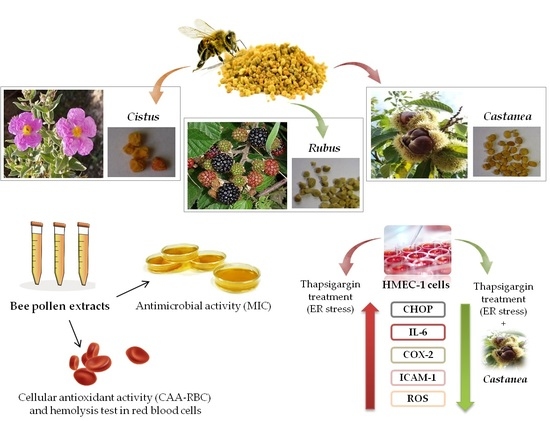
Antimicrobial Activity and Protective Effect of Tuscan Bee Pollens on Oxidative and Endoplasmic Reticulum Stress in Different Cell-Based Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Extraction
2.3. Antibacterial Activity
2.3.1. Pathogenic Bacterial Strains and Growth Conditions
2.3.2. Minimum Inhibitory Concentration (MIC)
2.4. Ex Vivo Biological Activities
2.4.1. Preparation of Human Erythrocytes
2.4.2. Cellular Antioxidant Activity (CAA) in Red Blood Cells
2.4.3. Erythrocytes Oxidative Hemolysis
2.5. Human Microvascular Endothelial Cells (HMEC-1) Treatments and Viability
2.6. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.7. Reactive Oxygen Species (ROS) Production
2.8. Statistical Analysis
3. Results and Discussion
3.1. Bee Pollen Antibacterial Potential
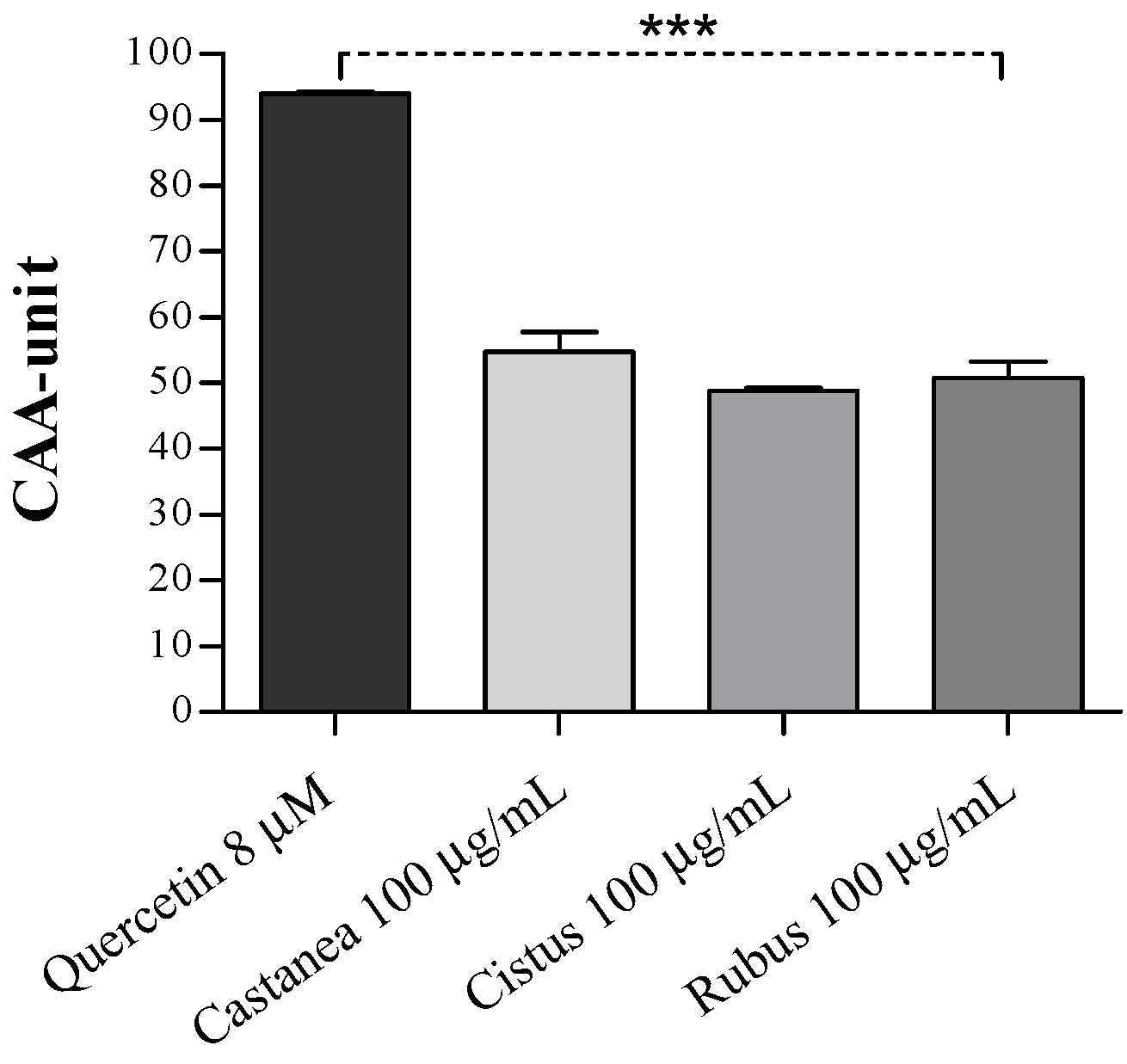
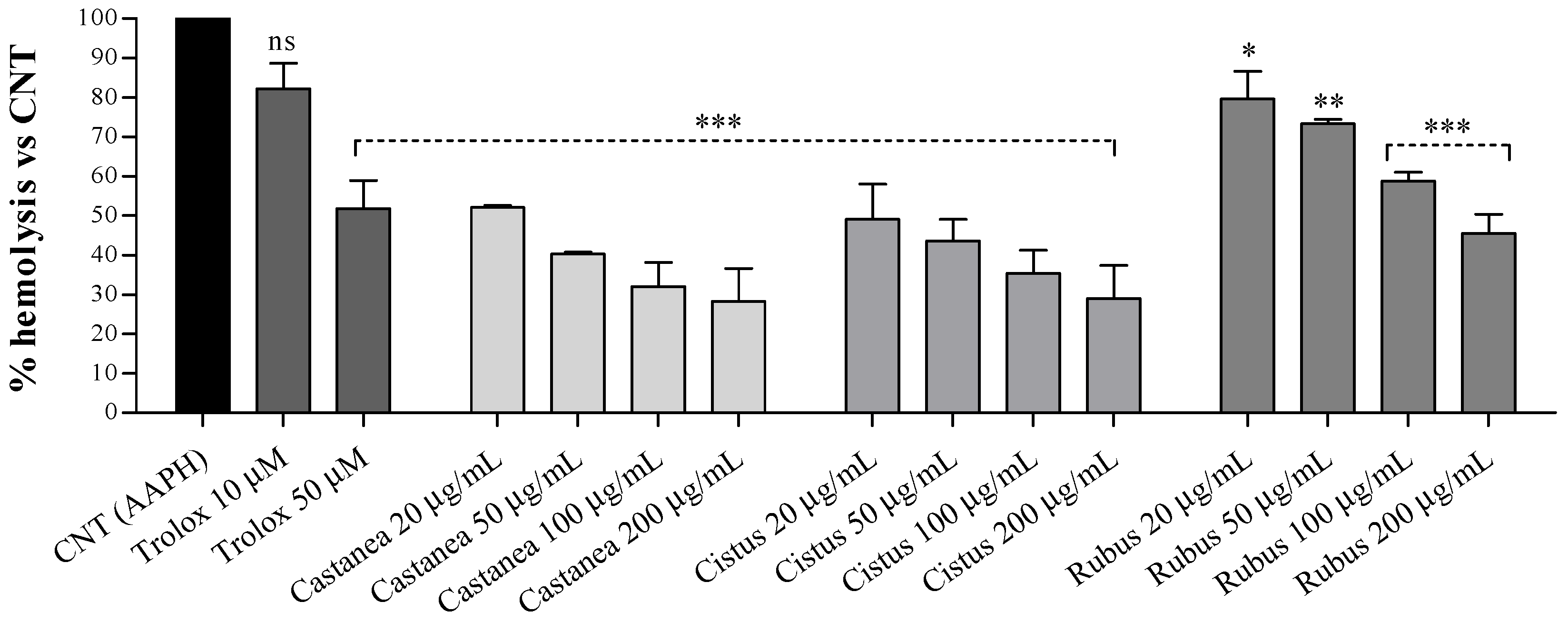
3.2. Bee Pollen Samples Protect Human Erythrocytes from Free Radicals Damage
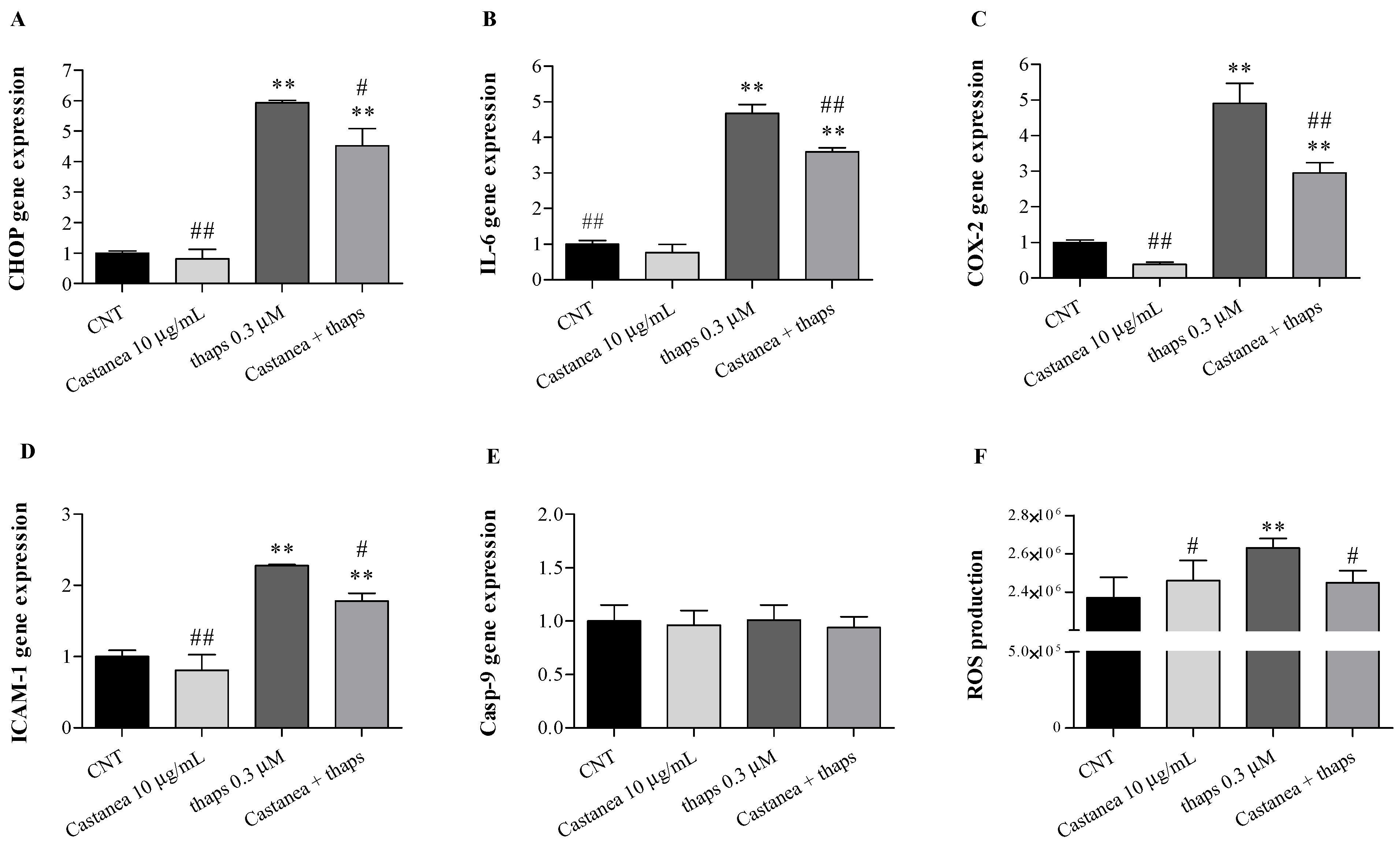
3.3. Castanea Bee Pollen Counteracts Thapsigargin Induced-ER Stress in HMEC-1 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zerdani, I.; Abouda, Z.; Kalalou, I.; Faid, M.; Ahami, M. The Antibacterial Activity of Moroccan Bee Bread and Bee-Pollen (Fresh and Dried) against Pathogenic Bacteria. Res. J. Microbiol. 2011, 6, 376–384. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Domenici, V.; Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Pucci, L. Phytochemical composition and antioxidant activity of Tuscan bee pollen of different botanic origins. Ital. J. Food Sci. 2015, 27, 27. [Google Scholar]
- Nogueira, C.; Iglesias, A.; Feás, X.; Estevinho, L.M. Commercial Bee Pollen with Different Geographical Origins: A Comprehensive Approach. Int. J. Mol. Sci. 2012, 13, 11173–11187. [Google Scholar] [CrossRef] [PubMed]
- Arruda, V.A.S.D.; Pereira, A.A.S.; Freitas, A.D.S.; Barth, O.M.; Almeida-Muradian, L.B.D. Dried bee pollen: B complex vitamins, physi-cochemical and botanical composition. J. Food Compos. Anal. 2013, 29, 100–105. [Google Scholar] [CrossRef]
- Almeida-Muradian, L.B.; Pamplona, L.C.; Coimbra, S.L.; Barth, O.M. Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food Compos. Anal. 2005, 18, 105–111. [Google Scholar] [CrossRef]
- Pérez-Pérez, E.M.; Vit, P.; Rivas, E.; Sciortino, R.; Sosa, A.; Tejada, D.; Rodríguez-Malaver, A.J. Antioxidant activity of four color fractions of bee pollen from Mérida, Venezuela. Arch. Latinoam. Nutr. 2012, 62, 376–380. [Google Scholar]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feás, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Izuta, H.; Shimazawa, M.; Tsuruma, K.; Araki, Y.; Mishima, S.; Hara, H. Bee products prevent VEGF-induced angiogenesis in human umbilical vein endothelial cells. BMC Complement. Altern. Med. 2009, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Gotoh, T.; Endo, M.; Oike, Y. Endoplasmic Reticulum Stress-Related Inflammation and Cardiovascular Diseases. Int. J. Inflamm. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nat. Cell Biol. 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Digaleh, H.; Kiaei, M.; Khodagholi, F. Nrf2 and Nrf1 signaling and ER stress crosstalk: Implication for proteasomal degradation and autophagy. Cell. Mol. Life Sci. 2013, 70, 4681–4694. [Google Scholar] [CrossRef] [PubMed]
- Frassinetti, S.; Gabriele, M.; Moccia, E.; Longo, V.; Di Gioia, D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. LWT 2020, 124, 109149. [Google Scholar] [CrossRef]
- Frassinetti, S.; Gabriele, M.; Caltavuturo, L.; Longo, V.; Pucci, L. Antimutagenic and antioxidant activity of a selected lectin-free common bean (Phaseolus vulgaris L.) in two cell-based models. Plant foods Hum. Nutr. 2015, 70, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Gabriele, M.; Frassinetti, S.; Caltavuturo, L.; Montero, L.; Dinelli, G.; Longo, V.; Gioia, D.D.; Puccia, L. Citrus bergamia powder: Antioxidant, antimi-crobial and anti-inflammatory properties. J. Funct. Foods. 2017, 31, 255–265. [Google Scholar] [CrossRef]
- Gabriele, M.; Pucci, L.; La Marca, M.; Lucchesi, D.; Della Croce, C.M.; Longo, V.; Lubrano, V. A fermented bean flour extract downregulates LOX-1, CHOP and ICAM-1 in HMEC-1 stimulated by ox-LDL. Cell. Mol. Biol. Lett. 2016, 21, 1–11. [Google Scholar] [CrossRef]
- Giusti, L.; Gabriele, M.; Penno, G.; Garofolo, M.; Longo, V.; Del Prato, S.; Lucchesi, D.; Pucci, L. A Fermented Whole Grain Prevents Lipopolysaccha-rides-Induced Dysfunction in Human Endothelial Progenitor Cells. Oxidative Med. Cell. Longev. 2017, 2017, 1026268. [Google Scholar] [CrossRef]
- Gabriele, M.; Pucci, L.; Árvay, J.; Longo, V. Anti-inflammatory and antioxidant effect of fermented whole wheat on TNFα-stimulated HT-29 and NF-κB signaling pathway activation. J. Funct. Foods. 2018, 45, 392–400. [Google Scholar] [CrossRef]
- Cabrera, C.; Montenegro, G. Pathogen control using a natural Chilean bee pollen extract of known botanical origin. Cienc. Investig. Agrar. 2013, 40, 223–230. [Google Scholar] [CrossRef]
- Carpes, S.; Begnini, R.; Alencar, S.; Masson, M. Study of Preparations Of Bee Pollen Extracts, Antioxidant And Antibacterial Activity Estudo das preparações de extratos de pólen apícola, atividade antioxidante e antibacteriana. In Ciência e Agrotecnologia; SciELO: São Paulo, Brazil, 2007; Volume 31, p. 1818. [Google Scholar]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.; Pereira, E.L. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernàndez-Busquets, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A.M.; Manconi, M. Stability, biocompatibility and antioxidant activity of PEG-modified liposomes containing resveratrol. Int. J. Pharm. 2018, 538, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Baltazar, A.F.; Henrique, R.; Estevinho, L.; Carvalho, M. Biological activities of Portuguese propolis: Protection against free radical-induced erythrocyte damage and inhibition of human renal cancer cell growth in vitro. Food Chem. Toxicol. 2011, 49, 86–92. [Google Scholar] [CrossRef]
- Barbieri, D.; Gabriele, M.; Summa, M.; Colosimo, R.; Leonardi, D.; Domenici, V.; Pucci, L. Antioxidant, Nutraceutical Properties, and Fluorescence Spectral Profiles of Bee Pollen Samples from Different Botanical Origins. Antioxidants 2020, 9, 1001. [Google Scholar] [CrossRef]
- Araújo, J.S.; Chambó, E.D.; Costa, M. Cavalcante da Silva SMP, Lopes de Carvalho CA, L ME. Chemical Composition and Biological Activities of Mono- and Heterofloral Bee Pollen of Different Geographical Origins. Int. J. Mol. Sci. 2017, 18, 921. [Google Scholar] [CrossRef]
- Campos, J.F.; Dos Santos, U.P.; Da Rocha, P.S.; Damião, M.J.; Balestieri, J.B.; Cardoso, C.A.; Paredes-Gamero, E.J.; Estevinho, L.M.; Picoli Souza, K.; Dos Santos, E.J. Antimicrobial, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities of Propolis from the Stingless Bee Tetragonisca fiebrigi (Jataí). Evid. Based Complementary Altern. Med. 2015, 2015, 296186. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.L.; Murphy, J.T. Reactive oxygen species mediate endotoxin-induced human dermal endothelial NF-kappaB activation. J. Surg. Res. 2003, 111, 120–126. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentão, P.; Domínguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated Analysis of COX-2 and iNOS Derived Inflammatory Mediators in LPS-Stimulated RAW Macrophages Pre-Exposed to Echium plantagineum L. Bee Pollen Extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G.; Padro, T. Nutraceuticals and atherosclerosis: Human trials. Cardiovasc. Ther. 2010, 28, 202–215. [Google Scholar] [CrossRef] [PubMed]
| MIC Values | ||||
|---|---|---|---|---|
| Gram negative strains | Gentamicin | Castanea | Cistus | Rubus |
| Escherichia coli | 0.05 mg/mL | 10 mg/mL | 10 mg/mL | - |
| Salmonella typhimurium | 0.05 mg/mL | 10 mg/mL | 10 mg/mL | - |
| Enterobacter erogene | 0.05 mg/mL | - | 10 mg/mL | - |
| Gram positive strains | Vancomycin | Castanea | Cistus | Rubus |
| Enterococcus faecalis | 0.05 mg/mL | - | 5 mg/mL | 10 mg/mL |
| Staphylococcus aureus | 0.05 mg/mL | 10 mg/mL | 5 mg/mL | 10 mg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabriele, M.; Frassinetti, S.; Pucci, L. Antimicrobial Activity and Protective Effect of Tuscan Bee Pollens on Oxidative and Endoplasmic Reticulum Stress in Different Cell-Based Models. Foods 2021, 10, 1422. https://doi.org/10.3390/foods10061422
Gabriele M, Frassinetti S, Pucci L. Antimicrobial Activity and Protective Effect of Tuscan Bee Pollens on Oxidative and Endoplasmic Reticulum Stress in Different Cell-Based Models. Foods. 2021; 10(6):1422. https://doi.org/10.3390/foods10061422
Chicago/Turabian StyleGabriele, Morena, Stefania Frassinetti, and Laura Pucci. 2021. "Antimicrobial Activity and Protective Effect of Tuscan Bee Pollens on Oxidative and Endoplasmic Reticulum Stress in Different Cell-Based Models" Foods 10, no. 6: 1422. https://doi.org/10.3390/foods10061422
APA StyleGabriele, M., Frassinetti, S., & Pucci, L. (2021). Antimicrobial Activity and Protective Effect of Tuscan Bee Pollens on Oxidative and Endoplasmic Reticulum Stress in Different Cell-Based Models. Foods, 10(6), 1422. https://doi.org/10.3390/foods10061422