. Int. J. Food Microbiol. 2006, 112, 83–95. [Google Scholar] [CrossRef]
- Estrada, E.M.; Hamilton, A.M.; Sullivan, G.B.; Wiedmann, M.; Critzer, F.J.; Strawn, L.K. Prevalence, Persistence, and Diversity of Listeria monocytogenes and Listeria Species in Produce Packinghouses in Three US States. J. Food Prot. 2020, 83, 277–286. [Google Scholar] [CrossRef]
- Sullivan, G.; Wiedmann, M. Detection and prevalence of Listeria in US produce packinghouses and fresh-cut facilities. J. Food Prot. 2020. [Google Scholar] [CrossRef]
- Ilic, S.; Odomeru, J.; LeJeune, J.T. Coliforms and Prevalence of Escherichia coli and Foodborne Pathogens on Minimally Processed Spinach in Two Packing Plants. J. Food Prot. 2008, 71, 2398–2403. [Google Scholar] [CrossRef]
- John, J.; Joy, W.C.; Jovana, K. Prevalence of Listeria spp. in produce handling and processing facilities in the Pacific Northwest. Food Microbiol. 2020, 90, 103468. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.A.; Harrison, M.A. Comparison of the microflora on organically and conventionally grown spring mix from a California processor. J. Food Prot. 2005, 68, 1143–1146. [Google Scholar] [CrossRef]
- Murugesan, L.; Kucerova, Z.; Knabel, S.J.; LaBorde, L.F. Predominance and Distribution of a Persistent Listeria monocytogenes Clone in a Commercial Fresh Mushroom Processing Environment. J. Food Prot. 2015, 78, 1988–1998. [Google Scholar] [CrossRef]
- Jarvis, K.G.; Daquigan, N.; White, J.R.; Morin, P.M.; Howard, L.M.; Manetas, J.E.; Ottesen, A.; Ramachandran, P.; Grim, C.J. Microbiomes Associated With Foods From Plant and Animal Sources. Front. Microbiol. 2018, 9, 2540. [Google Scholar] [CrossRef]
- Churchill, K.J.; Sargeant, J.M.; Farber, J.M.; O’Connor, A.M. Prevalence of Listeria monocytogenes in Select Ready-to-Eat Foods-Deli Meat, Soft Cheese, and Packaged Salad: A Systematic Review and Meta-Analysis. J. Food Prot. 2019, 82, 344–357. [Google Scholar] [CrossRef]
- Korir, R.C.; Parveen, S.; Hashem, F.; Bowers, J. Microbiological quality of fresh produce obtained from retail stores on the Eastern Shore of Maryland, United States of America. Food Microbiol. 2016, 56, 29–34. [Google Scholar] [CrossRef]
- Burnett, J.; Wu, S.T.; den Bakker, H.C.; Cook, P.W.; Veenhuizen, D.R.; Hammons, S.R.; Singh, M.; Oliver, H.F. Listeria monocytogenes is prevalent in retail produce environments but Salmonella enterica is rare. Food Control 2020, 113, 11. [Google Scholar] [CrossRef]
- Gombas, D.E.; Chen, Y.H.; Clavero, R.S.; Scott, V.N. Survey of Listeria monocytogenes in ready-to-eat foods. J. Food Prot. 2003, 66, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, J.B.; Chen, Y.H.; Porto-Fett, A.C.S.; Pouillot, R.; Shoyer, B.A.; Johnson-DeRycke, R.; Eblen, D.R.; Hoelzer, K.; Shaw, W.K.; Van Doren, J.M.; et al. Survey for Listeria monocytogenes in and on Ready-to-Eat Foods from Retail Establishments in the United States (2010 through 2013): Assessing Potential Changes of Pathogen Prevalence and Levels in a Decade. J. Food Prot. 2017, 80, 903–921. [Google Scholar] [CrossRef]
- Kim, C.; Nartea, T.J.; Pao, S.; Li, H.; Jordan, K.L.; Xu, Y.; Stein, R.A.; Sismour, E.N. Evaluation of Microbial Loads on Dried and Fresh Shiitake Mushrooms (Lentinula edodes) as Obtained from Internet and Local Retail Markets, Respectively. Food Saf. 2016, 4, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Strapp, C.M.; Shearer, A.E.H.; Joerger, R.D. Survey of retail alfalfa sprouts and mushrooms for the presence of Escherichia coli O157:H7, Salmonella, and Listeria with BAX, and evaluation of this polymerase chain reaction-based system with experimentally contaminated samples. J. Food Prot. 2003, 66, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Samadpour, M.; Barbour, M.W.; Nguyen, T.; Cao, T.M.; Buck, F.; Depavia, G.A.; Mazengia, E.; Yang, P.; Alfi, D.; Lopes, M.; et al. Incidence of enterohemorrhagic Escherichia coli, Escherichia coli O157, Salmonella, and Listeria monocytogenes in retail fresh ground beef, sprouts, and mushrooms. J. Food Prot. 2006, 69, 441–443. [Google Scholar] [CrossRef]
- Zhang, G.D.; Chen, Y.; Hu, L.J.; Melka, D.; Wang, H.; Laasri, A.; Brown, E.W.; Strain, E.; Allard, M.; Bunning, V.K.; et al. Survey of Foodborne Pathogens, Aerobic Plate Counts, Total Coliform Counts, and Escherichia coli Counts in Leafy Greens, Sprouts, and Melons Marketed in the United States. J. Food Prot. 2018, 81, 400–411. [Google Scholar] [CrossRef]
- Signs, R.J.; Darcey, V.L.; Carney, T.A.; Evans, A.A.; Quinlan, J.J. Retail Food Safety Risks for Populations of Different Races, Ethnicities, and Income Levels. J. Food Prot. 2011, 74, 1717–1723. [Google Scholar] [CrossRef]
- Petran, R.L.; Zottola, E.A.; Gravani, R.B. Incidence of Listeria monocytogenes in Market Samples of Fresh and Frozen Vegetables. J. Food Sci. 1988, 53, 1238–1240. [Google Scholar] [CrossRef]
- Heisick, J.E.; Wagner, D.E.; Nierman, M.L.; Peeler, J.T. Listeria spp. found on fresh market produce. Appl. Environ. Microbiol. 1989, 55, 1925–1927. [Google Scholar] [CrossRef]
- Li, K.W.; Weidhaas, J.; Lemonakis, L.; Khouryieh, H.; Stone, M.; Jones, L.; Shen, C.L. Microbiological quality and safety of fresh produce in West Virginia and Kentucky farmers’ markets and validation of a post-harvest washing practice with antimicrobials to inactivate Salmonella and Listeria monocytogenes. Food Control 2017, 79, 101–108. [Google Scholar] [CrossRef]
- Scheinberg, J.A.; Dudley, E.G.; Campbell, J.; Roberts, B.; DiMarzio, M.; DebRoy, C.; Cutter, C.N. Prevalence and Phylogenetic Characterization of Escherichia coli and Hygiene Indicator Bacteria Isolated from Leafy Green Produce, Beef, and Pork Obtained from Farmers’ Markets in Pennsylvania. J. Food Prot. 2017, 80, 237–244. [Google Scholar] [CrossRef]
- Thunberg, R.L.; Tran, T.T.; Bennett, R.W.; Matthews, R.N.; Belay, N. Microbial evaluation of selected fresh produce obtained at retail markets. J. Food Prot. 2002, 65, 677–682. [Google Scholar] [CrossRef]
- Roth, L.; Simonne, A.; House, L.; Ahn, S. Microbiological analysis of fresh produce sold at Florida farmers’ markets. Food Control 2018, 92, 444–449. [Google Scholar] [CrossRef]
- Borrusso, P.A.; Quinlan, J.J. Prevalence of Pathogens and Indicator Organisms in Home Kitchens and Correlation with Unsafe Food Handling Practices and Conditions. J. Food Prot. 2017, 80, 590–597. [Google Scholar] [CrossRef]
- Gorski, L.; Parker, C.T.; Liang, A.S.; Walker, S.; Romanolo, K.F. The Majority of Genotypes of the Virulence Gene inlA Are Intact among Natural Watershed Isolates of Listeria monocytogenes from the Central California Coast. PLoS ONE 2016, 11, e167566. [Google Scholar] [CrossRef]
- Chen, Y.; Gonzalez-Escalona, N.; Hammack, T.S.; Allard, M.W.; Strain, E.A.; Brown, E.W. Core Genome Multilocus Sequence Typing for Identification of Globally Distributed Clonal Groups and Differentiation of Outbreak Strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2016, 82, 6258–6272. [Google Scholar] [CrossRef]
- Burall, L.S.; Grim, C.J.; Mammel, M.K.; Datta, A.R. A Comprehensive Evaluation of the Genetic Relatedness of Listeria monocytogenes Serotype 4b Variant Strains. Front. Public Health 2017, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.; Costolo, B.; Brown, P.; Kathariou, S. Penicillin-binding protein encoded by pbp4 is involved in mediating copper stress in Listeria monocytogenes. FEMS Microbiol. Lett. 2017, 364, fnx207. [Google Scholar] [CrossRef]
- Laksanalamai, P.; Joseph, L.A.; Silk, B.J.; Burall, L.S.; Tarr, C.L.; Gerner-Smidt, P.; Datta, A.R. Genomic Characterization of Listeria monocytogenes Strains Involved in a Multistate Listeriosis Outbreak Associated with Cantaloupe in US. PLoS ONE 2012, 7, e42448. [Google Scholar] [CrossRef]
- Price, R.; Jayeola, V.; Niedermeyer, J.; Parsons, C.; Kathariou, S. The Listeria monocytogenes Key Virulence Determinants hly and prfA are involved in Biofilm Formation and Aggregation but not Colonization of Fresh Produce. Pathogens 2018, 7, 18. [Google Scholar] [CrossRef]
- Wang, Y.; Pettengill, J.B.; Pightling, A.; Timme, R.; Allard, M.; Strain, E.; Rand, H. Genetic Diversity of Salmonella and Listeria Isolates from Food Facilities. J. Food Prot. 2018, 81, 2082–2089. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Yeh, E.; Hall, G.; Cripe, J.; Bhagwat, A.A.; Meng, J.H. Characterization of Listeria monocytogenes isolated from retail foods. Int. J. Food Microbiol. 2007, 113, 47–53. [Google Scholar] [CrossRef]
- Ratani, S.S.; Siletzky, R.M.; Dutta, V.; Yildirim, S.; Osborne, J.A.; Lin, W.; Hitchins, A.D.; Ward, T.J.; Kathariou, S. Heavy Metal and Disinfectant Resistance of Listeria monocytogenes from Foods and Food Processing Plants. Appl. Environ. Microbiol. 2012, 78, 6938–6945. [Google Scholar] [CrossRef] [PubMed]
- Li, K.W.; Khouryieh, H.; Jones, L.; Etienne, X.; Shen, C.L. Assessing farmers market produce vendors’ handling of containers and evaluation of the survival of Salmonella and Listeria monocytogenes on plastic, pressed-card, and wood container surfaces at refrigerated and room temperature. Food Control 2018, 94, 116–122. [Google Scholar] [CrossRef]
- Schardt, J.; Jones, G.; Müller-Herbst, S.; Schauer, K.; D’Orazio, S.E.; Fuchs, T.M. Comparison between Listeria sensu stricto and Listeria sensu lato strains identifies novel determinants involved in infection. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Gerner-Smidt, P.; Besser, J.; Concepcion-Acevedo, J.; Folster, J.P.; Huffman, J.; Joseph, L.A.; Kucerova, Z.; Nichols, M.C.; Schwensohn, C.A.; Tolar, B. Whole Genome Sequencing: Bridging One-Health Surveillance of Foodborne Diseases. Front. Public Health 2019, 7, 172. [Google Scholar] [CrossRef]
- Mohammad, Z.H.; Yu, H.Y.; Neal, J.A.; Gibson, K.E.; Sirsat, S.A. Food Safety Challenges and Barriers in Southern United States Farmers Markets. Foods 2020, 9, 12. [Google Scholar] [CrossRef]
- Pollard, S.; Boyer, R.; Chapman, B.; di Stefano, J.; Archibald, T.; Ponder, M.A.; Rideout, S. Identification of risky food safety practices at southwest Virginia farmers’ markets. Food Prot. Trends 2016, 36, 168–175. [Google Scholar]
- Harrison, J.A.; Gaskin, J.W.; Harrison, M.A.; Cannon, J.L.; Boyer, R.R.; Zehnder, G.W. Survey of Food Safety Practices on Small to Medium-Sized Farms and in Farmers Markets. J. Food Prot. 2013, 76, 1989–1993. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.F.; Angulo, F.J. Eating in restaurants: A risk factor for foodborne disease? Clin. Infect. Dis. 2006, 43, 1324–1328. [Google Scholar] [CrossRef]
- Gould, L.H.; Rosenblum, I.; Nicholas, D.; Phan, Q.; Jones, T.F. Contributing Factors in Restaurant- Associated Foodborne Disease Outbreaks, FoodNet Sites, 2006 and 2007. J. Food Prot. 2013, 76, 1824–1828. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Report on the Occurrence of Foodborne Illness Risk Factors in Fast Food and Full Service Restaurants, 2013–2014. 2018. Available online: https://www.fda.gov/food/cfsan-constituent-updates/fda-releases-report-occurrence-foodborne-illness-risk-factors-fast-food-and-full-service-restaurants (accessed on 22 April 2020).
- Byrd-Bredbenner, C.; Berning, J.; Martin-Biggers, J.; Quick, V. Food Safety in Home Kitchens: A Synthesis of the Literature. Int. J. Environ. Res. Public Health 2013, 10, 4060–4085. [Google Scholar] [CrossRef]
- O’Beirne, D.; Gomez-Lopez, V.; Tudela, J.A.; Allende, A.; Gil, M.I. Effects of oxygen-depleted atmospheres on survival and growth of Listeria monocytogenes on fresh-cut Iceberg lettuce stored at mild abuse commercial temperatures. Food Microbiol. 2015, 48, 17–21. [Google Scholar] [CrossRef]
- Thomas, C.; Prior, O.; O’Beirne, D. Survival and growth of Listeria species in a model ready-to-use vegetable product containing raw and cooked ingredients as affected by storage temperature and acidification. Int. J. Food Sci. Technol. 1999, 34, 317–324. [Google Scholar] [CrossRef]
- Ndraha, N.; Hsiao, H.I.; Vlajic, J.; Yang, M.F.; Lin, H.T.V. Time-temperature abuse in the food cold chain: Review of issues, challenges, and recommendations. Food Control 2018, 89, 12–21. [Google Scholar] [CrossRef]
- Jayeola, V.; Jeong, S.; Almenar, E.; Marks, B.P.; Vorst, K.L.; Brown, J.W.; Ryser, E.T. Predicting the Growth of Listeria monocytogenes and Salmonella Typhimurium in Diced Celery, Onions, and Tomatoes during Simulated Commercial Transport, Retail Storage, and Display. J. Food Prot. 2019, 82, 287–300. [Google Scholar] [CrossRef]
- Huang, J.W.; Luo, Y.G.; Zhou, B.; Zheng, J.; Nou, X.W. Growth and survival of Salmonella enterica and Listeria monocytogenes on fresh-cut produce and their juice extracts: Impacts and interactions of food matrices and temperature abuse conditions. Food Control 2019, 100, 300–304. [Google Scholar] [CrossRef]
- Huang, J.W.; Luo, Y.G.; Nou, X.W. Growth of Salmonella enterica and Listeria monocytogenes on Fresh-Cut Cantaloupe under Different Temperature Abuse Scenarios. J. Food Prot. 2015, 78, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
| Associated Food | Source of Contamination | Year | Number of States Affected | Cases | Hospitalizations | Deaths | Reference |
|---|---|---|---|---|---|---|---|
| Enoki mushrooms | TBD | 2020 | 17 | 36 | 30 | 4 | [18] |
| Frozen vegetables | Processing environment | 2016 | 4 | 9 | 9 | 3 | [19] |
| Packaged salad | Processing environment | 2016 | 9 | 19 | 19 | 1 | [20] |
| Caramel apples | Processing environment | 2014 | 12 | 35 | 34 | 7 | [16] |
| Mung bean sprouts | Production environment | 2014 | 2 | 5 | 5 | 2 | [21] |
| Peaches and nectarines | Packinghouse environment | 2014 | 4 | 2 | 2 | 1 | [17,22] |
| Cantaloupes | Processing environment | 2011 | 28 | 147 | 143 | 33 | [14] |
| Chopped celery | Processing environment | 2010 | 1 | 10 | 10 | 5 | [15] |
| Sprouts | Production environment | 2008 | Unknown | 20 | 16 | 0 | [23] |
| Raw celery, tomatoes, and lettuce | Unknown | 1979 | 1 | 20 | 20 | 5 | [23,24] |
| Geographical Location(s) | Sampling Site(s) | Study Duration | Type of Sample(s) | Target Organism(s) | Presence (Yes/Not Detected) | Prevalence | Positive Sample Type(s) | Strain/Serotype | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Natural Environment and Outdoor Production | |||||||||
| Colorado | Wilderness areas (n = 5) | Two years | Soil, water, sediment, surface soil, and wildlife feces (n = 572) | Listeria spp. (excludes Lm) | Yes | 19/572 (3.32) | All sample types | L. welshimeri and undetermined strains | [27] |
| Listeria monocytogenes | Yes | 3/572 (0.52) | Feces and water | 1/2a, 1/2b, 3a, 3b, or 7 | |||||
| Washington, Oregon, and California | Compost facilities (n = 94) | Three weeks | Market-ready, organic compost (n = 94) | Listeria spp. (all) | Yes | 22/47 (46.81) | Compost from OR and CA | Not identified | [28] |
| Arizona, California, Georgia, Kentucky, Maryland, New York, North Carolina, South Carolina, Tennessee | Farms, outside locations, commercial operations | Four years | Organic fertilizers (n = 103) | Listeria monocytogenes | ND | N/A | N/A | N/A | [29] |
| New York | Natural environments (n = 5) | Two years | Composite soil, drag swab, water, and wildlife/domestic animal feces (n = 1322) | Listeria spp. (excludes Lm) | Yes | 186/734 (25.34) | All sample types | L. innocua, L. seeligeri, L. welshimeri, and L. marthii | [25] |
| Listeria monocytogenes | Yes | 59/734 (8.04) | Not specified | Not identified | |||||
| California | Watershed areas near produce field (n = 30) | Two years | Surface water (n = 1405) | Listeria monocytogenes | Yes | ~ 604/1405 (43) | Surface water | 1/2a, 1/2b, 3a, 4d, and 4e | [30] |
| California | Watershed areas near produce field (n = 14) | Ten months | Surface water | Listeria monocytogenes | Yes | Culture positive: 22/36 (61.1) PCR positive: 21/36 (58.3) | Surface water | Not identified | [31] |
| California | Watershed areas near produce field (n = 30) | Three months | Surface water (n = 206) | Listeria monocytogenes | Yes | 62/206 (30.1) | Surface water | 1/2a, 1/2b, and 4b | [32] |
| California | Watershed areas (n > 30) | Two years | Surface water (n = 860) | Listeria monocytogenes | Yes | 381/860 (44.3) | Surface water | Not identified | [33] |
| Ohio | Experimental treatments (n = 5) with varying amendments (sawdust, straw, and water) to manure | Sampling occurred over 57-day period | Compost testing occurred on days 0, 14, 28, and 56 | Listeria spp. (all) | Yes | Not specified | Day 0: Five compost treatments. Day 3: Three compost treatments. Day 14: One compost treatment | Not identified | [34] |
| Pennsylvania | Six parallel plots (5 m × 2 m) | Not specified | Irrigation water and spinach | Listeria spp. (all) | ND | N/A | N/A | N/A | [35] |
| Maine | Randomized complete block split plot design with five replications completed in duplicate | Two years | Liquid dairy, pig, chicken, cow manure, and potatoes grown with and without liquid dairy manure | Listeria monocytogenes | Yes | Total: 3/24 (12.5) Liquid dairy: 2/6 (33.3) Cow: 1/6 (16.7) | Liquid dairy and cow manure | N/A | [36] |
| Maryland | Mixed produce and dairy farm; 12 sampling sites | 14 months | Cow feces, cow feed, cow drinking water, bird feces, bird gathering areas, raw liquid manure, water from lagoon, raw separated solids, partially composted material, fully composed material, surface water, and soil from vegetable production area and cow pasture (n = 159) | Listeria spp. (excludes Lm) | Yes | 8/159 (5.03) | Cow feed, cow feces, raw separated solid, windrow compost, finished compost, bird feces, pasture soil | Not identified | [37] |
| Listeria monocytogenes | Yes | 2/159 (1.26) | Cow feed and pasture soil | Not identified | |||||
| Rio Grande Valley and Texas | Cabbage farms with packing sheds and separate packing sheds (n = 6) | Seven months | Cabbage (n = 425), water (n = 205), and environmental (n = 225) | Listeria spp. (excludes Lm) | Yes | 24/855 (2.81) | All sample types | L. grayi, L. innocua, L. ivanoii, and L. welshimeri | [38] |
| Listeria monocytogenes | Yes | 26/855 (3.04) | All sample types | Not identified | |||||
| Maryland | Non-tidal freshwater creek | One year | Creek water, soil, radishes, and kale | Listeria monocytogenes | Yes | 0.04 and 0.07 MPN/L | Creek water | Not identified | [39] |
| New York | Spinach fields (n = 2) | Seven weeks | Feces, leaves, soil, and surface water (n = 1492) | Listeria spp. (excludes Lm) | Yes | 74/1492 (4.96) | All sample types | Not identified | [40] |
| Listeria monocytogenes | Yes | 130/1492 (8.71) | Not identified | ||||||
| New York | Natural (n = 4) and urban (n = 4) sites | Two years | Soil, vegetation, surface water, floors, sidewalks, and human contact surfaces (n = 1805) | Listeria monocytogenes | Yes | Urban: 67/898 (7.5) Natural: 13/907 (1.4) | Urban: soil, vegetation, water, sidewalk/floor, and human contact surfaces Natural: soil, vegetation, and water | Not identified | [41] |
| New York | Natural (n = 4) and urban (n = 4) sites | Two years | Soil, vegetation, surface water, floors, sidewalks, and human contact surfaces (n = 1805) | Listeria spp. (excludes Lm) | Yes | 362/1805 (20.1) | Variety from natural and urban sites | L. marthii, L. innocua, L. seeligeri, and L. welshimeri | [42] |
| Listeria monocytogenes | Yes | 80/1805 (4.43) | Not identified | ||||||
| Arizona and New York | Watershed areas (n = 9) | Eleven months | Surface water (n = 1053) | Listeria spp. (excludes Lm) | Yes | AZ: 0/76 (0) NY: 58/257 (22.57) | Streams in NY | L. booriae, L. innocua, L. marthii, L. seeligeri, and L. welshimeri | [43] |
| Listeria monocytogenes | Yes | AZ: 3/76 (3.95) NY: 30/257 (11.67) | Canals in AZ and streams in NY | Not identified | |||||
| Arizona | Five sites in two wastewater treatment plants | 20 months | Reclaimed and return flow water (n = 28) | Listeria monocytogenes | ND | N/A | N/A | N/A | [44] |
| Mid-Atlantic U.S. | Convention water sources (n = 6) | Three years | Tidal freshwater river (n = 34), non-tidal freshwater creek (n = 32), reclaimed water holding pond (n = 25), pond water sites (n = 69), and produce wash water (n = 10) | Listeria monocytogenes | Yes | 53/170 (31.18) | All sample types | Not identified | [45] |
| Washington | Red raspberry field with individual plots (22.86 m × 3.05 m; n = 4) with buffer rows between treatment plots; completed in duplicate | Two years | Fertilizer, soil, foliar, and raspberry fruit | Listeria monocytogenes | ND | N/A | N/A | N/A | [46] |
| New York | Produce farms (n = 5) | 27 months | Soil, water (engineered and surface), feces, and drag swabs (n = 588) | Listeria monocytogenes | Yes | 88/588 (15.0) | All sample types except for engineered water | Not identified | [47] |
| New York | Produce farms (n = 21) | Five weeks | Fields (n = 263) and environmental samples (soil, drag swab, and water; n = 600) | Listeria monocytogenes | Yes | Field: 46/263 (17.5) Soil: 30/263 (11) Drag: 21/263 (8) Water: 22/74 (30) | All sample types | Nine allelic types representing lineages I, II, and IIIa | [48] |
| New York | Produce farms (n = 10) | Six weeks | Terrestrial, water, and fecal (n = 124) | Listeria spp. (excludes Lm) | Yes | 24/124 (19.35) | All sample types | L. seeligeri, L. welshimeri, and L. innocua | [49] |
| Listeria monocytogenes | Yes | 28/124 (22.58) | All sample types | Not identified | |||||
| Packinghouse, Indoor Production, and Processing | |||||||||
| Pennsylvania | Small scale mushroom production facility (n = 1) | Two months | NFCS, such as shovels, drains, doors, floors, conveyor belts, brooms, dust pans, etc. (n = 184) | Listeria spp. (excludes Lm) | Yes | 26/184 (14.13) | Phase I composting, phase II composting, tray filling line, and growing rooms | L. innocua, L. welshimeri, and L. grayi | [50] |
| Listeria monocytogenes | Yes | 3/184 (1.63) | Phase I composting | Not identified | |||||
| Maryland | Organic farms (n = 7) | Two years | Produce (tomatoes, leafy greens, peppers, cucumbers, etc.), well water, and surface water (n = 206) | Listeria monocytogenes | ND | N/A | N/A | N/A | [51] |
| Southern U.S. | Farms (n = 13) and packing sheds (n = 5) | 19 months | Produce (leafy greens, herbs, and cantaloupe; n = 398) | Listeria monocytogenes | ND | N/A | N/A | N/A | [52] |
| Northeast U.S. | Apple and other tree fruit packinghouses (n = 3) | Six months | NFCS (n = 117) | Listeria monocytogenes | Yes | 66/117 (56.41) | Washing, drying, and waxing areas of all facilities | Not identified | [53] |
| Southern U.S. | Packinghouses (n = 8) | 14 months | Leafy greens (n = 109), herbs (n = 165), and cantaloupe (n = 36) | Listeria monocytogenes | Yes | Leafy greens: 3/43 (6.98) | Cabbage | Not identified | [54] |
| Southeastern U.S. | Packinghouses (n = 11) | Nine months | NFCS, such as forklift wheels, drains, dump tank legs, cold room floors, etc. | Listeria spp. (excludes Lm) | Yes | 52/1588 (3.27) | Drains, cold storage rooms, wet NFCS, mobile NFCS, dry NFCS, and outside packing/handling area | L. innouca, L. marthii, L. seeligeri, and L. welshimeri | [55] |
| Listeria monocytogenes | Yes | 60/1588 (3.78) | Not identified | ||||||
| California | Stone fruits (n = 105; from seven lots) | Not specified | White nectarines (n = 30), yellow nectarines (n = 30), white peaches (n = 30), and yellow peaches (n = 15) | Listeria monocytogenes | Yes; 11.3 CFU/fruit (geometric mean) | Total: 56/105 (53.3) Nectarines: 15/60 (25) Peaches: 41/45 (91.1) | All sample types | IVb-v1 and 1/2b | [17] |
| Four U.S. states | Packinghouses (n = 3) and fresh-cut facilities (n = 5) | One year | Sponge samples (n = 2014) | Listeria spp. (excludes Lm) | Yes | Packinghouse: 5/252 (2) to 8/171 (4.7) Fresh-cut: 0/249 (0) to 5/325 (1.5) | Zones 2 and 3 for packinghouse and only zone 3 for fresh-cut | Not identified | [56] |
| Listeria monocytogenes | Packinghouse: 2/252 (0.8) to 10/171 (5.8) Fresh-cut: 0/249 (0) to 4/246 (1.6) | Zones 2 and 3 for packinghouse and only zone 3 for fresh-cut | Not identified | ||||||
| Multiregional | Processing plants (n = 2) | 14 months | Baby spinach (n = 409) | Listeria spp. (excludes Lm) | Yes | 2/409 (0.49) | Processed samples | L. seeligeri | [57] |
| Listeria monocytogenes | Yes | 3/409 (0.73) | One processed and two minimally processed baby spinach samples | Not identified | |||||
| Pacific Northwest U.S. | Produce handling and processing facilities (n = 7) | One year | Environmental sponge samples (n = 350) | Listeria spp. (excludes Lm) | Yes | 11/350 (3.14) | Drain, entry point, floor, forklift tire, forklift traffic area, equipment leg | L. innocua, L. ivanoii, and L. welshimeri | [58] |
| Listeria monocytogenes | Yes | 15/350 (4.29) | 1/2a, 3a, 4b, 4d, 4e | ||||||
| California | Grower (n = 1) | Four months | Conventional and organic spring mix | Listeria monocytogenes | ND | N/A | N/A | N/A | [59] |
| Not specified | Fresh mushroom slicing and packaging operation; 98 sampling sites within the facility | 14 months | NFCS, such as loading dock doors, floors, walls, pallets, drains, squeegees, electrical utility boxes, forklifts, plastic curtains, etc. (n = 255) | Listeria spp. (excludes Lm) | Yes | 16/255 (6.27) | Receiving and staging, washing and slicing, packaging, and shipping sites | L. innocua and L. grayi | [60] |
| Listeria monocytogenes | Yes | 48/255 (18.8) | Receiving and staging, washing and slicing, and packaging sites | 1/2a, 1/2b, and 1/2c | |||||
| Retail | |||||||||
| Michigan and New Jersey | Distribution (cilantro), retail (cilantro and mung bean sprouts) and farm (cucumber) | Not specified | Cilantro (pre-retail and retail), cucumbers, and mung bean sprouts | Listeria spp. and Listeria monocytogenes | No live isolates obtained; however, species level proportional abundances illustrate presence of Listeria DNA | L. monocytogenes DNA present in two cilantro samples | Not identified | [61] | |
| South America, North America, Europe, Africa, and Asia | Published studies (n = 25) | Not specified | Packaged salads (n = 20,904), including packaged greens (n = 1212), packaged RTE (n = 11,978), unsure if packaged (n = 2637), and packaged with meat (n = 5077) | Listeria monocytogenes | Yes | 543/20,904 (2.60) | All sample types, except for some unsure if packaged samples | Not identified | [62] |
| Maryland | Retail stores (n = 3) | One year | Basil, cilantro, lettuce, scallion, spinach, and parsley (n = 414) | Listeria monocytogenes | Yes | Not specified | Spinach | Not identified | [63] |
| California, Texas, Iowa, Minnesota, Ohio, Massachusetts, and Florida | Retail grocery produce departments (n = 30) | Eight months | FCS and NFCS | Listeria monocytogenes | Yes | Total: 226/5112 (4.42) NFCS: 178/2205 (8.1) FCS: 48/2907 (1.7) | Drain (cold room storage), standing water, drain (produce area), squeegee/floor cleaners, floor (cold room storage), etc. | Not identified | [64] |
| Maryland and California | Retail markets | Over 14 to 23 months | Bagged salads (n = 2966) | Listeria monocytogenes | Yes | Total: 22/2966 (0.74) MD: 8/1465 (0.55) CA: 14/1501 (0.93) | Bagged salads | Not identified | [65] |
| California, Maryland, Connecticut, and Georgia | Retail stores (n = 1042) | Two years | Produce, including cut vegetables (raw), low-acid cut fruit, and sprouts (n = 6749) | Listeria monocytogenes | Yes | 36/6749 (0.53) | All sample types | Not identified | [66] |
| Virginia | Retail markets | Two months | Whole (n = 20) and sliced (n = 8) shiitake mushrooms | Listeria spp. (excludes Lm) | Yes | Total: 3/28 (10.71) Whole: 1/20 (5) Sliced: 2/8 (25) | Whole and sliced mushrooms | Not identified | [67] |
| Listeria monocytogenes | ND | N/A | N/A | N/A | |||||
| Delaware | Grocery stores (number not specified) | 15 months | Mushrooms (n = 202) and alfalfa sprouts (n = 206) | Listeria spp. (excludes Lm) | Yes | Total: 24/408 (5.88) Mushroom: 17/202 (8.42) Sprouts: 7/206 (3.40) | Mushroom and sprouts | L. welshimeri, L. innocua, and L. seeligeri | [68] |
| Listeria monocytogenes | Total: 1/408 (0.25) Mushroom: 0/202 Sprouts: 1/206 (0.49) | Only sprouts | Not identified | ||||||
| Seattle, Washington | Retail stores | One year | Sprouts (n = 200) and mushrooms (n = 100) | Listeria monocytogenes | Yes | 1/100 (1) | Mushroom | Not identified | [69] |
| Colorado, Connecticut, Georgia, Maryland, Minnesota, California, Texas, and Washington | Retail locations | Six years | Leafy greens (n = 14,183), sprouts (2652), and melons (3411) | Listeria monocytogenes | Yes | Leafy greens: (0.11) Sprouts: (0.11) Melons: (0.23) | Spinach, romaine, alfalfa sprouts, broccoli sprouts, cucumber, and mango | Not identified | [70] |
| Philadelphia, Pennsylvania | Retail food establishments (n = 60) | Two years | RTE fresh fruit, greens, and herbs | Listeria monocytogenes | ND | N/A | N/A | N/A | [71] |
| Minnesota | Supermarket | Not specified | Produce (lettuce, potato peels, corn husks, broccoli stems, cabbage outer leaves, carrot peels, cauliflower stems, mushroom stems, spinach, beet peels, and frozen green beans, pea pods, green peas, and spinach) | Listeria monocytogenes | ND | N/A | N/A | N/A | [72] |
| Minneapolis, Minnesota | Supermarkets (n = 2) | One year | Produce (broccoli, cabbage, carrots, cauliflower, cucumbers, lettuce, mushrooms, potatoes, radishes, and tomatoes; n = 1000) | Listeria spp. and Listeria monocytogenes | Yes | 97/1000 (9.7) | Lettuce, cabbage, cucumbers, mushrooms, potatoes, radishes | L. monocytogenes, L. innocua, L. welshimeri, and L. seeligeri | [73] |
| Farmers’ Markets | |||||||||
| West Virginia and Kentucky | Farmers’ markets (n = 2) | Four months | Produce (tomatoes, peppers, cucumber, cantaloupe, and spinach; n = 212) | Listeria spp. (excludes Lm) | Yes | 4/212 (1.89) | Peppers and cantaloupes | Not identified | [74] |
| Listeria monocytogenes | Yes | 4/212 (1.89) | Tomatoes, cucumbers, and cantaloupes | Not identified | |||||
| Pennsylvania | Farmers’ markets (n = 25) and vendors (n = 58) | 8 months | Leafy greens (n = 50 each of lettuce, spinach, and kale) | Listeria spp. (excludes Lm) | Yes | 5/152 (3.30) | Kale, lettuce, and spinach | Not identified | [75] |
| Listeria monocytogenes | Yes | 1/152 (0.66) | Spinach | ||||||
| Washington D.C. | Farmers’ markets and supermarkets | Not specified | Produce (alfalfa sprouts, beets, broccoli, broccoli sprouts, cauliflower, celery, cilantro, cucumbers, field cress, green peppers, lettuce, mung bean sprouts, potatoes, soybean sprouts, watercress, yams; n = 127) | Listeria spp. (excludes Lm) | Yes | 19/127 (14.96) | Celery, field cress, lettuce, mung bean sprouts, potatoes, soybean sprouts, watercress, yams | L. innocua, L. welshimeri, and L. grayi | [76] |
| Listeria monocytogenes | Yes | 6/127 (4.72) | Field cress and potatoes | N/A | |||||
| Florida | Farmers’ markets (n = 9) and supermarkets (n = 12) | 10 months | Leafy greens (n = 103), berries (n = 106), spinach (n = 77), and tomatoes (n = 115) | Listeria monocytogenes | Yes | 4/401 (1) | Leafy greens and spinach from farmer’s markets | Not identified | [77] |
| Domestic | |||||||||
| Philadelphia, Pennsylvania | Homes (n = 100) | One year | Refrigerator door handle, bottom shelf, meat drawer; kitchen counter near sink; used kitchen sponge or dishcloth | Listeria spp. (excludes Lm) | Yes | 12/557 (2.15) | Meat drawer | L. innocua, L. welshimeri, L. grayi, and L. seeligeri | [78] |
| Listeria monocytogenes | Yes | 4/557 (0.72) | Refrigerator door handle, refrigerator drawer, kitchen sink, and dishcloth/sponge | Not identified | |||||
| Stage of Supply Chain | Listeria spp. Prevalence (%) a,b | Identified Listeria Species | Lm Prevalence (%) | Identified Lm Serovars c | ||
|---|---|---|---|---|---|---|
| Low | High | Low | High | |||
| Natural environment and outdoor production | ND | 46.81 [28] | L. welshimeri, L. innocua, L. seeligeri, L. grayi, L. ivanoii, L. marthii, L. booriae | ND | 61.1 [31] | 1/2a, 1/2b, 3a, 3b, 4d, 4e, 7 |
| Packinghouse, indoor production, and processing | 0.08 | 14.13 [50] | L. welshimeri, L. innocua, L. seeligeri, L. grayi, L. ivanoii, L. marthii | ND | 56.41 [53] | 1/2a, 1/2b, 1/2c, 3a, 4b, 4c, 4d, IVb-v1 |
| Retail | 6.13 [68] | 10.71 [67] | L. innocua, L. welshimeri, L. seeligeri | ND | 4.42 [64] | NI |
| Farmers’ markets | ND | 14.96 [76] | L. welshimeri, L. innocua, L. seeligeri, L. grayi | ND | 4.72 [76] | NI |
| Domestic | 2.15 [78] | L. welshimeri, L. innocua, L. seeligeri, L. grayi | 0.72 [78] | NI | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Townsend, A.; Strawn, L.K.; Chapman, B.J.; Dunn, L.L. A Systematic Review of Listeria Species and Listeria monocytogenes Prevalence, Persistence, and Diversity throughout the Fresh Produce Supply Chain. Foods 2021, 10, 1427. https://doi.org/10.3390/foods10061427
Townsend A, Strawn LK, Chapman BJ, Dunn LL. A Systematic Review of Listeria Species and Listeria monocytogenes Prevalence, Persistence, and Diversity throughout the Fresh Produce Supply Chain. Foods. 2021; 10(6):1427. https://doi.org/10.3390/foods10061427
Chicago/Turabian StyleTownsend, Anna, Laura K. Strawn, Benjamin J. Chapman, and Laurel L. Dunn. 2021. "A Systematic Review of Listeria Species and Listeria monocytogenes Prevalence, Persistence, and Diversity throughout the Fresh Produce Supply Chain" Foods 10, no. 6: 1427. https://doi.org/10.3390/foods10061427
APA StyleTownsend, A., Strawn, L. K., Chapman, B. J., & Dunn, L. L. (2021). A Systematic Review of Listeria Species and Listeria monocytogenes Prevalence, Persistence, and Diversity throughout the Fresh Produce Supply Chain. Foods, 10(6), 1427. https://doi.org/10.3390/foods10061427






