Inhibition Mechanism of L-Cysteine on Maillard Reaction by Trapping 5-Hydroxymethylfurfural
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Inhibitory Effect of Sulfhydryl Compounds on MR
2.3. HPLC-DAD
2.4. Preparation of the Adduct of HMF and L-Cys
2.5. UPLC-Q-TOF-MS for DCH Identification
2.6. Nuclear Magnetic Resonance (NMR)
2.7. Detection of DCH during MR
2.8. Cytotoxicity and Biological Activity of DCH in RAW 264.7 Cells
2.8.1. Cell Culture
2.8.2. Cytotoxicity Assay
2.8.3. Anti-Inflammatory Activity
2.8.4. Relieving H2O2-Induced Oxidative Stress in RAW 264.7 Cells
2.9. Free Radical Scavenging Ability
2.9.1. Relative DPPH Radical Scavenging Capacity (RDSC)
2.9.2. ABTS Cation Radical (ABTS•+) Scavenging Capacity Assay (ASCA)
2.9.3. Oxygen Radical Absorbance Capacity (ORAC)
2.10. Statistical Analysis
3. Results and Discussion
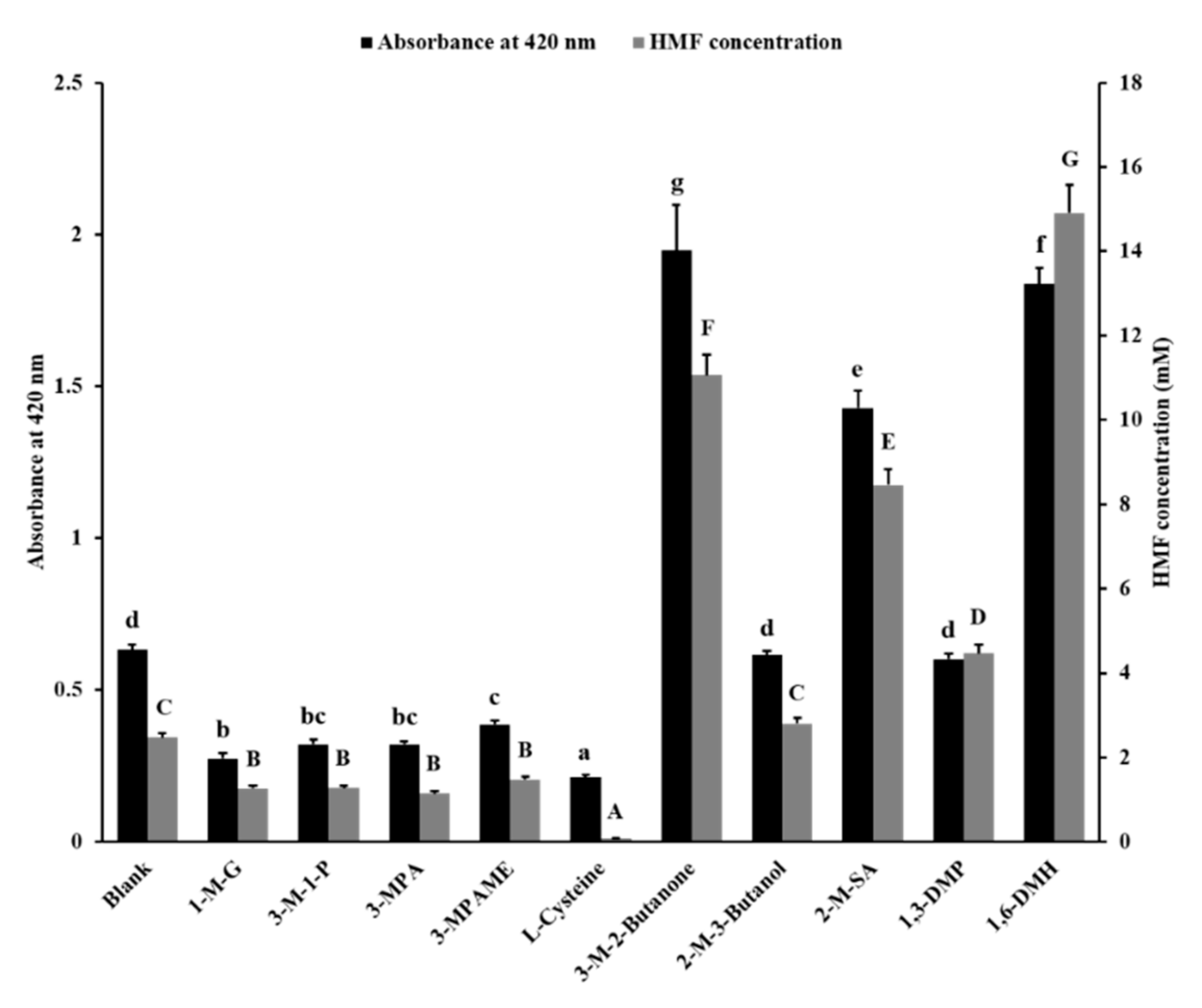
3.1. Inhibitory Effect of Sulfhydryl Compounds on MR
3.2. Structural Identification of the Adduct of HMF and L-Cys
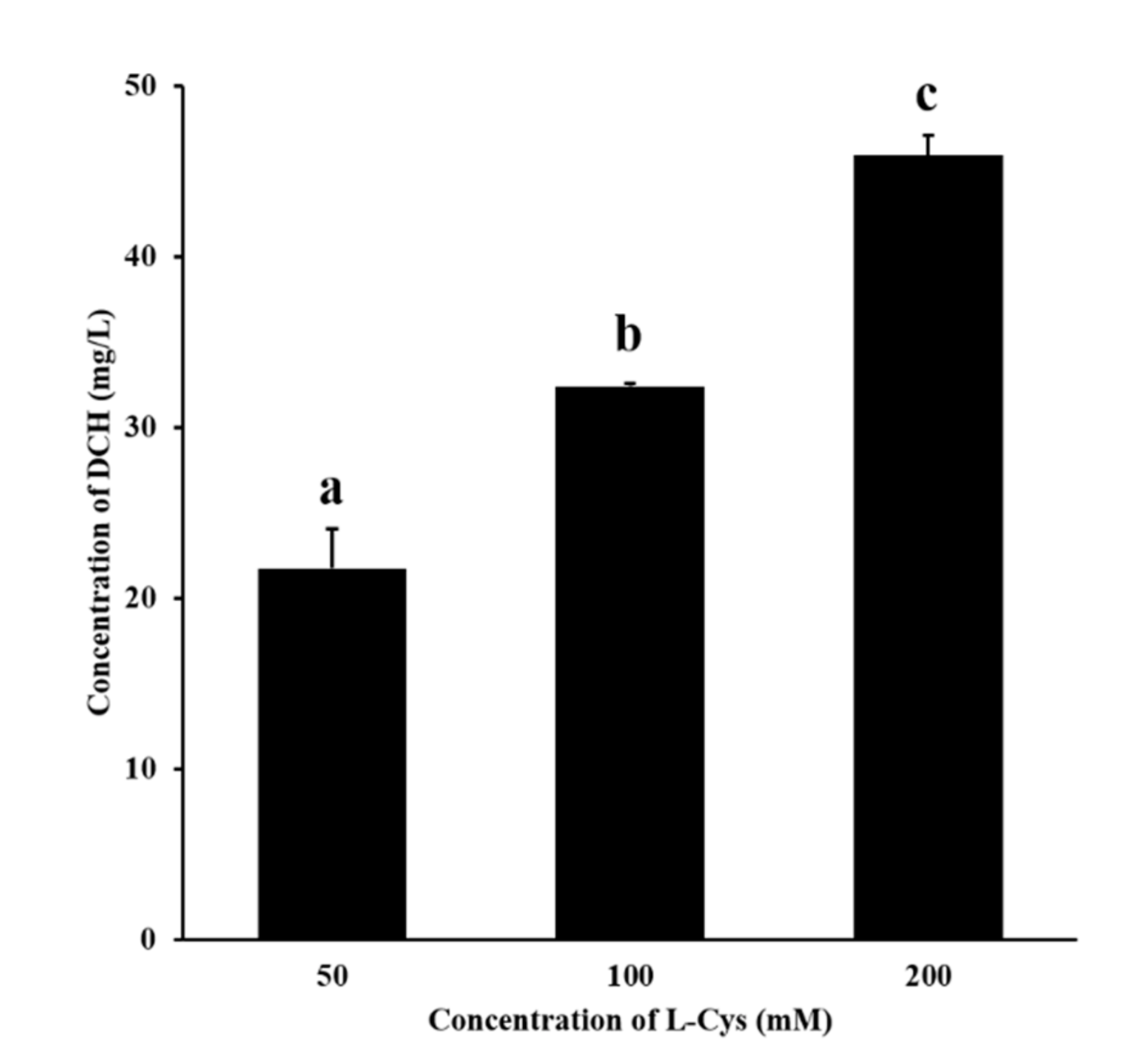
3.3. Confirmation of the Generation of DCH during MR Added with L-Cys
3.4. The Cytotoxicity of HMF and DCH on RAW 264.7 Cells
3.5. Anti-Inflammatory Activity Comparison between HMF and DCH
3.6. Extracellular Antioxidant Activity Comparison between HMF and DCH
3.7. Intracellular Antioxidant Activity Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Newton, A.E.; Fairbanks, A.J.; Golding, M.; Andrewes, P.; Gerrard, J.A. The role of the Maillard reaction in the formation of flavour compounds in dairy products—Not only a deleterious reaction but also a rich source of flavour compounds. Food Funct. 2012, 3, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Perez-Locas, C.; Yaylayan, V.A. The Maillard reaction and food quality deterioration. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 70–94. [Google Scholar]
- Chutintrasri, B.; Noomhorm, A. Color degradation kinetics of pineapple puree during thermal processing. LWT Food Sci. Technol. 2007, 40, 300–306. [Google Scholar] [CrossRef]
- Jaeger, H.; Janositz, A.; Knorr, D. The Maillard reaction and its control during food processing. The potential of emerging technologies. Pathol. Biol. 2010, 58, 207–213. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, ageing, diabetes: A short history of the maillard reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, Q.; Liu, Y.; Guo, Y.; Xie, Y.; Zhou, W.; Yao, W. Recent advances of ultrasound-assisted Maillard reaction. Ultrason. Sonochem. 2020, 64, 104844. [Google Scholar] [CrossRef]
- Pischetsrieder, M.; Henle, T. Glycation products in infant formulas: Chemical, analytical and physiological aspects. Amino Acids 2012, 42, 1111–1118. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Glatt, H.; Schneider, H.; Liu, Y. V79-hCYP2E1-hSULT1A1, a cell line for the sensitive detection of genotoxic effects induced by carbohydrate pyrolysis products and other food-borne chemicals. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2005, 580, 41–52. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Hu, T.; Li, Y.; Liu, R.; Zhang, J.; He, H. Potential neurotoxicity of 5-hydroxymethylfurfural and its oligomers: Widespread substances in carbohydrate-containing foods. Food Funct. 2020, 11, 4216–4223. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-Hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Tannenbaum, S.R. Activation of the Maillard reaction product 5-(hydroxymethyl) furfural to strong mutagens via allylic sulfonation and chlorination. Chem. Res. Toxicol. 1994, 7, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Høie, A.H.; Svendsen, C.; Brunborg, G.; Glatt, H.; Alexander, J.; Meinl, W.; Husøy, T. Genotoxicity of three food processing contaminants in transgenic mice expressing human sulfotransferases 1A1 and 1A2 as assessed by the in vivo alkaline single cell gel electrophoresis assay. Environ. Mol. Mutagenesis 2015, 56, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Bauer-Marinovic, M.; Taugner, F.; Florian, S.; Glatt, H. Toxicity studies with 5-hydroxymethylfurfural and its metabolite 5-sulphooxymethylfurfural in wild-type mice and transgenic mice expressing human sulphotransferases 1A1 and 1A2. Arch. Toxicol. 2012, 86, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Janzowski, C.; Glaab, V.; Samimi, E.; Schlatter, J.; Eisenbrand, G. 5-Hydroxymethylfurfural: Assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem. Toxicol. 2000, 38, 801–809. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Ameur, L.A.; Mathieu, O.; Lalanne, V.; Trystram, G.; Birlouez-Aragon, I. Comparison of the effects of sucrose and hexose on furfural formation and browning in cookies baked at different temperatures. Food Chem. 2007, 101, 1407–1416. [Google Scholar] [CrossRef]
- Czerwonka, M.; Opiłka, J.; Tokarz, A. Evaluation of 5-hydroxymethylfurfural content in non-alcoholic drinks. Eur. Food Res. Technol. 2018, 244, 11–18. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Wajda, Ł.; Tarko, T.; Sroka, P.; Satora, P. A review of the interactions between acrylamide, microorganisms and food components. Food Funct. 2016, 7, 1282–1295. [Google Scholar] [CrossRef]
- Mousavi, R.; Alizadeh, M.; Saleh-Ghadimi, S. Consumption of 5-hydroxymethylfurfural-rich dried fruits is associated with reduction in urinary excretion of 8-hydroxy-2′-deoxyguanosine: A randomized clinical trial. Eur. Food Res. Technol. 2016, 242, 677–684. [Google Scholar] [CrossRef]
- Chevalier, F.; Chobert, J.-M.; Genot, C.; Haertlé, T. Scavenging of free radicals, antimicrobial, and cytotoxic activities of the maillard reaction products of β-lactoglobulin glycated with several sugars. J. Agric. Food Chem. 2001, 49, 5031–5038. [Google Scholar] [CrossRef]
- Chevalier, F.; Chobert, J.-M.; Popineau, Y.; Nicolas, M.G.; Haertlé, T. Improvement of functional properties of β-lactoglobulin glycated through the Maillard reaction is related to the nature of the sugar. Int. Dairy J. 2001, 11, 145–152. [Google Scholar] [CrossRef]
- Yin, J.; Hedegaard, R.V.; Skibsted, L.H.; Andersen, M.L. Epicatechin and epigallocatechin gallate inhibit formation of intermediary radicals during heating of lysine and glucose. Food Chem. 2014, 146, 48–55. [Google Scholar] [CrossRef]
- Li, Y.; Jongberg, S.; Andersen, M.L.; Davies, M.J.; Lund, M.N. Quinone-induced protein modifications: Kinetic preference for reaction of 1,2-benzoquinones with thiol groups in proteins. Free Radic. Biol. Med. 2016, 97, 148–157. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard React. Foods: Strategies and Chem. Mech. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef]
- Erbas, M.; Sekerci, H.; Arslan, S.; Durak, A.N. Effect of sodium metabisulfite addition and baking temperature on Maillard reaction in bread. J. Food Qual. 2012, 35, 144–151. [Google Scholar] [CrossRef]
- Davies, S.S.; Zhang, L.S. Reactive carbonyl species scavengers-novel therapeutic approaches for chronic diseases. Curr. Pharmacol. Rep. 2017, 3, 51–67. [Google Scholar] [CrossRef]
- Paravisini, L.; Peterson, D.G. Mechanisms non-enzymatic browning in orange juice during storage. Food Chem. 2019, 289, 320–327. [Google Scholar] [CrossRef]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.W.; Rychlik, M.; Taylor, A.J.; Schmitt-Kopplin, P. Insights into the chemistry of non-enzymatic browning reactions in different ribose-amino acid model systems. Sci. Rep. 2018, 8, 16879. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar] [CrossRef]
- Jung, H.; Kwak, H.K.; Hwang, K.T. Antioxidant and antiinflammatory activities of cyanidin-3-glucoside and cyanidin-3-rutinoside in hydrogen peroxide and lipopolysaccharide-treated RAW264.7 cells. Food Sci. Biotechnol. 2014, 23, 2053–2062. [Google Scholar] [CrossRef]
- Cheng, Z.; Moore, J.; Yu, L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Hao, Z.; Zhou, K.; Luther, M.; Costa, J.; Yu, L. Carotenoid, tocopherol, phenolic acid, and antioxidant properties of Maryland-grown soft wheat. J. Agric. Food Chem. 2005, 53, 6649–6657. [Google Scholar] [CrossRef]
- Sartori, G.; Ballini, R.; Bigi, F.; Bosica, G.; Maggi, R.; Righi, P. Protection (and deprotection) of functional groups in organic synthesis by heterogeneous catalysis. Chem. Rev. 2004, 104, 199–250. [Google Scholar] [CrossRef]
- Zou, Y.; Pei, K.; Peng, X.; Bai, W.; Huang, C.; Ou, S. Possible adducts formed between hydroxymethylfurfural and selected amino acids, and their release in simulated gastric model. Int. J. Food Sci. Technol. 2016, 51, 1002–1009. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Formation and elimination reactions of 5-hydroxymethylfurfural during in vitro digestion of biscuits. Food Res. Int. 2017, 99, 308–314. [Google Scholar] [CrossRef]
- Zhao, Q.; Zou, Y.; Huang, C.; Lan, P.; Zheng, J.; Ou, S. Formation of a hydroxymethylfurfural–cysteine adduct and its absorption and cytotoxicity in caco-2 cells. J. Agric. Food Chem. 2017, 65, 9902–9908. [Google Scholar] [CrossRef]
- Wang, G.; Liu, P.; He, J.; Yin, Z.; Yang, S.; Zhang, G.; Ou, S.; Yang, X.; Zheng, J. Identification of a 5-hydroxymethylfurfural–lysine schiff base and its cytotoxicity in three cell lines. J. Agric. Food Chem. 2019, 67, 10214–10221. [Google Scholar] [CrossRef]
- Hwang, B.Y.; Lee, J.-H.; Koo, T.H.; Kim, H.S.; Hong, Y.S.; Ro, J.S.; Lee, K.S.; Lee, J.J. Furanoligularenone, an eremophilane from Ligularia fischeri, inhibits the LPS-induced production of nitric oxide and prostaglandin E2 in macrophage RAW264. 7 cells. Planta Med. 2002, 68, 101–105. [Google Scholar] [CrossRef]
- Fang, S.-C.; Hsu, C.-L.; Yen, G.-C. Anti-inflammatory effects of phenolic compounds isolated from the fruits of artocarpus heterophyllus. J. Agric. Food Chem. 2008, 56, 4463–4468. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I.H.; Newsholme, P. Molecular events linking oxidative stress and inflammation to insulin resistance and β-cell dysfunction. Oxidative Med. Cell. Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Rodríguez-Iturbe, B. Mechanisms of Disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006, 2, 582–593. [Google Scholar] [CrossRef]
- Cottone, S.; Lorito, M.C.; Riccobene, R.; Nardi, E.; Mulè, G.; Buscemi, S.; Geraci, C.; Guarneri, M.; Arsena, R.; Cerasola, G. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J. Nephrol. 2008, 21, 175–179. [Google Scholar]
- Hensley, K.; Robinson, K.A.; Gabbita, S.P.; Salsman, S.; Floyd, R.A. Reactive oxygen species, cell signaling, and cell injury. Free. Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Forman, H.J. Reactive oxygen species and alpha, beta-unsaturated aldehydes as second messengers in signal transduction. Ann. N. Y. Acad. Sci. 2010, 1203, 35–44. [Google Scholar] [CrossRef]
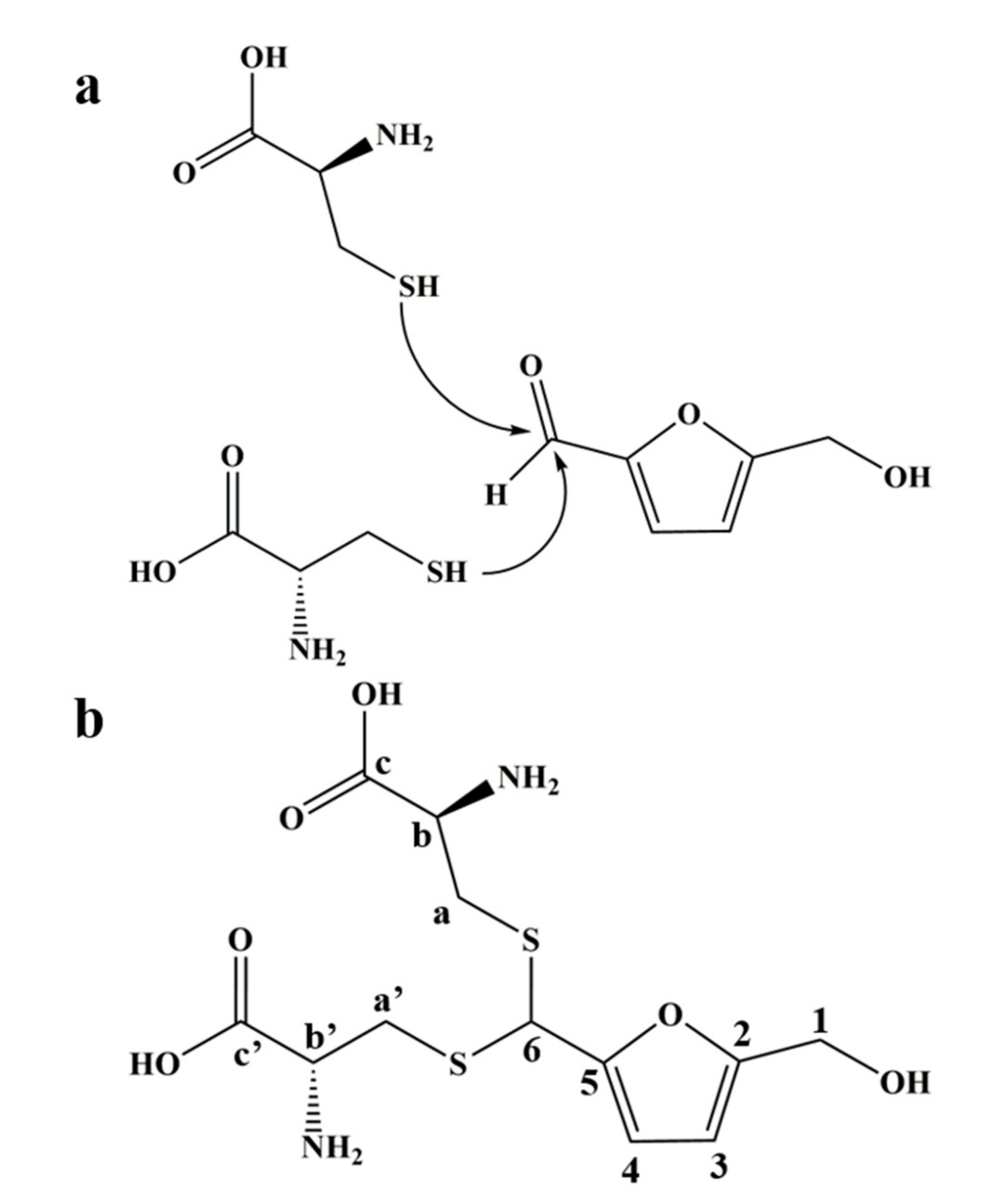

| Carbon Label * | δH (ppm) | δC (ppm) |
|---|---|---|
| 1 | 4.55 (s, 2H) | 55.67 (s) |
| 2 | 154.45 (s) | |
| 3 | 6.51 (d, J = 3.2 Hz, 1H) | 109.30 (s) |
| 4 | 6.39 (d, J = 3.2 Hz, 1H) | 110.32 (s) |
| 5 | 149.62 (s) | |
| 6 | 5.37 (s, 1H) | 45.09 (s) |
| a | 3.17 (dd, J =14.7, 7.7 Hz, 1H) 3.06 (dd, J = 14.9, 7.5 Hz, 1H) | 32.51 (s) |
| b | 3.82 (ddd, J = 11.6, 7.6, 4.4 Hz, 1H) | 53.79 (s) |
| c | 172.39 (s) | |
| a’ | 3.28–3.19 (m, 2H) | 32.04 (s) |
| b’ | 3.82 (ddd, J = 11.6, 7.6, 4.4 Hz, 1H) | 53.57 (s) |
| c’ | 4.55 (s, 2H) | 172.43 (s) |
| Experiment Name (Free Radical Type) | RDSC (DPPH•) * | ASCA (ABTS•+) * | ORAC (ROO•) * |
|---|---|---|---|
| HMF | 215.14 ± 24.17 | 293.92 ± 81.91 | −439.04 ± 119.17 a |
| DCH | 2110.04 ± 100.80 | 2924.47 ± 70.05 | 3260.25 ± 97.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhang, Z.; Li, J.; Niu, Y.; Yu, L.L. Inhibition Mechanism of L-Cysteine on Maillard Reaction by Trapping 5-Hydroxymethylfurfural. Foods 2021, 10, 1391. https://doi.org/10.3390/foods10061391
Yang S, Zhang Z, Li J, Niu Y, Yu LL. Inhibition Mechanism of L-Cysteine on Maillard Reaction by Trapping 5-Hydroxymethylfurfural. Foods. 2021; 10(6):1391. https://doi.org/10.3390/foods10061391
Chicago/Turabian StyleYang, Shiqiang, Zhongfei Zhang, Jiaoyong Li, Yuge Niu, and Liangli Lucy Yu. 2021. "Inhibition Mechanism of L-Cysteine on Maillard Reaction by Trapping 5-Hydroxymethylfurfural" Foods 10, no. 6: 1391. https://doi.org/10.3390/foods10061391
APA StyleYang, S., Zhang, Z., Li, J., Niu, Y., & Yu, L. L. (2021). Inhibition Mechanism of L-Cysteine on Maillard Reaction by Trapping 5-Hydroxymethylfurfural. Foods, 10(6), 1391. https://doi.org/10.3390/foods10061391







