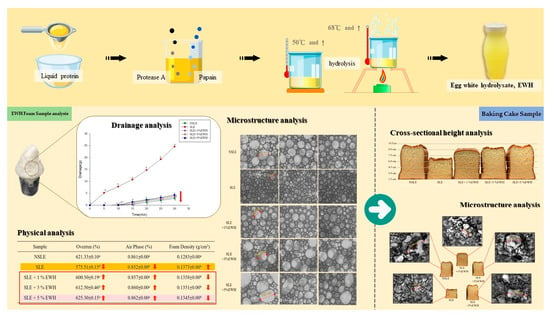
Improvement of Foaming Characteristics and Stability of Sterilized Liquid Egg with Egg White Hydrolysate (EWH)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Egg White Hydrolysate Preparation
2.2.1. Protein Content Analysis
2.2.2. Analysis of Degree of Hydrolysis (DH)
2.3. Sample Preparation
2.3.1. Zeta Potential Analysis
2.3.2. Surface Tension Analysis
2.3.3. Viscosity Analysis
2.3.4. Color Analysis
2.4. Foam Sample Preparation
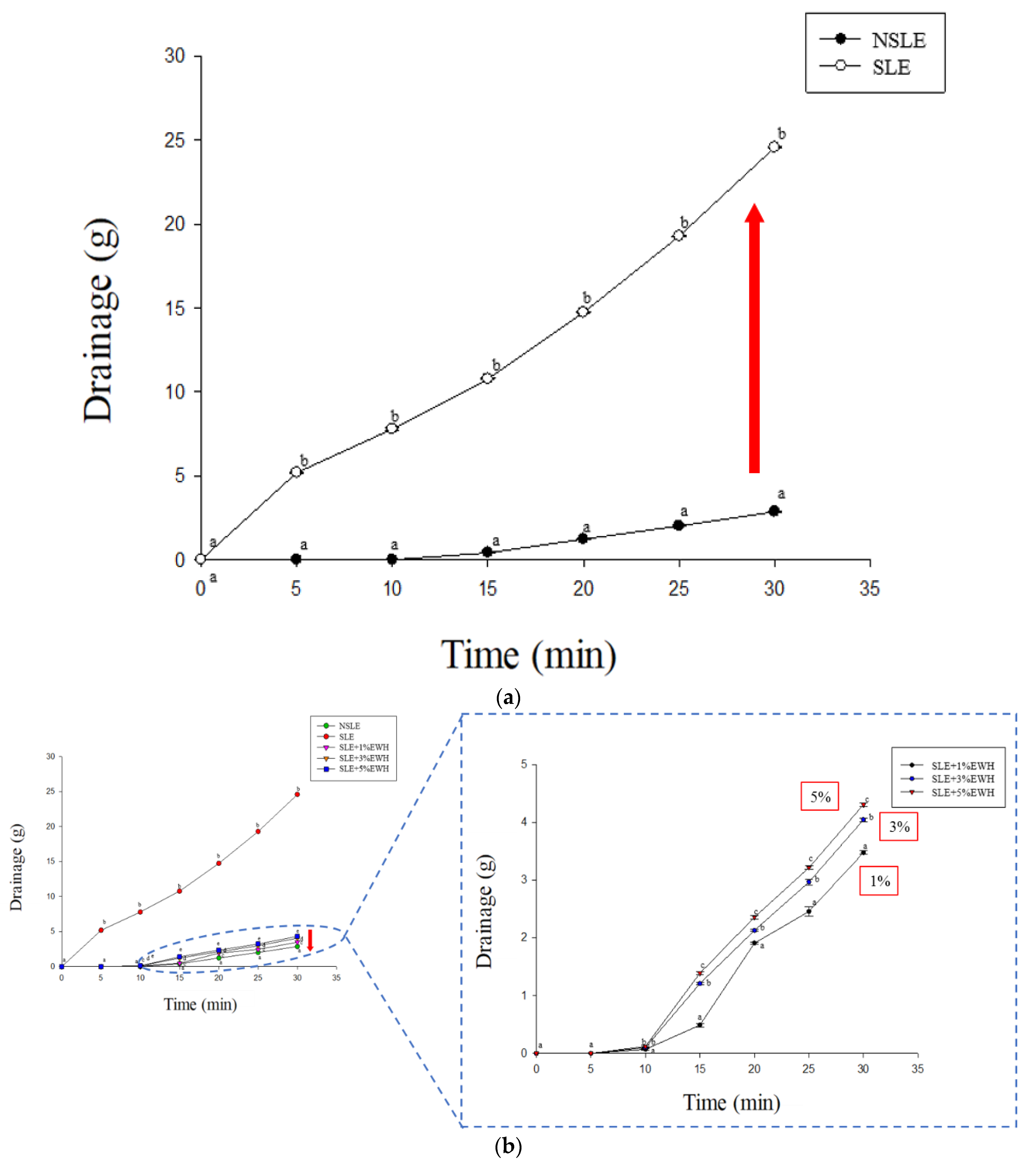
2.4.1. Drainage Analysis
2.4.2. Physical Analysis
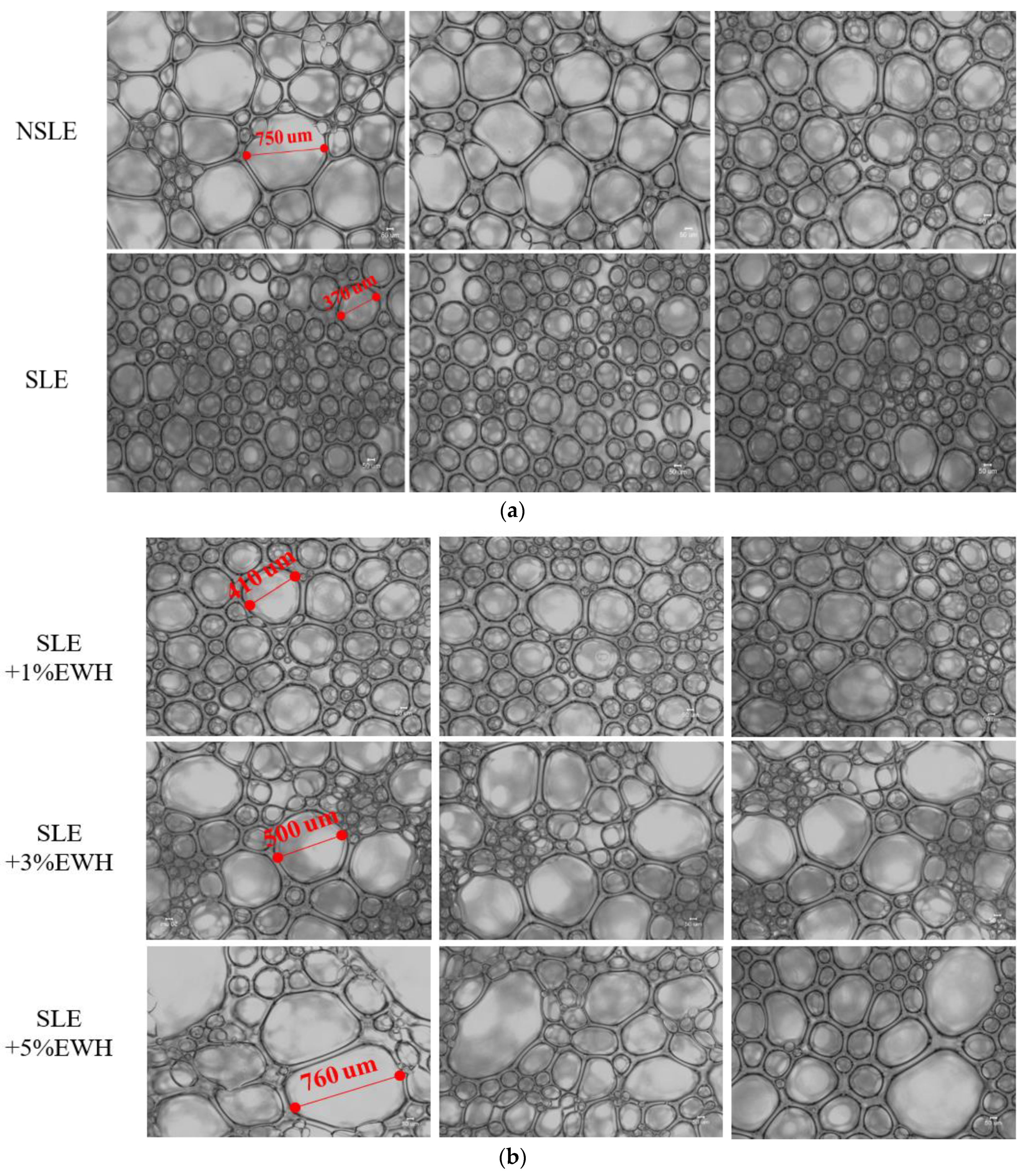
2.4.3. Microstructure Analysis
2.5. Baking Cake Sample
2.5.1. Color Analysis
2.5.2. Microstructure Analysis
2.5.3. Cross-Sectional Height Analysis
2.5.4. Organoleptic Analysis
2.6. Statistical Analyses
3. Results
3.1. Analysis of Egg White Hydrolysate
3.2. Analysis of Stability
3.3. Analysis of Foaming Characteristics
3.4. Organoleptic Analysis
3.5. Color Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pande, V.V.; Gole, V.C.; McWhorter, A.R.; Abraham, S.; Chousalkar, K.K. Antimicrobial resistance of non-typhoidal Salmonella isolates from egg layer flocks and egg shells. Int. J. Food Microbiol. 2015, 203, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Lechevalier, V.; Guérin-Dubiard, C.; Anton, M.; Beaumal, V.; Briand, E.D.; Gillard, A.; LeGouar, Y.; Musikaphun, N.; Tanguy, G.; Pasco, M.; et al. Pasteurisation of liquid whole egg: Optimal heat treatments in relation to its functional, nutritional and allergenic properties. J. Food Eng. 2017, 195, 137–149. [Google Scholar] [CrossRef]
- Hegg, P.-O.; Löfqvist, B. The protective effect of small amounts of anionic detergents on the thermal aggregation of crude ovalbumin. J. Food Sci. 1974, 39, 1231–1236. [Google Scholar] [CrossRef]
- Lomakina, K.; Míková, K. A study of the factors affecting the foaming properties of egg white—A review. Czech J. Food Sci. 2006, 24, 110–118. [Google Scholar] [CrossRef]
- Janssen, H.J.L. Influence of pasteurisation, freezing and storage on properties of egg products made from eggs stored for 7 and for 28 days. J. Sci. Food Agric. 1971, 22, 491–495. [Google Scholar] [CrossRef]
- Monfort, S.; Mañas, P.; Condón, S.; Raso, J.; Álvarez, I. Physicochemical and functional properties of liquid whole egg treated by the application of Pulsed Electric Fields followed by heat in the presence of triethyl citrate. Food Res. Int. 2012, 48, 484–490. [Google Scholar] [CrossRef]
- Robertson, W.R.; Muriana, P.M. Reduction of Salmonella by two commercial egg white pasteurization methods. J. Food Prot. 2004, 67, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Romanoff, A.L.; Romanoff, A. The Avian Egg. Bird-Banding 1949, 20, 121. [Google Scholar] [CrossRef]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Blecker, C.; Delcour, J.A.; Murray, B.S. Foaming and air-water interfacial characteristics of solutions containing both gluten hydrolysate and egg white protein. Food Hydrocoll. 2018, 77, 176–186. [Google Scholar] [CrossRef]
- Dabestani, M.; Yeganehzad, S. Effect of Persian gum and Xanthan gum on foaming properties and stability of pasteurized fresh egg white foam. Food Hydrocoll. 2019, 87, 550–560. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Murray, B.S.; Sarkar, A. Combination of egg white protein and microgels to stabilize foams: Impact of processing treatments. J. Food Eng. 2020, 275, 109860. [Google Scholar] [CrossRef]
- Lahl, W.J.; Braun, S.D. Enzymatic production of protein hydrolysates for food use. Food Technol. (Chicago) 1994, 48, 68–71. [Google Scholar]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Chang, C.; Wang, C.; Zhang, M.; Su, Y.; Yang, Y. Foaming characterization of fresh egg white proteins as a function of different proportions of egg yolk fractions. Food Hydrocoll. 2019, 90, 118–125. [Google Scholar] [CrossRef]
- Horimoto, Y.; Lim, L.T. Effects of different proteases on iron absorption property of egg white hydrolysates. Food Res. Int. 2017, 95, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Li, X.; Su, Y.; Yang, Y.; Yu, X. Effects of pH and NaCl on the physicochemical and interfacial properties of egg white/yolk. Food Biosci. 2018, 23, 115–120. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Esparza, Y.; Temelli, F.; Wu, J. Physicochemical and functional properties of livetins fraction from hen egg yolk. Food Biosci. 2017, 18, 38–45. [Google Scholar] [CrossRef]
- Xue, Z.; Worthen, A.; Qajar, A.; Robert, I.; Bryant, S.L.; Huh, C.; Prodanović, M.; Johnston, K.P. Viscosity and stability of ultra-high internal phase CO2-in-water foams stabilized with surfactants and nanoparticles with or without polyelectrolytes. J. Colloid Interface Sci. 2016, 461, 383–395. [Google Scholar] [CrossRef]
- Akhtar, T.F.; Ahmed, R.; Elgaddafi, R.; Shah, S.; Amani, M. Rheological behavior of aqueous foams at high pressure. J. Pet. Sci. Eng. 2018, 162, 214–224. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Warkentin, T.D.; Tyler, R.T.; Nickerson, M.T. Physicochemical and functional properties of protein isolates obtained from several pea cultivars. Cereal Chem. 2017, 94, 89–97. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y. Study on the interaction between surfactin and alkaline protease in aqueous solution. Int. J. Biol. Macromol. 2018, 118, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, X.; Cao, M.; Wang, Y. Micellization of surfactin and its effect on the aggregate conformation of amyloid β(1–40). J. Phys. Chem. B 2008, 112, 15195–15201. [Google Scholar] [CrossRef] [PubMed]
- Mokni Ghribi, A.; Maklouf Gafsi, I.; Sila, A.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Effects of enzymatic hydrolysis on conformational and functional properties of chickpea protein isolate. Food Chem. 2015, 187, 322–330. [Google Scholar] [CrossRef]
- Ai, M.; Tang, T.; Zhou, L.; Ling, Z.; Guo, S.; Jiang, A. Effects of different proteases on the emulsifying capacity, rheological and structure characteristics of preserved egg white hydrolysates. Food Hydrocoll. 2019, 87, 933–942. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Chang, C.; Wang, C.; Yang, Y.; Su, Y. Effect of enzymatic hydrolysis on heat stability and emulsifying properties of egg yolk. Food Hydrocoll. 2019, 97, 105224. [Google Scholar] [CrossRef]
- Giacintucci, V.; DiMattia, C.; Sacchetti, G.; Neri, L.; Pittia, P. Role of olive oil phenolics in physical properties and stability of mayonnaise-like emulsions. Food Chem. 2016, 213, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Raikos, V.; Campbell, L.; Euston, S.R. Effects of sucrose and sodium chloride on foaming properties of egg white proteins. Food Res. Int. 2007, 40, 347–355. [Google Scholar] [CrossRef]
- Zmudziński, D.; Ptaszek, P.; Kruk, J.; Kaczmarczyk, K.; Roznowski, W.; Berski, W.; Ptaszek, A.; Grzesik, M. The role of hydrocolloids in mechanical properties of fresh foams based on egg white proteins. J. Food Eng. 2014, 121, 128–134. [Google Scholar] [CrossRef]
- Daugelaite, D.; Guillermic, R.M.; Scanlon, M.G.; Page, J.H. Quantifying liquid drainage in egg-white sucrose foams by resistivity measurements. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 241–248. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.-C.; Wang, H.; Shangguan, X.; Zhang, L.; Niu, P.; Sha, X.-M. Promotion of foam properties of egg white protein by subcritical water pre-treatment and fish scales gelatin. Colloids Surf. A Physicochem. Eng. Asp. 2017, 512, 171–177. [Google Scholar] [CrossRef]
- Noorlaila, A.; Hasanah, H.N.; Asmeda, R.; Yusoff, A. The effects of xanthan gum and hydroxypropylmethylcellulose on physical properties of sponge cakes. J. Saudi Soc. Agric. Sci. 2020, 19, 128–135. [Google Scholar] [CrossRef]
- Hill, C.; Eastoe, J. Foams: From nature to industry. Adv. Colloid Interface Sci. 2017, 247, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
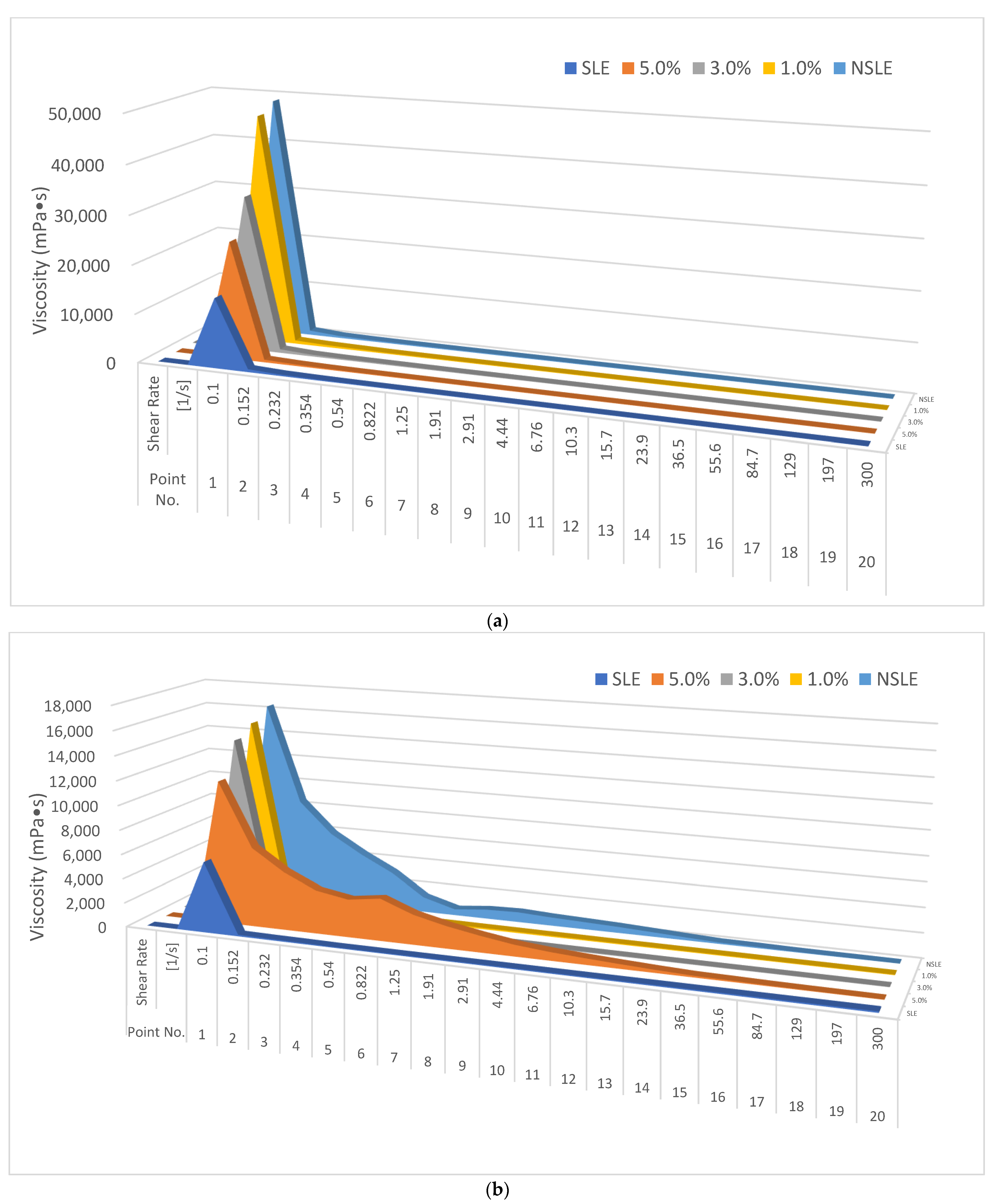


| Sample * | Zeta Potential (mV) | Surface Tension (erg/cm2) | Viscosity (mPa·s) |
|---|---|---|---|
| NSLE | −14.91 ± 0.16 a | 66.24 ± 0.48 a | 7356.67 ± 172.14 a |
| SLE | −0.93 ± 0.11 e | 56.64 ± 0.48 e | 4056.67 ± 100.17 e |
| SLE + 1% EWH | −12.65 ± 0.24 b | 63.52 ± 0.28 b | 6093.33 ± 41.63 b |
| SLE + 3% EWH | −8.55 ± 0.12 c | 62.40 ± 0.48 c | 5580.00 ± 95.39 c |
| SLE + 5% EWH | −4.16 ± 0.04 d | 61.12 ± 0.48 d | 5320.00 ± 65.57 d |
| Sample * | Overrun (%) | Air Phase (%) | Foam Density (g/cm3) |
|---|---|---|---|
| NSLE | 621.33 ± 0.10 a | 0.861 ± 0.00 a | 0.1283 ± 0.00 a |
| SLE | 575.51 ± 0.15 d | 0.852 ± 0.00 b | 0.1373 ± 0.00 b |
| SLE + 1% EWH | 600.50 ± 0.19 c | 0.857 ± 0.00 a | 0.1358 ± 0.00 b |
| SLE + 3% EWH | 612.50 ± 0.46 b | 0.860 ± 0.00 a | 0.1351 ± 0.00 b |
| SLE + 5% EWH | 625.30 ± 0.15 a | 0.862 ± 0.00 a | 0.1345 ± 0.00 b |
| Sample * | Appearance Acceptability | Aroma Acceptability | Sweetness Acceptability | Bitterness Acceptability | Texture Acceptability | Overall Acceptability |
|---|---|---|---|---|---|---|
| NSLE | 6.38 ± 2.24 a | 6.40 ± 1.92 a | 6.50 ± 1.73 a | 8.20 ± 1.40 a | 6.63 ± 1.59 a | 6.75 ± 1.77 a |
| SLE | 5.83 ± 1.43 d | 5.88 ± 1.75 c | 5.88 ± 1.87 c | 7.85 ± 1.63 c | 5.58 ± 1.61 c | 6.08 ± 1.56 e |
| SLE + 1% EWH | 6.37 ± 1.82 a | 6.40 ± 2.02 a | 6.50 ± 1.50 a | 8.10 ± 1.74 b | 6.35 ± 1.66 b | 6.70 ± 1.45 b |
| SLE + 3% EWH | 6.28 ± 1.90 b | 6.38 ± 1.50 a | 6.70 ± 1.45 b | 8.08 ± 1.47 b | 6.35 ± 1.60 b | 6.20 ± 1.70 d |
| SLE + 5% EWH | 6.20 ± 2.24 c | 6.31 ± 1.58 b | 6.69 ± 1.54 b | 8.06 ± 1.98 b | 6.60 ± 1.78 a | 6.40 ± 1.82 c |
| Sample * | L | a | b | ΔE |
|---|---|---|---|---|
| NSLE | 87.46 ± 0.41 a | −1.10 ± 0.19 a | 16.61 ± 0.15 a | 0.44 ± 0.39 a |
| SLE | 86.31 ± 0.34 b | −1.07 ± 0.08 a | 17.24 ± 0.39 a | 1.00 ± 0.53 a |
| SLE + 1% EWH | 80.99 ± 0.34 c | 0.68 ± 0.48 b | 22.40 ± 1.38 b | 9.33 ± 1.37 b |
| SLE + 3% EWH | 79.51 ± 0.26 d | 0.89 ± 0.31 b,c | 23.08 ± 0.60 b | 9.92 ± 1.85 b |
| SLE + 5% EWH | 77.21 ± 0.17 e | 1.31 ± 0.06 c | 23.69 ± 0.05 b | 12.10 ± 0.15 c |
| Cake Sample * | L | a | b | ΔE |
|---|---|---|---|---|
| NSLE | 70.45 ± 0.46 a | 3.01 ± 0.17 a | 24.56 ± 0.21 a | 4.46 ± 0.26 a |
| SLE | 68.66 ± 0.26 b | 3.94 ± 0.75 a | 25.58 ± 0.90 a | 2.58 ± 0.95 b |
| SLE + 1% EWH | 67.73 ± 0.21 c | 4.83 ± 0.17 b | 26.99 ± 0.54 b | 0.63 ± 0.42 d |
| SLE + 3% EWH | 66.39 ± 0.33 d | 4.64 ± 0.56 b | 26.45 ± 0.94 b | 1.48 ± 0.60 c |
| SLE + 5% EWH | 65.12 ± 0.10 e | 4.65 ± 1.19 b | 26.78 ± 1.19 b | 2.65 ± 0.28 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, H.-Y.; Ciou, J.-Y.; Qiu, Y.-T.; Hsieh, S.-L.; Shih, M.-K.; Chen, M.-H.; Tu, C.-W.; Hsieh, C.-W.; Hou, C.-Y. Improvement of Foaming Characteristics and Stability of Sterilized Liquid Egg with Egg White Hydrolysate (EWH). Foods 2021, 10, 1326. https://doi.org/10.3390/foods10061326
Ho H-Y, Ciou J-Y, Qiu Y-T, Hsieh S-L, Shih M-K, Chen M-H, Tu C-W, Hsieh C-W, Hou C-Y. Improvement of Foaming Characteristics and Stability of Sterilized Liquid Egg with Egg White Hydrolysate (EWH). Foods. 2021; 10(6):1326. https://doi.org/10.3390/foods10061326
Chicago/Turabian StyleHo, Hen-Yo, Jhih-Ying Ciou, Yi-Ting Qiu, Shu-Ling Hsieh, Ming-Kuei Shih, Min-Hung Chen, Chao-Wen Tu, Chang-Wei Hsieh, and Chih-Yao Hou. 2021. "Improvement of Foaming Characteristics and Stability of Sterilized Liquid Egg with Egg White Hydrolysate (EWH)" Foods 10, no. 6: 1326. https://doi.org/10.3390/foods10061326
APA StyleHo, H.-Y., Ciou, J.-Y., Qiu, Y.-T., Hsieh, S.-L., Shih, M.-K., Chen, M.-H., Tu, C.-W., Hsieh, C.-W., & Hou, C.-Y. (2021). Improvement of Foaming Characteristics and Stability of Sterilized Liquid Egg with Egg White Hydrolysate (EWH). Foods, 10(6), 1326. https://doi.org/10.3390/foods10061326