Transformation of Inferior Tomato into Preservative: Fermentation by Multi-Bacteriocin Producing Lactobacillus paracasei WX322
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibacterial Activity of L. paracasei WX322 Fermentation Products from 12 Kinds of Vegetables
2.1.1. Screening of Vegetable Fermentation with Good Antibacterial Activity
2.1.2. Verification of Antibacterial Activity from L. paracasei WX322 Metabolites
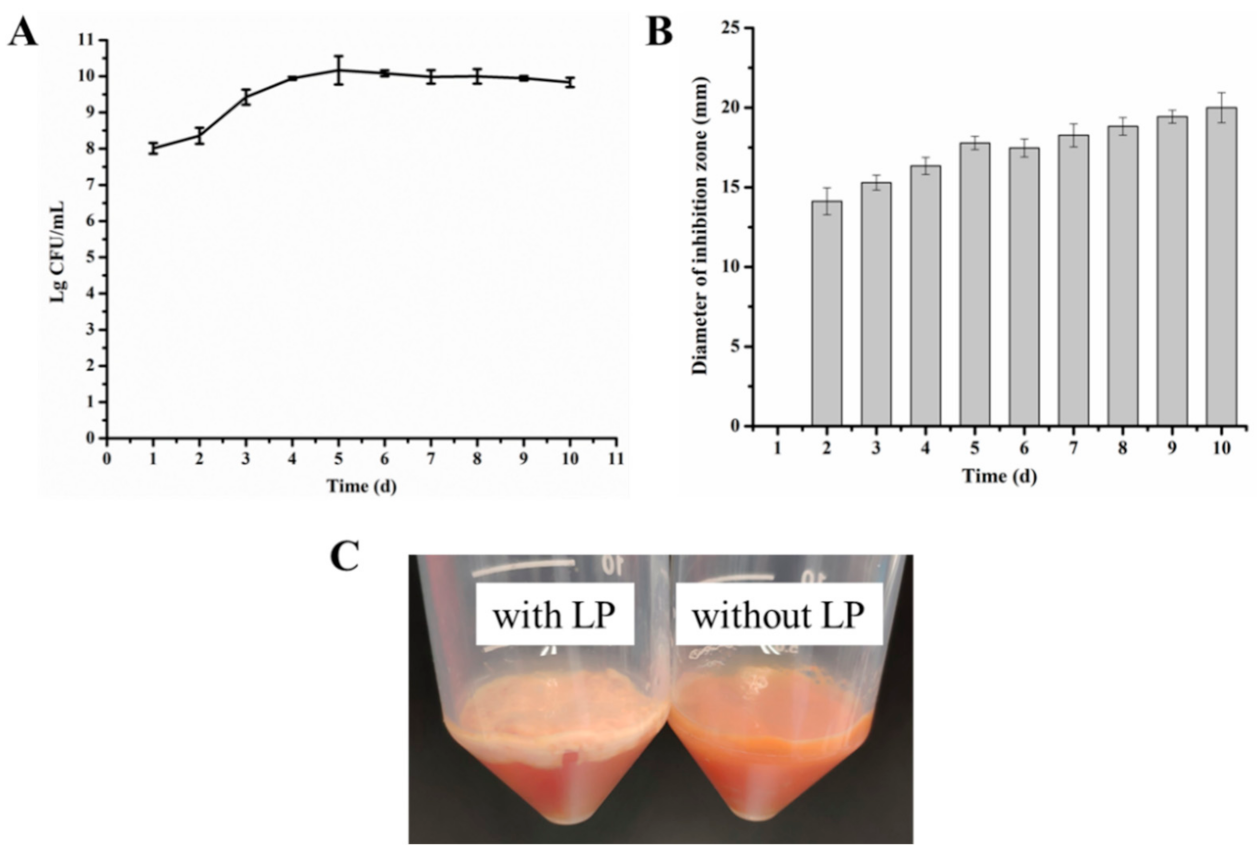
2.2. Microbial Growth of L. paracasei WX322 in Tomato Juice and Antibacterial Activity Analysis
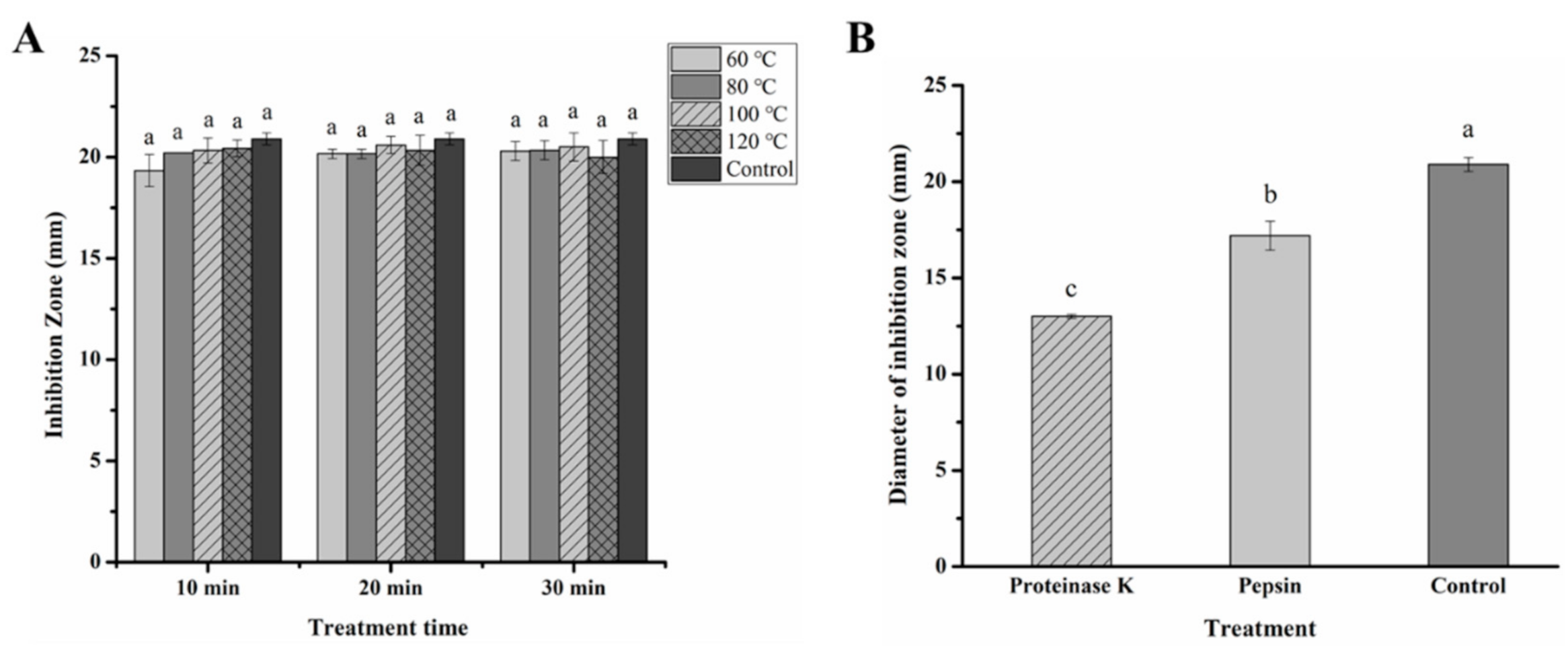
2.3. Sensitivity of Fermentation Product of L. paracasei WX322 in Tomato Juice to Heat and Proteinases
2.4. Preparation of Bacteriocin Sample and Its Antibacterial Activity Determination
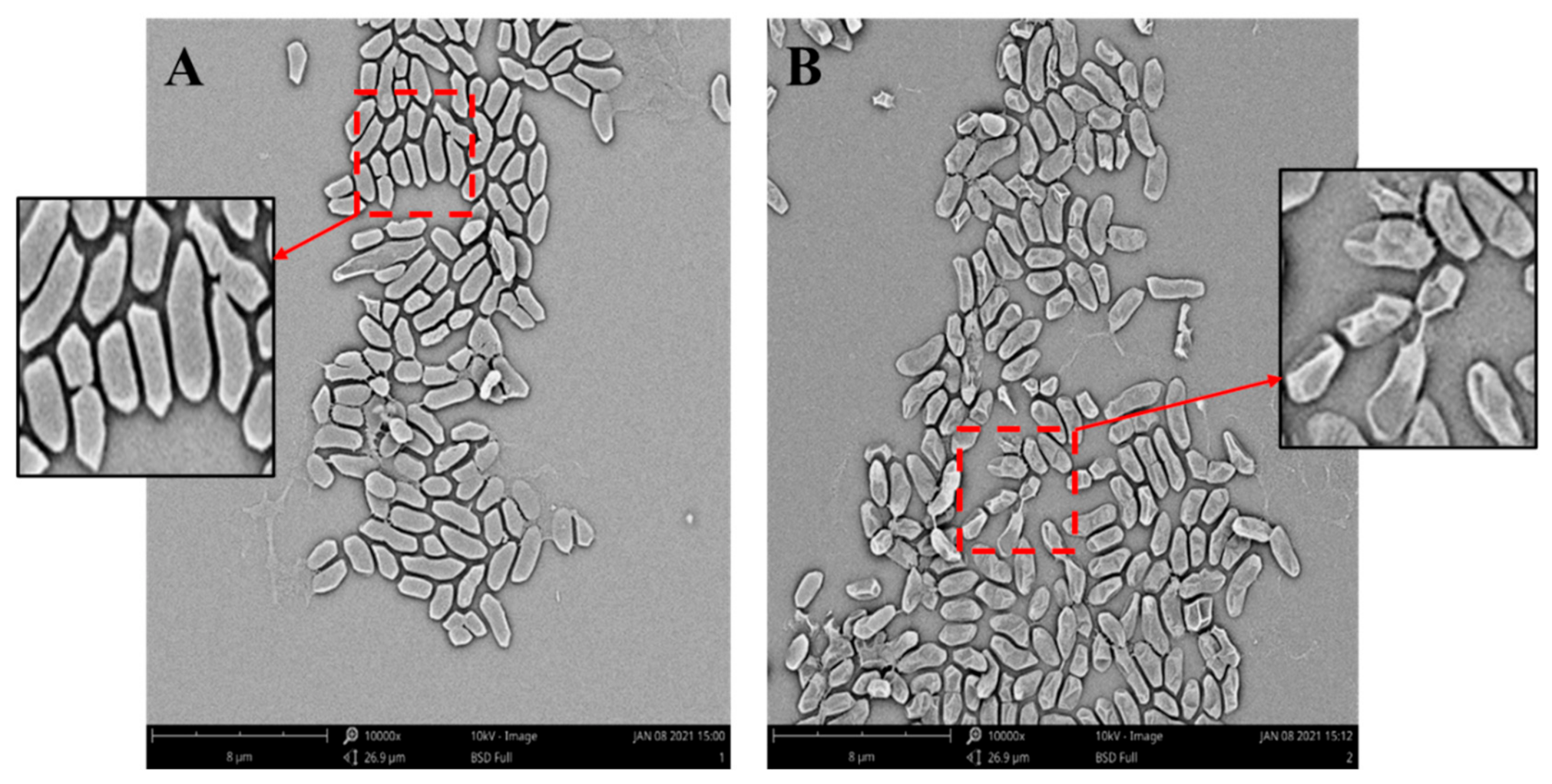
2.5. Scanning Electron Microscope (SEM) Observation
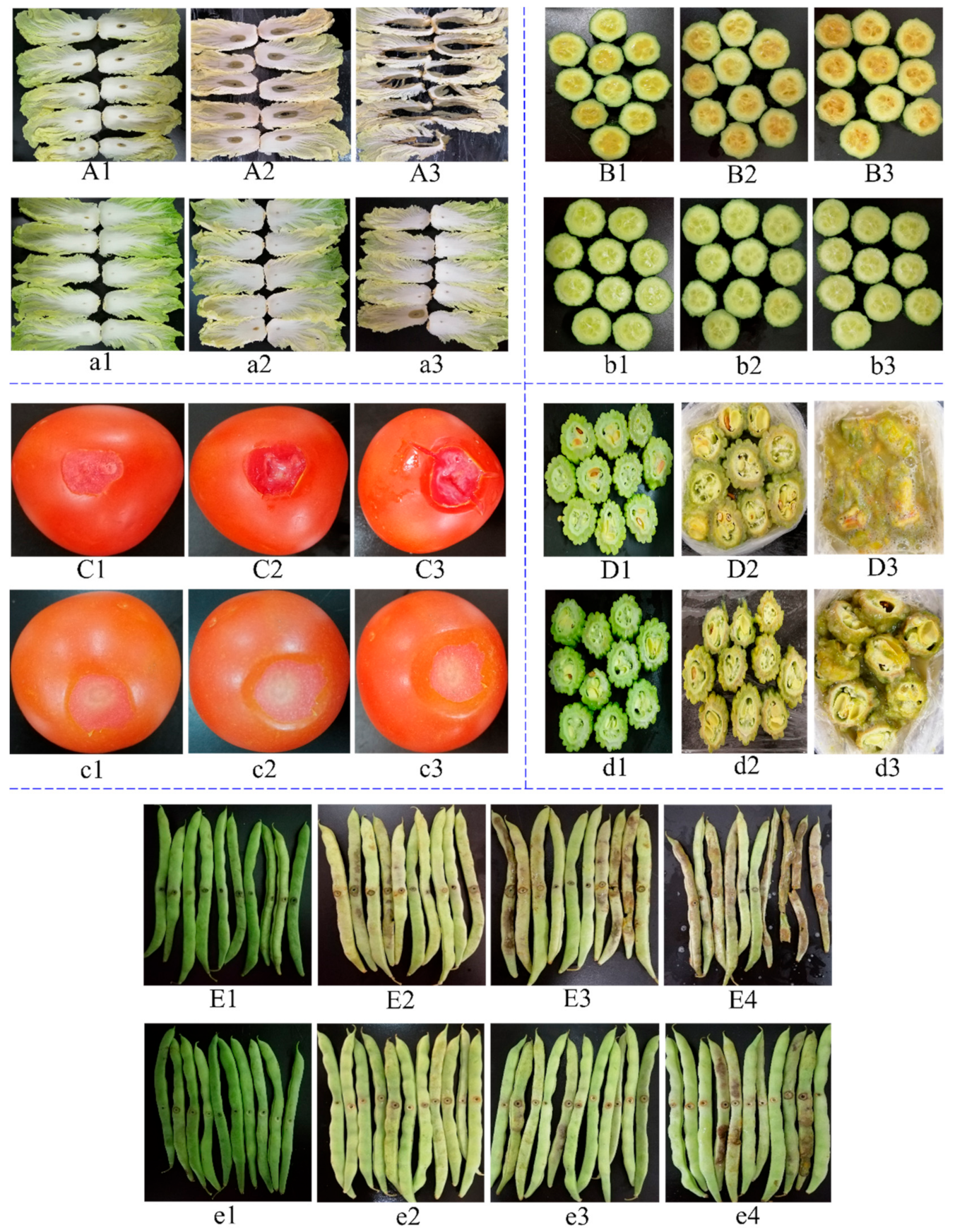
2.6. Controlling Bacterial Soft Rot of Five Vegetables
2.7. Statistical Analysis
3. Results and Discussion
3.1. Screening Vegetable Alternative to Man-Rogosa-Sharpe Broth among 12 Kinds of Vegetables
3.2. Microbial Growth of L. paracasei WX322 and Antibacterial Activity of Fermented Product in Tomato Juice
3.3. Sensitivity of Fermentation Product of L. paracasei WX322 from Tomato Juice to Heat and Proteinase
3.4. Effect of Bacteriocin of L. paracasei WX322 from Tomato Juice on Cell Morphology of Pcb BZA12
3.5. Controlling Bacterial Soft Rot of Five Kinds of Vegetables by Bacteriocin from Tomato Juice Fermentation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gebbers, J.-O. Atherosclerosis, cholesterol, nutrition, and statins—A critical review. GMS Ger. Med. Sci. 2007, 5, Doc04. [Google Scholar] [PubMed]
- Jin, W.; Chen, X.; Huo, Q.; Cui, Y.; Yu, Z.; Yu, L. Aerial parts of maca (Lepidium meyenii Walp.) as functional vegetables with gastrointestinal prokinetic efficacy in vivo. Food Funct. 2018, 9, 3456–3465. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: Meta-analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.; Miller, F.; Brandão, T.; Teixeira, P.; Silva, C. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Kalmpourtzidou, A.; Eilander, A.; Talsma, E.F. Global vegetable intake and supply compared to recommendations: A systematic review. Nutrients 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Porat, R.; Lichter, A.; Terry, L.; Harker, R.; Buzby, J. Postharvest losses of fruit and vegetables during retail and in consumers’ homes: Quantifications, causes, and means of prevention. Postharvest Biol. Technol. 2018, 139, 135–149. [Google Scholar] [CrossRef]
- Salim, N.S.M.; Singh, A.; Raghavan, V. Potential utilization of fruit and vegetable wastes for food through drying or extraction techniques. Nov. Tech. Nutr. Food Sci. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Ferreira, M.S.L.; Santos, M.C.P.; Moro, T.M.A.; Basto, G.J.; Andrade, R.M.S.; Goncalves, E. Formulation and characterization of functional foods based on fruit and vegetable residue flour. J. Food Sci. Technol. 2015, 52, 822–830. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Qi, T.; Wang, S.; Deng, L.; Yi, L.; Zeng, K. Controlling pepper soft rot by Lactobacillus paracasei WX322 and identification of multiple bacteriocins by complete genome sequencing. Food Control 2021, 121, 107629. [Google Scholar] [CrossRef]
- Viswanath, H.; Rather, R.A.; Bhat, K.; Peerzada, S. Management of post-harvest bacterial soft rot of potato caused by Pectobacterium carotovorum using bacterial bio-agents. Pharma Innov. J. 2020, 9, 31–35. [Google Scholar]
- Hadizadeh, I.; Peivastegan, B.; Hannukkala, A.; Van Der Wolf, J.M.; Nissinen, R.; Pirhonen, M. Biological control of potato soft rot caused by Dickeya solani and the survival of bacterial antagonists under cold storage conditions. Plant Pathol. 2018, 68, 297–311. [Google Scholar] [CrossRef]
- Godfrey, S.A.C.; Marshall, J.W. Identification of cold-tolerant Pseudomonas viridiflava and P. marginalis causing severe carrot postharvest bacterial soft rot during refrigerated export from New Zealand. Plant Pathol. 2002, 51, 155–162. [Google Scholar] [CrossRef]
- Lo, H.-H.; Liao, C.-T.; Li, C.-E.; Chiang, Y.-C.; Hsiao, Y.-M. The clpX gene plays an important role in bacterial attachment, stress tolerance, and virulence in Xanthomonas campestris pv. campestris. Arch. Microbiol. 2019, 202, 597–607. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanun, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Wiryawan, F.S.; Marimin; Djatna, T. Value chain and sustainability analysis of fresh-cut vegetable: A case study at SSS Co. J. Clean. Prod. 2020, 260, 121039. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Azpilicueta, C.A.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Yi, L.; Dang, Y.; Wu, J.; Zhang, L.; Liu, X.; Liu, B.; Zhou, Y.; Lu, X. Purification and characterization of a novel bacteriocin produced by Lactobacillus crustorum MN047 isolated from koumiss from Xinjiang, China. J. Dairy Sci. 2016, 99, 7002–7015. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; O’Connor, P.M.; Colquhoun, I.J.; Vior, N.M.; Rodríguez, J.M.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Production of multiple bacteriocins, including the novel bacteriocin gassericin M, by Lactobacillus gasseri LM19, a strain isolated from human milk. Appl. Microbiol. Biotechnol. 2020, 104, 3869–3884. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Miao, L.; Ma, H.; Bai, F.; Lin, Y.; Sun, M.; Li, J. Purification, characterization and action mechanism of plantaricin JY22, a novel bacteriocin against Bacillus cereus produced by Lactobacillus plantarum JY22 from golden carp intestine. Food Sci. Biotechnol. 2018, 27, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Qi, T.; Hong, Y.; Deng, L.; Zeng, K. Screening of bacteriocin-producing lactic acid bacteria in Chinese homemade pickle and dry-cured meat, and bacteriocin identification by genome sequencing. LWT Food Sci. Technol. 2020, 125, 109177. [Google Scholar] [CrossRef]
- Yi, L.; Qi, T.; Ma, J.; Zeng, K. Genome and metabolites analysis reveal insights into control of foodborne pathogens in fresh-cut fruits by Lactobacillus pentosus MS031 isolated from Chinese Sichuan Paocai. Postharvest Biol. Technol. 2020, 164, 111150. [Google Scholar] [CrossRef]
- Vallejo, C.V.; Minahk, C.J.; Rollán, G.C.; Rodríguez-Vaquero, M.J. Inactivation of Listeria monocytogenes and Salmonella Typhimurium in strawberry juice enriched with strawberry polyphenols. J. Sci. Food Agric. 2021, 101, 441–448. [Google Scholar] [CrossRef]
- Masoud, K. Antibacterial properties of natural compounds extracted from plants compared to chemical preservatives against Salmonella spp.—A Systematic Review. Int. J. Food Nutr. Saf. 2017, 8, 13–31. [Google Scholar]
- Ai, C.; Meng, H.; Lin, J.; Zhang, T.; Guo, X. Combined membrane filtration and alcohol-precipitation of alkaline soluble polysaccharides from sugar beet pulp: Comparision of compositional, macromolecular, and emulsifying properties. Food Hydrocoll. 2020, 109, 106049. [Google Scholar] [CrossRef]
- Williams, K.T.; Bevenue, A. Vegetable components, some carbohydrate components of tomato. J. Agric. Food Chem. 1954, 2, 472–474. [Google Scholar] [CrossRef]
- Sabo, S.; Converti, A.; Ichiwaki, S.; Oliveira, R.P. Bacteriocin production by Lactobacillus plantarum ST16Pa in supplemented whey powder formulations. J. Dairy Sci. 2019, 102, 87–99. [Google Scholar] [CrossRef]
- Li, T.; Liu, Q.; Wang, D.; Li, J. Characterization and antimicrobial mechanism of CF-14, a new antimicrobial peptide from the epidermal mucus of catfish. Fish Shellfish. Immunol. 2019, 92, 881–888. [Google Scholar] [CrossRef]
- Hajian-Maleki, H.; Baghaee-Ravari, S.; Moghaddam, M. Efficiency of essential oils against Pectobacterium carotovorum subsp. carotovorum causing potato soft rot and their possible application as coatings in storage. Postharvest Biol. Technol. 2019, 156, 110928. [Google Scholar] [CrossRef]
- Tsuda, K.; Tsuji, G.; Higashiyama, M.; Ogiyama, H.; Umemura, K.; Mitomi, M.; Kubo, Y.; Kosaka, Y. Biological control of bacterial soft rot in Chinese cabbage by Lactobacillus plantarum strain BY under field conditions. Biol. Control 2016, 100, 63–69. [Google Scholar] [CrossRef]
- Ma, J.; Hong, Y.; Deng, L.; Yi, L.; Zeng, K. Screening and characterization of lactic acid bacteria with antifungal activity against Penicillium digitatum on citrus. Biol. Control 2019, 138, 104044. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Moayedi, A.; Mora, L.; Aristoy, M.C.; Safari, M.; Hashemi, M.; Toldrá, F. Peptidomic analysis of antioxidant and ACE-inhibitory peptides obtained from tomato waste proteins fermented using Bacillus subtilis. Food Chem. 2018, 250, 180–187. [Google Scholar] [CrossRef]
- Kum, E.; Ince, E. Genome-guided investigation of secondary metabolites produced by a potential new strain Streptomyces BA2 isolated from an endemic plant rhizosphere in Turkey. Arch. Microbiol. 2021, 1–8. [Google Scholar] [CrossRef]
- Russell, J.B.; Mantovani, H.C. The bacteriocins of ruminal bacteria and their potential as an alternative to antibiotics. J. Mol. Microbiol. Biotechnol. 2002, 4, 347–355. [Google Scholar]
- Hols, P.; Ledesma, L.; Gabant, P.; Mignolet, J. Mobilization of microbiota commensals and their bacteriocins for therapeutics. Trends Microbiol. 2019, 27, 690–702. [Google Scholar] [CrossRef]
- Pokhrel, R.; Bhattarai, N.; Baral, P.; Gerstman, B.S.; Park, J.H.; Handfield, M.; Chapagain, P.P. Molecular mechanisms of pore formation and membrane disruption by the antimicrobial lantibiotic peptide Mutacin 1140. Phys. Chem. Chem. Phys. 2019, 21, 12530–12539. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Circular and leaderless bacteriocins: Biosynthesis, mode of action, applications, and prospects. Front. Microbiol. 2018, 9, 2085. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, A.S.; Valyshev, A.V. Pore-forming bacteriocins: Structural–functional relationships. Arch. Microbiol. 2018, 201, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; He, P.; Munir, S.; He, P.; He, Y.; Li, X.; Yang, L.; Wang, B.; Wu, Y.; He, P. Biocontrol of soft rot of Chinese cabbage using an endophytic bacterial strain. Front. Microbiol. 2019, 10, 1471. [Google Scholar] [CrossRef]
- Kyeremeh, A.G.; Kikumoto, T.; Chuang, D.-Y.; Gunji, Y.; Takahara, Y.; Ehara, Y. Biological control of soft rot of Chinese cabbage using single and mixed treatments of bacteriocin-producing avirulent mutants of Erwinia carotovora subsp. carotovora. J. Gen. Plant Pathol. 2000, 66, 264–268. [Google Scholar] [CrossRef]
- Onkendi, E.M.; Ramesh, A.M.; Kwenda, S.; Naidoo, S.; Moleleki, L.N. Draft genome sequence of a virulent Pectobacterium carotovorum subsp. brasiliense isolate causing soft rot of cucumber. Genome Announc. 2016, 4, e01530-15. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, C.; Wang, H.; Guan, T.; Liu, L.; Yu, S. Bioinformatic analysis of the complete genome sequence of Pectobacterium carotovorum subsp. brasiliense BZA12 and candidate effector screening. J. Plant Pathol. 2018, 101, 39–49. [Google Scholar] [CrossRef]
- Ahmed, F.A.; Arif, M.; Alvarez, A.M. Antibacterial effect of potassium tetraborate tetrahydrate against soft rot disease agent Pectobacterium carotovorum in tomato. Front. Microbiol. 2017, 8, 1728. [Google Scholar] [CrossRef]
- Kubo, H.; Kanehashi, K.; Shinohara, H.; Negishi, H.; Matsuyama, N.; Suyama, K. Bacterial soft rot, a new disease of balsam pear (Momordica charantia L.) caused by Erwinia carotovora subsp. carotovora. Jpn. J. Phytopathol. 2009, 75, 173–175. [Google Scholar] [CrossRef]
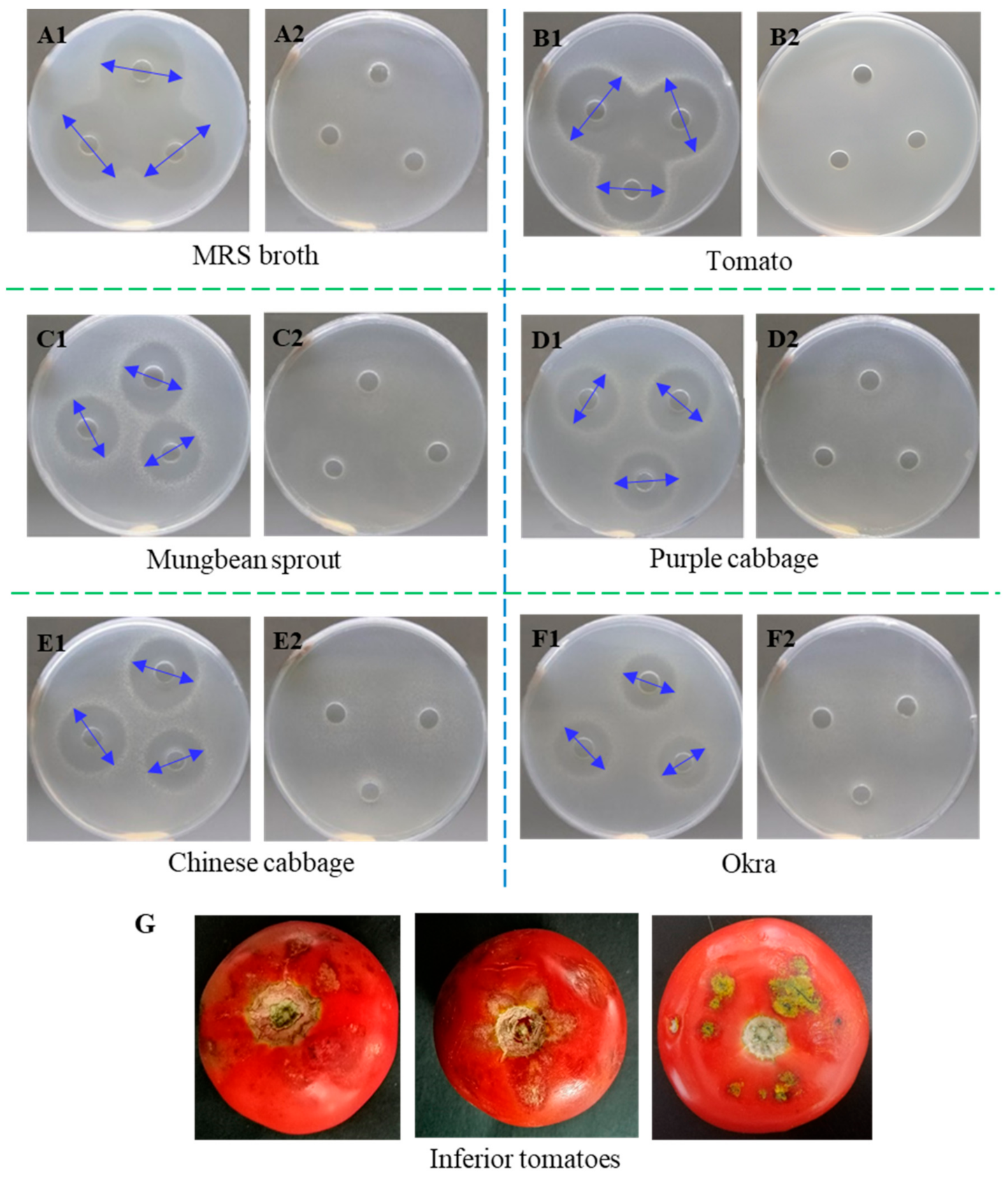
| No. | Vegetables | Diameter of Inhibition Zone (mm) * |
|---|---|---|
| 1 | Tomato | 47.3 ± 1.6 a |
| 2 | Cucumber | 42.7 ± 1.2 b |
| 3 | Man-Rogosa-Sharpe broth | 40.5 ± 0.4 c |
| 4 | Cabbage | 40.3 ± 0.4 cd |
| 5 | Soybean sprout | 39.9 ± 0.8 cde |
| 6 | Mungbean sprout | 39.4 ± 1.8 cde |
| 7 | Okra | 39.2 ± 0.6 cdef |
| 8 | Garlic chives | 38.7 ± 1.0 cdef |
| 9 | Green bean | 38.5 ± 1.3 def |
| 10 | Carrot | 38.4 ± 0.8 def |
| 11 | Balsam pear | 38.2 ± 0.9 ef |
| 12 | White ground | 38.2 ± 0.5 ef |
| 13 | Purple cabbage | 37.4 ± 1.2 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, R.; Liu, X.; Li, X.; Zeng, K.; Yi, L. Transformation of Inferior Tomato into Preservative: Fermentation by Multi-Bacteriocin Producing Lactobacillus paracasei WX322. Foods 2021, 10, 1278. https://doi.org/10.3390/foods10061278
Zhu R, Liu X, Li X, Zeng K, Yi L. Transformation of Inferior Tomato into Preservative: Fermentation by Multi-Bacteriocin Producing Lactobacillus paracasei WX322. Foods. 2021; 10(6):1278. https://doi.org/10.3390/foods10061278
Chicago/Turabian StyleZhu, Rong, Xiaoqing Liu, Xiaofen Li, Kaifang Zeng, and Lanhua Yi. 2021. "Transformation of Inferior Tomato into Preservative: Fermentation by Multi-Bacteriocin Producing Lactobacillus paracasei WX322" Foods 10, no. 6: 1278. https://doi.org/10.3390/foods10061278
APA StyleZhu, R., Liu, X., Li, X., Zeng, K., & Yi, L. (2021). Transformation of Inferior Tomato into Preservative: Fermentation by Multi-Bacteriocin Producing Lactobacillus paracasei WX322. Foods, 10(6), 1278. https://doi.org/10.3390/foods10061278






