“Sfogliatella Riccia Napoletana”: Realization of a Lard-Free and Palm Oil-Free Pastry
Abstract
1. Introduction
2. Materials and Methods
2.1. Fats
2.1.1. Free Acidity
2.1.2. Peroxide Value
2.1.3. Fatty Acids Composition
2.1.4. Triacylglycerols and Cholesterol
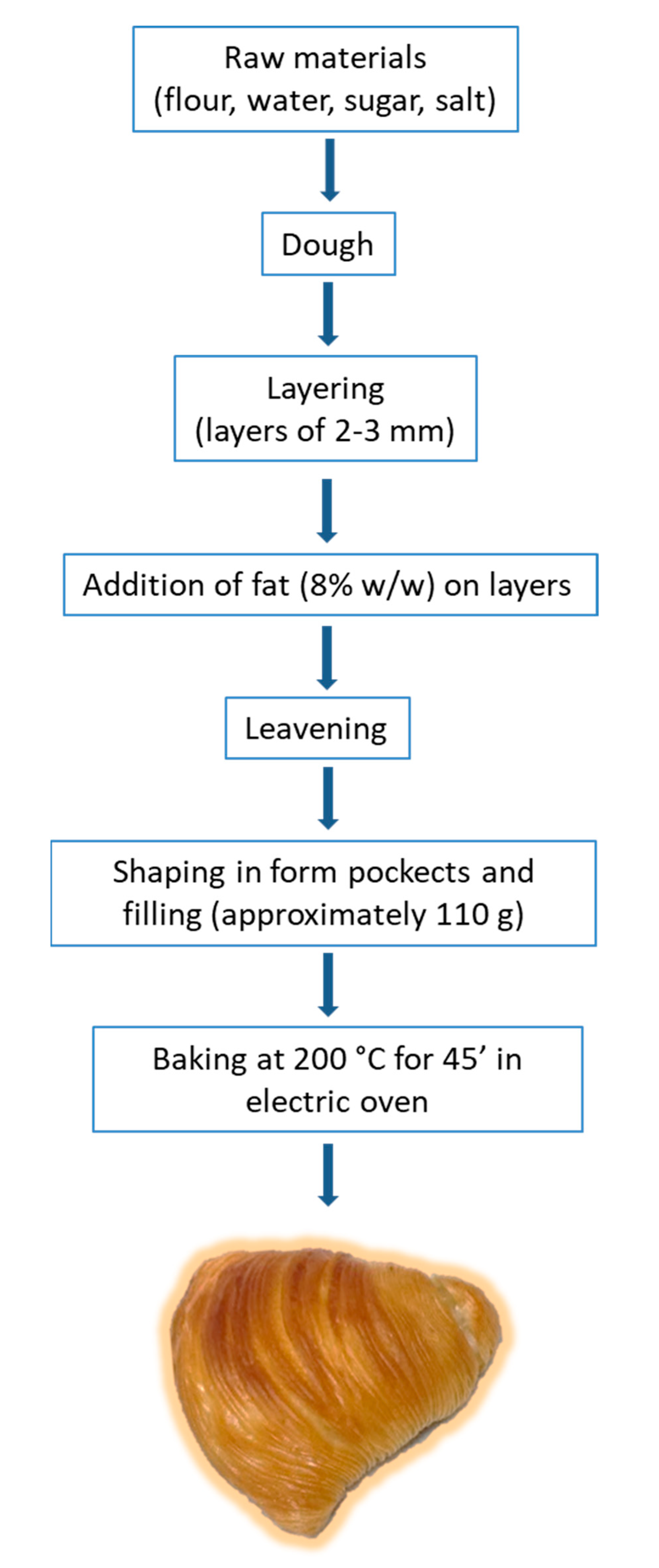
2.2. SRN Formulation
2.2.1. Moisture Content
- a = weight of cap with dried sample;
- b = weight of cap with not-dried sample;
- p = weight of sample in grams.
2.2.2. Water Activity (aw)
2.2.3. Fat Extraction
2.2.4. Free Acidity, Peroxide Value and Fatty Acids
2.2.5. Total Polar Compounds
- mNP = weight in grams of nonpolar fraction
- mp = weight in grams of polar fraction
- m = weight in grams of sample
2.2.6. Sensory Evaluation
2.3. Statistical Analysis
3. Results and Discussion
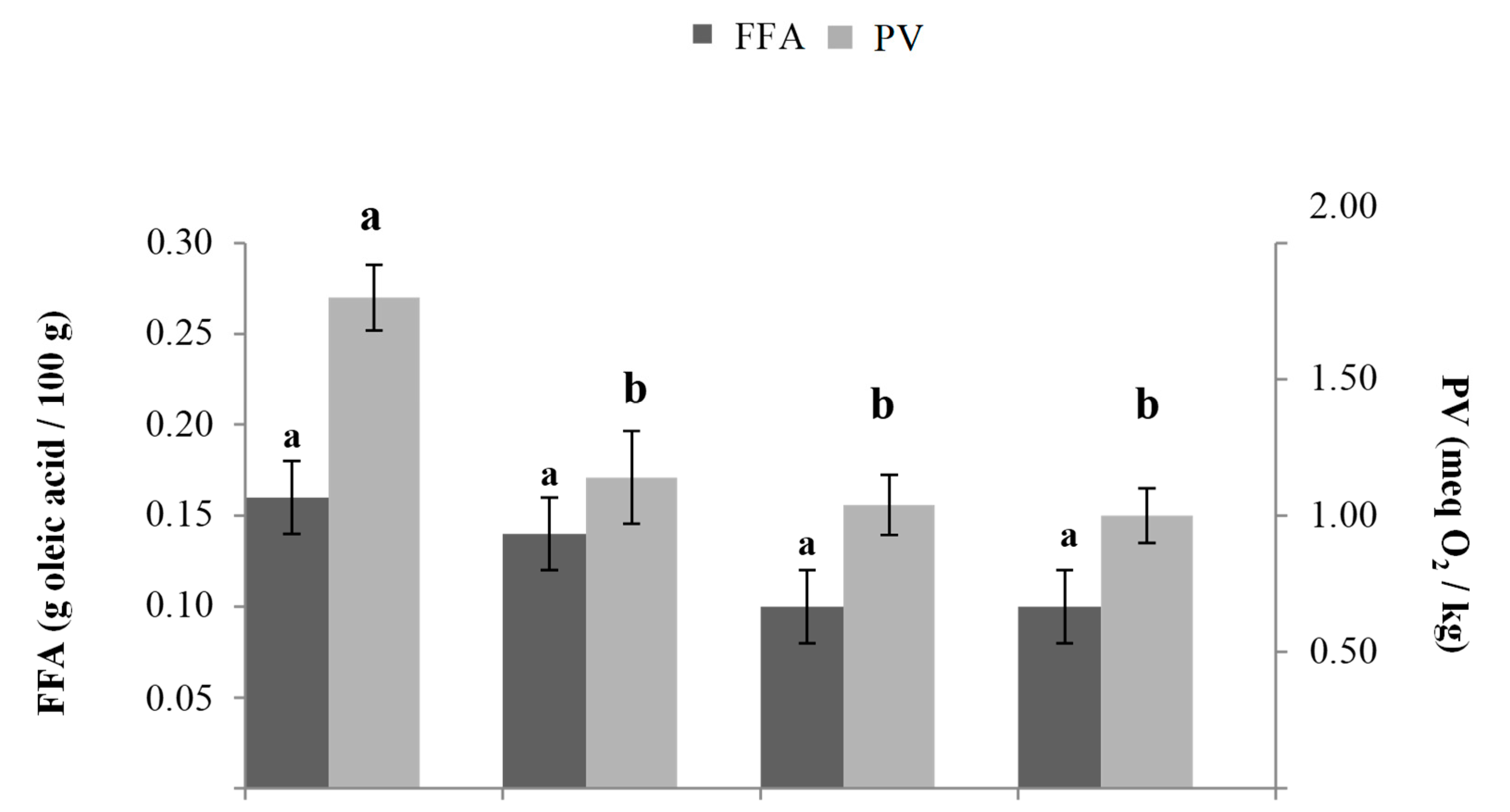
3.1. FFA and PV in Fat Blends
3.2. FA composition of Fat Blends
3.3. Triacylglycerols and Cholesterol in Fat Blends
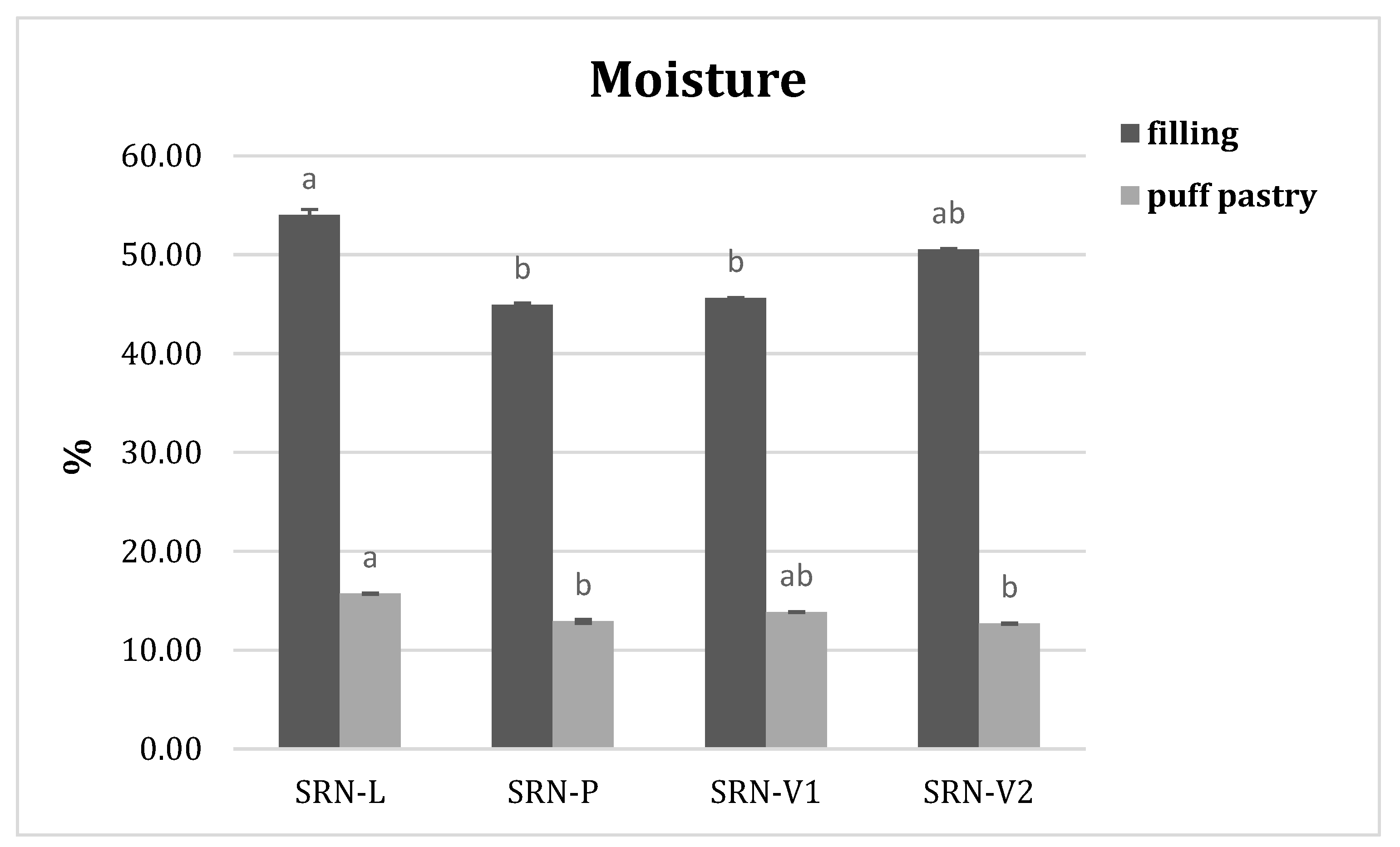
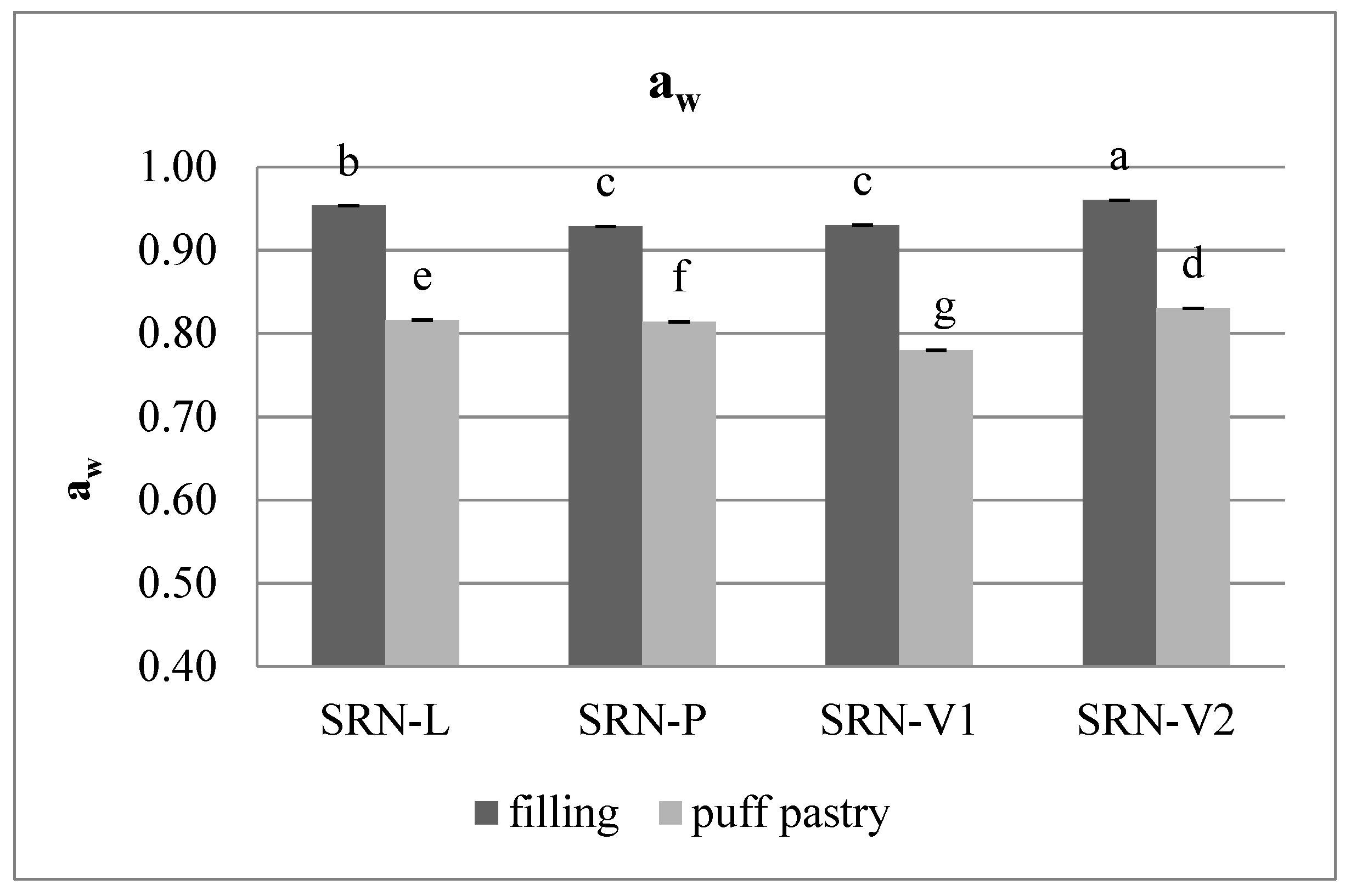
3.4. Moisture Content and Water Activity in SRN
3.5. FFA, PV and TPC in SRN
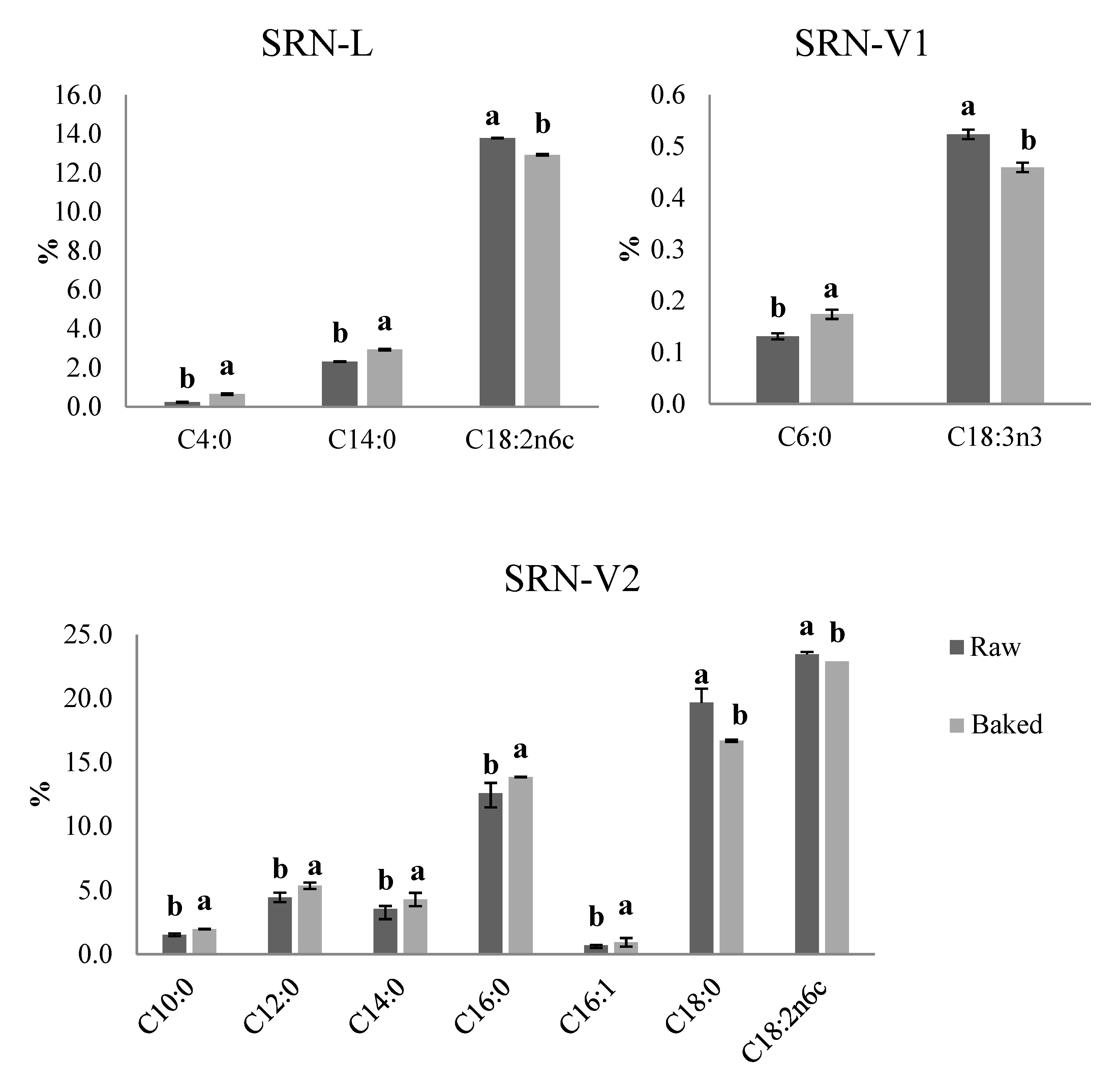
3.6. FA Composition of SRN
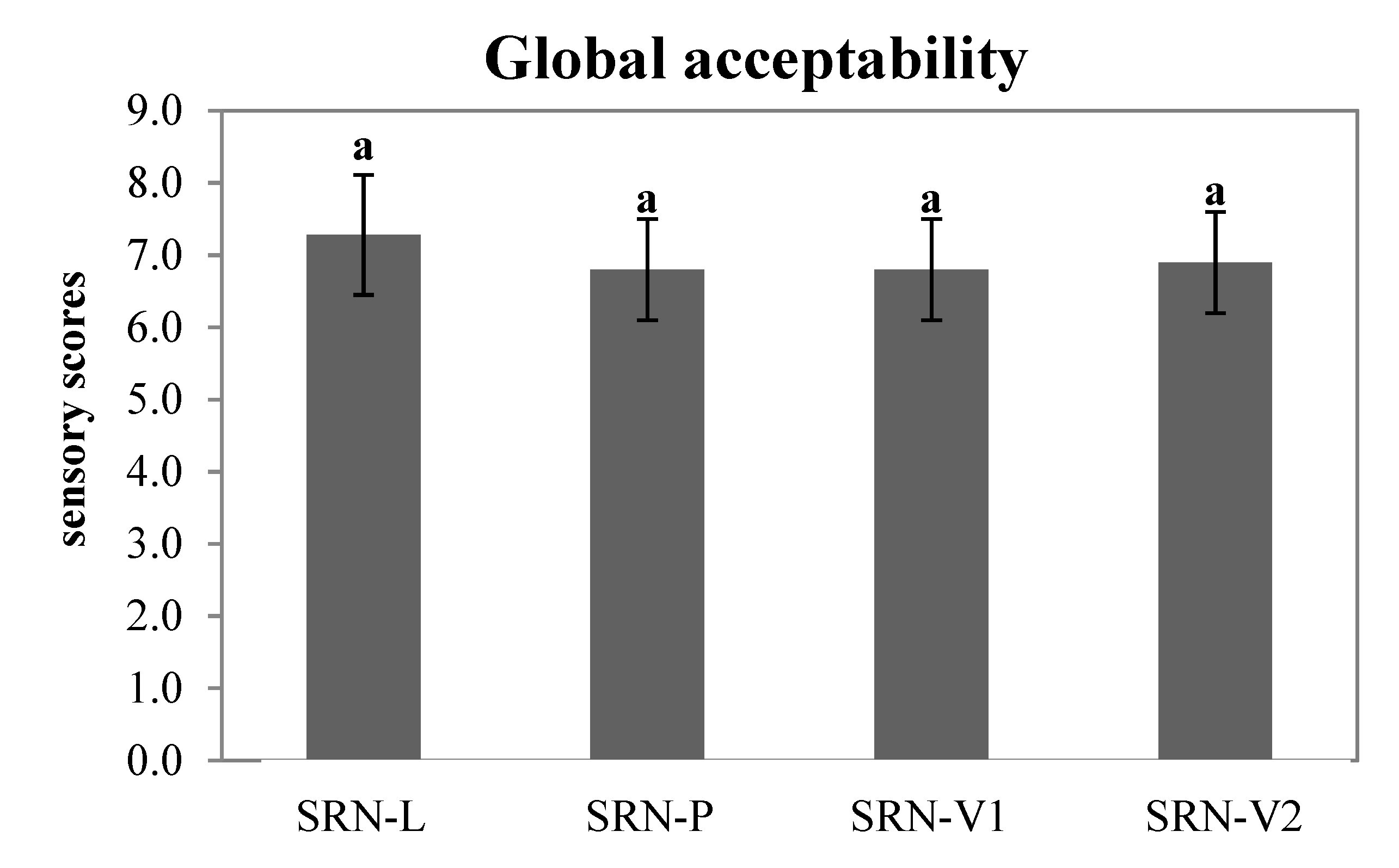
3.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gazzetta Ufficiale Della Repubblica Italiana (2001) N. 161 del 13 Luglio 2001; Istituto Poligrafico e Zecca dello Stato: Rome, Italy, 2001.
- Pagani, M.A.; Bottega, G.; Mariotti, M. Tecnologia dei prodotti lievitati da forno. In Biotecnologia dei Prodotti Lievitati da Forno; Gobbetti, M., Corsetti, A., Eds.; Publisher Ambrosiana: Milan, Italy, 2010; pp. 39–69. [Google Scholar]
- Wickramarachchi, K.S.; Sissons, M.J.; Cauvain, P.S. Puff pastry and trends in fat reduction: An update. Int. J. Food Sci. Technol. 2015, 50, 1065–1075. [Google Scholar] [CrossRef]
- Tarancón, P.; Salvador, A.; Sanz, T. Sunflower oil-water-cellulose ether emulsions as trans-fatty acid-free fat replacers in biscuits: Texture and acceptability study. Food Bioprocess Technol. 2013, 6, 2389–2398. [Google Scholar] [CrossRef]
- Conte, L.; Nicoli, M.C. Impiego di oli e Grassi nella Formulazione; Area Science Park: Trieste, Italy, 2003; pp. 1–109. [Google Scholar]
- Kamel, B.S. Characteristics of bread and buns made with lard and vegetable oils of different iodine values. J. Am. Oil Chem. Soc. 1992, 69, 794–796. [Google Scholar] [CrossRef]
- Nur Illiyin, M.R.; Marikkar, J.M.N.; Shuhaimi, M.; Mahiran, B.; Miskandar, M.S. A Comparison of the Thermo–Physical Behavior of Engkabang (Shorea macrophylla) Seed Fat–Canola Oil Blends and Lard. J. Am. Oil Chem. Soc. 2013, 90, 1485–1493. [Google Scholar] [CrossRef]
- Dijkstra, A.J. Modification Processes and Food Uses. The Lipid Handbook, 3rd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 263–353. [Google Scholar] [CrossRef]
- Pajin, B.; Soronja-Simovic, D.; Seres, Z.; Gyura, J.; Radujko, I.; Sakac, M. Physicochemical and textural properties of puff pastry margarines. Eur. J. Lipid Sci. Technol. 2011, 113, 262–268. [Google Scholar] [CrossRef]
- Dianda, M.; Bayala, J.; Diop, T.; Ouédraogo, S.J. Improving growth of shea butter tree (Vitellaria paradoxa CF Gaertn.) seedlings using mineral N, P and arbuscular mycorrhizal (AM) fungi. Biotechnol. Agron. Soc. 2009, 13, 93–102. [Google Scholar]
- Leakey, R.R.B. Potential for novel food products from agroforestry trees: A review. Food Chem. 1999, 66, 1–4. [Google Scholar] [CrossRef]
- Kante, A.; Igo, C.G.; Frick, M.J. Enhancing effectiveness of extension efforts: A case study of Malian shea butter producers. J. Int. Agric. Ext. Educ. 2009, 16, 05–15. [Google Scholar] [CrossRef]
- Teklehaimanot, Z. Exploiting the potential of indigenous agroforestry trees: Parkia biglobosa and Vitellaria paradoxa in sub-Saharan Africa. Agrofor. Syst. 2004, 61, 207–220. [Google Scholar]
- Bongers, P.; Almeida-Rivera, C. Dynamic modelling of the margarine production process. Comput. Aided Chem. Eng. 2011, 29, 1301–1305. [Google Scholar] [CrossRef]
- Chrysan, M.M. Margarines and spreads. In Bailey‘s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 1–41. [Google Scholar] [CrossRef]
- Miskandar, M.S.; Man, Y.C.; Yusoff, M.S.A.; Rahman, R.A. Quality of margarine: Fats selection and processing parameters. Asia Pac. J. Clin. Nutr. 2005, 14, 387–395. [Google Scholar]
- Vijay, V.; Pimm, S.L.; Jenkins, C.N.; Smith, S.J. Palm oil is everywhere—But where did it come from? Environ. Sci. J. Teens 2016, 12, 1–6. [Google Scholar]
- Grossi, M.; Di Lecce, G.; Gallina Toschi, T.; Riccò, B. Fast and accurate determination of olive oil acidity by electrochemical impedance spectroscopy. IEEE Sens. J. 2014, 14, 2947–2954. [Google Scholar] [CrossRef]
- EECR. European Economic Community Regulation 2568/91. Off. J. Eur. Communities L 1991, 248, 1–83. [Google Scholar]
- Grossi, M.; Di Lecce, G.; Arru, M.; Gallina Toschi, T.; Riccò, B. An opto-electronic system for in-situ determination of peroxide value and total phenol content in olive oil. J. Food Eng. 2015, 146, 1–7. [Google Scholar] [CrossRef]
- European Commission Regulation 1989/2003 amending Regulation (EEC)No 2568/91 on the characteristics of olive oil and olive-residue oil and on the re-levant methods of analysis. Off. J. Eur. Union 2003, L 295, 57–77.
- International Union of Pure and Applied Chemistry (IUPAC). Standard Methods for the Analysis of Oils, Fats and Derivatives; Pergamon Press: Oxford, UK, 1990. [Google Scholar]
- Romano, R.; Santini, A.; Le Grottaglie, L.; Manzo, N.; Visconti, A.; Ritieni, A. Identification markers based on fatty acid composition to differentiate between roasted Arabica and Canephora (Robusta) coffee varieties in mixtures. J. Food Compos. Anal. 2014, 35, 1–9. [Google Scholar] [CrossRef]
- Molkentin, J. Authentication of organic milk using δ13C and the α-linolenic acid content of milk fat. J. Agric. Food Chem. 2009, 57, 785–790. [Google Scholar] [CrossRef]
- Romano, R.; Giordano, A.; Chianese, L.; Addeo, F.; Spagna Musso, S. Triacylglycerols, fatty acids and conjugated linoleic acids in Italian Mozzarella di Bufala Campana cheese. J. Food Compos. Anal. 2011, 24, 244–249. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists AACC. Approved Methods of the AACC, 10th ed.; Methods 44-15A; The Association: St. Paul, MN, USA, 2000. [Google Scholar]
- International Standard 5B. Cheese and Processed Cheese Products–Determination of Fat Content (Schmid-Bondzynski-Ratzlaff Reference Method); International Dairy Federation: Brussels, Belgium, 1986. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; Method Nr. 92307; AOAC: Washington, DC, USA, 1999. [Google Scholar]
- Dobarganes, M.C.; Velasco, J.; Dieffenbacher, A. Determination of polar compounds, polymerized and oxidized triacylglycerols, and diacylglycerols in oils and fats: Results of collaborative studies and the standardized method (Technical report). Pure Appl. Chem. 2000, 72, 1563–1575. [Google Scholar] [CrossRef]
- Cocci, E.; Sacchetti Vallicelli, M.; Angioloni, A.; Dalla Rosa, M. Spaghetti cooking by microwave oven: Cooking kinetics and product quality. J. Food Eng. 2008, 85, 537–546. [Google Scholar] [CrossRef]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Shahidi, F.; Zhong, Y. Lipid oxidation: Measurement methods. In Bailey‘s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; pp. 357–385. [Google Scholar] [CrossRef]
- Calligaris, S.; Pieve, S.D.; Kravina, G.; Manzocco, L.; Nicoli, C.M. Shelf life prediction of bread sticks using oxidation indices: A validation study. J. Food Sci. 2008, 73, E51–E56. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Lembo, G.; Aretini, A.; Nag, A. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2007, 100, 998–1004. [Google Scholar] [CrossRef]
- Garcia-Macias, P.; Gordon, M.H.; Frazier, R.A.; Smith, K.; Gambelli, L. Performance of palm-based fat blends with a low saturated fat content in puff pastry. Eur. J. Lipid Sci. Technol. 2011, 113, 1474–1480. [Google Scholar] [CrossRef]
- Maranz, S.; Wiesman, Z.; Bisgaard, J.; Bianchi, G. Germplasm resources of Vitellaria paradoxa based on variations in fat composition across the species distribution range. AGROFOR Int. J. 2004, 60, 71–76. [Google Scholar] [CrossRef]
- Mensink, R.P. Effects of stearic acid on plasma lipid and lipoproteins in humans. Lipids 2005, 40, 1201–1205. [Google Scholar] [CrossRef]
- Chiesa, L.; Panseri, S.; Bonacci, S.; Procopio, A.; Zecconi, A.; Arioli, F.; Cuevas, F.j.; Moreno-Rojas, J.M. Authentication of Italian PDO lard using NIR spectroscopy, volatile profile and fatty acid composition combined with chemometrics. Food Chem. 2016, 212, 296–304. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2013; 89p. [Google Scholar]
- Nor Aini, I.; Miskandar, M. S Utilization of palm oil and palm products in shortenings and margarines. Eur. J. Lipid Sci. Technol. 2007, 109, 422–432. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of water activity (aw) on microbial stability as a hurdle in food preservation. Water Act. Foods Fundam. Appl. 2020, 323–355. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Gomes, T.; Nasti, R.; Caponio, F.; Summo, C. Effects of free fatty acids on the oxidative processes in purified olive oil. Food Res. Int. 2010, 43, 1389–1394. [Google Scholar] [CrossRef]
- Mahesar, S.A.; Sherazi, S.T.H.; Khaskheli, A.R.; Kandhro, A.A. Analytical approaches for the assessment of free fatty acids in oils and fats. Anal. Methods 2014, 6, 4956–4963. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.O.; Min, D.B. Effects and prooxidant mechanisms of oxidized α- tocopherol on the oxidative stability of soybean oil. J. Food Sci. 2007, 72, C223–C230. [Google Scholar] [CrossRef]
- DaĞlioĞlu, O.; Tasan, M.; Tuncel, B. Effects of microwave and conventional baking on the oxidative stability and fatty acid composition of puff pastry. J. Am. Oil Chem. Soc. 2000, 77, 543–545. [Google Scholar] [CrossRef]
- Abdulkarim, S.M.; Long, K.; Lai, O.M.; Muhammad, S.K.S.; Ghazali, H.M. Frying quality and stability of high-oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem. 2007, 105, 1382–1389. [Google Scholar] [CrossRef]
- Aladedunye, F.A.; Przybylski, R. Degradation and nutritional quality changes of oil during frying. J. Am. Oil Chem. Soc. 2009, 86, 149–156. [Google Scholar] [CrossRef]
- D’souza, V.; de Man, J.M. Chemical and physical properties of the high melting glyceride fractions of commercial margarines. J. Am. Oil Chem. Soc. 1991, 68, 153–162. [Google Scholar] [CrossRef]
- DaĞlioĞlu, O.; TaŞan, M.; TunÇel, B. Determination of fatty acid composition and total trans fatty acids in cereal-based Turkish foods. Turk. J. Chem. 2002, 26, 705–710. [Google Scholar]
- Yoshida, H.; Hirakawa, Y.; Abe, S.; Mizushina, Y. The Content of Tocopherols and Oxidative Quality of Oils Prepared from Sunflower (Helianthus Annuus L.) Seeds Roasted in a Microwave Oven. Eur. J. Lipid Sci. Technol. 2002, 104, 116–122. [Google Scholar] [CrossRef]
| Blend | Lard (%) | Palm Oil (%) | Sunflower Oil (%) | Coconut Oil (%) | Shea Butter (%) | Saturation (%) |
|---|---|---|---|---|---|---|
| L | 100 | 39.4 | ||||
| P | 80 | 20 | 42.9 | |||
| V1 | 60 | 15 | 25 | 40.4 | ||
| V2 | 40 | 20 | 40 | 42.4 |
| Fatty Acids (%) | L | P | V1 | V2 |
|---|---|---|---|---|
| C4:0 | nd | nd | 0.73 ± 0.64 | 0.43 ± 0.03 |
| C6:0 | nd | nd | 0.07 ± 0.01 | 0.05 ± 0.01 |
| C8:0 | nd | nd | 1.05 ± 0.03 | 0.74 ± 0.03 |
| C10:0 | 0.07 ± 0.02 | nd | 0.81 ± 0.05 | 0.54 ± 0.01 |
| C12:0 | 0.09 ± 0.01 | 0.21 ± 0.02 | 7.25 ± 0.06 | 4.76 ± 0.21 |
| C14:0 | 1.50 ± 0.10 | 1.02 ± 0.03 | 2.72 ± 0.08 | 1.85 ± 0.01 |
| C14:1 | 0.01 ± 0.01 | nd | nd | nd |
| C15:0 | 0.04 ± 0.01 | nd | nd | nd |
| C16:0 | 24.10 ± 0.11 | 35.31 ± 0.23 | 6.21 ± 0.23 | 5.87 ± 0.02 |
| C16:1 | 2.63 ± 0.14 | 0.17 ± 0.01 | 0.03 ± 0.02 | Nd |
| C17:0 | 0.27 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.00 | 0.06 ± 0.01 |
| C17:1 | 0.27 ± 0.01 | nd | 0.01 ± 0.01 | 0.01 ± 0.01 |
| C18:0 | 13.14 ± 0.09 | 5.56 ± 0.05 | 20.37 ± 0.19 | 26.95 ± 0.01 |
| C18:1n9t | 0.23 ± 0.01 | nd | 0.09 ± 0.14 | 0.18 ± 0.01 |
| C18:1n9c | 41.33 ± 0.17 | 36.84 ± 0.02 | 21.73 ± 0.04 | 29.59 ± 0.03 |
| C18:2n6t | 0.03 ± 0.01 | nd | 0.09 ± 0.09 | 0.07 ± 0.00 |
| C18:2n6c | 13.85 ± 0.05 | 19.57 ± 0.18 | 37.44 ± 0.02 | 27.32 ± 0.06 |
| C18:3n6 | nd | nd | 0.22 ± 0.03 | 0.35 ± 0.02 |
| C18:3n3 | 1.25 ± 0.02 | 0.20 ± 0.03 | nd | nd |
| C20:0 | 0.17 ± 0.01 | 0.44 ± 0.51 | 0.64 ± 0.12 | 0.79 ± 0.02 |
| C20:1 | nd | 0.34 ± 0.22 | nd | nd |
| C20:2 | 0.60 ± 0.10 | nd | 0.02 ± 0.01 | 0.03 ± 0.01 |
| C22:0 | 0.01 ± 0.01 | 0.26 ± 0.02 | 0.36 ± 0.13 | 0.29 ± 0.01 |
| C20:3n6 | 0.07 ± 0.01 | nd | nd | nd |
| C20:4n6 | 0.32 ± 0.01 | nd | nd | nd |
| C23:0 | nd | nd | 0.01 ± 0.01 | 0.01 ± 0.01 |
| C24:0 | nd | 0.02 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 |
| C22:6n3 | nd | nd | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Σ SFA | 39.40 b ± 0.19 | 42.97 a ± 0.28 | 40.35 b ± 0.31 | 42.43 a ± 0.23 |
| Σ MUFA | 44.47 a ± 0.17 | 37.01 b ± 0.04 | 21.86 d ± 0.10 | 29.78 c ± 0.04 |
| Σ PUFA | 16.13 d ± 0.03 | 20.03 c ± 0.10 | 37.79 a ± 0.08 | 27.79 b ± 0.02 |
| Σ UFA | 60.60 a ± 0.19 | 57.04 d ± 0.13 | 59.65 b ± 0.03 | 57.57 c ± 0.06 |
| UFA/SFA | 1.54 a ± 0.01 | 1.33 c ± 0.01 | 1.48 b ± 0.01 | 1.36 c ± 0.01 |
| MUFA/SFA | 1.13 a ± 0.01 | 0.86 b ± 0.01 | 0.54 d ± 0.01 | 0.70 c ± 0.01 |
| PUFA/SFA | 0.41 d ± 0.01 | 0.47 c ± 0.01 | 0.94 a ± 0.01 | 0.65 b ± 0.01 |
| TAG (%) | L | P | V1 | V2 |
|---|---|---|---|---|
| C24-C42 | 11.8 ± 0.3 | 0.3 ± 0.1 | 20.1 ± 0.2 | 20.5 ± 0.2 |
| C44 | 0.3 ± 0.1 | 0.2 ± 0.1 | 7.0 ± 0.2 | 5.1 ± 0.1 |
| C46 | 0.8 ± 0.1 | 1.0 ± 0.1 | 6.0 ± 0.2 | 5.0 ± 0.1 |
| C48 | 4.8 ± 0.3 | 7.6 ± 0.1 | 22.4 ± 1.7 | 13.0 ± 0.1 |
| C50 | 19.5 ± 0.6 | 32.0 ± 0.2 | nd | 5.6 ± 0.1 |
| C52 | 44.1 + 6±1.2 | 34.4 ± 0.4 | 10.4 ± 0.1 | 11.3 ± 0.1 |
| C54 | 18.7 ± 0.3 | 23.5 ± 0.2 | 32.8 ± 1.1 | 38.8 ± 0.9 |
| Others | nd | 1.0 ± 0.1 | 1.3 ± 0.1 | 0.7 ± 0.2 |
| Cholesterol (%) | 0.11 ± 0.3 | nd | nd | nd |
| Samples | FFA (g oleic acid/100 g oil) | PV (meq O2/kg oil) | TPC (%) |
|---|---|---|---|
| Raw SRN-L | 0.58 ± 0.1 c | 3.53 ± 0.1 e | 8.84 ± 0.2 fg |
| Baked SRN-L | 0.72 ± 0.1 b | 15.75 ± 0.3 a | 14.55 ± 0.1 c |
| Raw SRN-P | 0.20 ± 0.1 d | 3.23 ± 0.1 e | 9.17 ± 0.1 ef |
| Baked SRN-P | 0.35 ± 0.1 e | 9.13 ± 0.1 b | 15.22 ± 0.1 b |
| Raw SRN-V1 | 0.58 ± 0.1 c | 3.03 ± 0.1 e | 9.65 ± 0.1 e |
| Baked SRN-V1 | 0.83 ± 0.1 a | 7.25 ± 0.2 c | 16.44 ± 0.1 a |
| Raw SRN-V2 | 0.11 ± 0.1 e | 2.88 ± 0.1 f | 9.19 ± 0.1 ef |
| Baked SRN-V2 | 0.18 ± 0.1 e | 5.35 ± 0.16 d | 13.39 ± 0.1 d |
| Fatty Acids (%) | Raw SRN-L | Raw SRN-P | Raw SRN-V1 | Raw SRN-V2 |
|---|---|---|---|---|
| C4:0 | 0.23 ± 0.02 | 0.65 ± 0.04 | 0.14 ± 0.02 | 0.74 ± 0.13 |
| C6:0 | 0.16 ± 0.02 | 0.33 ± 0.02 | 0.13 ± 0.01 | 0.42 ± 0.06 |
| C8:0 | 0.10 ± 0.02 | 0.25 ± 0.02 | 0.73 ± 0.08 | 0.96 ± 0.16 |
| C10:0 | 0.28 ± 0.02 | 0.50 ± 0.02 | 0.66 ± 0.04 | 1.52 ± 0.11 |
| C12:0 | 0.32 ± 0.01 | 0.33 ± 0.07 | 4.21 ± 0.29 | 4.45 ± 0.38 |
| C14:0 | 2.31 ± 0.07 | 1.75 ± 0.13 | 2.68 ± 0.13 | 3.55 ± 0.23 |
| C14:1 | 0.11 ± 0.01 | 0.23 ± 0.01 | nd | 0.26 ± 0.01 |
| C15:0 | 0.15 ± 0.01 | 0.23 ± 0.01 | nd | 0.22 ± 0.02 |
| C16:0 | 28.21 ± 1.01 | 40.88 ± 2.30 | 17.06 ± 0.55 | 12.59 ± 0.80 |
| C16:1 | 2.63 ± 0.08 | 0.68 ± 0.04 | 1.27 ± 0.05 | 0.69 ± 0.04 |
| C17:0 | 0.35 ± 0.02 | 0.15 ± 0.01 | 0.19 ± 0.01 | 0.16 ± 0.01 |
| C17:1 | 0.37 ± 0.01 | nd | 0.16 ± 0.01 | nd |
| C18:0 | 12.54 ± 0.63 | 3.46 ± 0.59 | 13.78 ± 0.53 | 19.85 ± 1.11 |
| C18:1n9t | 0.42 ± 0.02 | 0.26 ± 0.05 | 0.23 ± 0.01 | 0.25 ± 0.03 |
| C18:1n9c | 34.85 ± 0.03 | 32.77 ± 1.43 | 31.07 ± 0.36 | 29.64 ± 0.44 |
| C18:2n6c | 13.79 ± 0.24 | 17.37 ± 1.53 | 25.54 ± 0.13 | 23.68 ± 0.19 |
| C18:3n6 | 0.22 ± 0.02 | nd | 0.39 ± 0.02 | nd |
| C18:3n3 | 0.80 ± 0.02 | 0.16 ± 0.06 | 0.52 ± 0.01 | 0.44 ± 0.02 |
| C21:0 | 1.00 ± 0.11 | nd | 0.49 ± 0.02 | 0.30 ± 0.04 |
| C22:0 | 1.16 ± 0.13 | nd | 0.49 ± 0.04 | nd |
| C22:1n9 | nd | nd | 0.10 ± 0.01 | nd |
| C20:4n6 | nd | nd | 0.16 ± 0.01 | 0.28 ± 0.03 |
| Σ SFA | 46.81 a ± 0.28 | 48.53 a ± 2.04 | 40.56 b ± 0.52 | 44.76 ab ± 0.73 |
| Σ MUFA | 38.38 a ± 0.06 | 33.94 b ± 1.43 | 32.83 b ± 0.33 | 30.284 b ± 0.49 |
| Σ PUFA | 14.81 b ± 0.31 | 17.53 b ± 1.61 | 26.61 a ± 0.19 | 24.40 a ± 0.24 |
| UFA/SFA | 1.14 a ± 0.00 | 1.06 a ± 0.11 | 1.47 a ± 0.03 | 1.23 a ± 0.04 |
| TFA | 0.42 a ± 0.03 | 0.26 ab ± 0.03 | 0.23 b ± 0.01 | 0.25 b ± 0.02 |
| Σ omega 3 | 0.80 a ± 0.14 | 0.16 c ± 0.06 | 0.52 ab ± 0.02 | 0.44 bc ± 0.01 |
| Σ omega 6 | 14.01 c ± 0.33 | 17.37 b ± 1.54 | 26.09 a ± 0.17 | 23.96 a ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, R.; Aiello, A.; De Luca, L.; Acunzo, A.; Montefusco, I.; Pizzolongo, F. “Sfogliatella Riccia Napoletana”: Realization of a Lard-Free and Palm Oil-Free Pastry. Foods 2021, 10, 1393. https://doi.org/10.3390/foods10061393
Romano R, Aiello A, De Luca L, Acunzo A, Montefusco I, Pizzolongo F. “Sfogliatella Riccia Napoletana”: Realization of a Lard-Free and Palm Oil-Free Pastry. Foods. 2021; 10(6):1393. https://doi.org/10.3390/foods10061393
Chicago/Turabian StyleRomano, Raffaele, Alessandra Aiello, Lucia De Luca, Alessandro Acunzo, Immacolata Montefusco, and Fabiana Pizzolongo. 2021. "“Sfogliatella Riccia Napoletana”: Realization of a Lard-Free and Palm Oil-Free Pastry" Foods 10, no. 6: 1393. https://doi.org/10.3390/foods10061393
APA StyleRomano, R., Aiello, A., De Luca, L., Acunzo, A., Montefusco, I., & Pizzolongo, F. (2021). “Sfogliatella Riccia Napoletana”: Realization of a Lard-Free and Palm Oil-Free Pastry. Foods, 10(6), 1393. https://doi.org/10.3390/foods10061393







