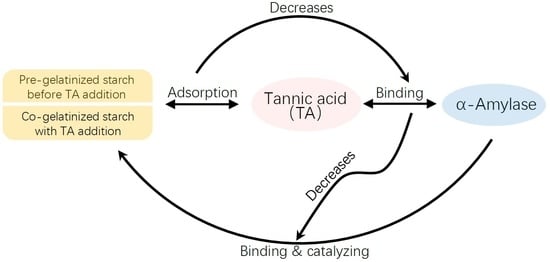
The Physical Adsorption of Gelatinized Starch with Tannic Acid Decreases the Inhibitory Activity of the Polyphenol against α-Amylase
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
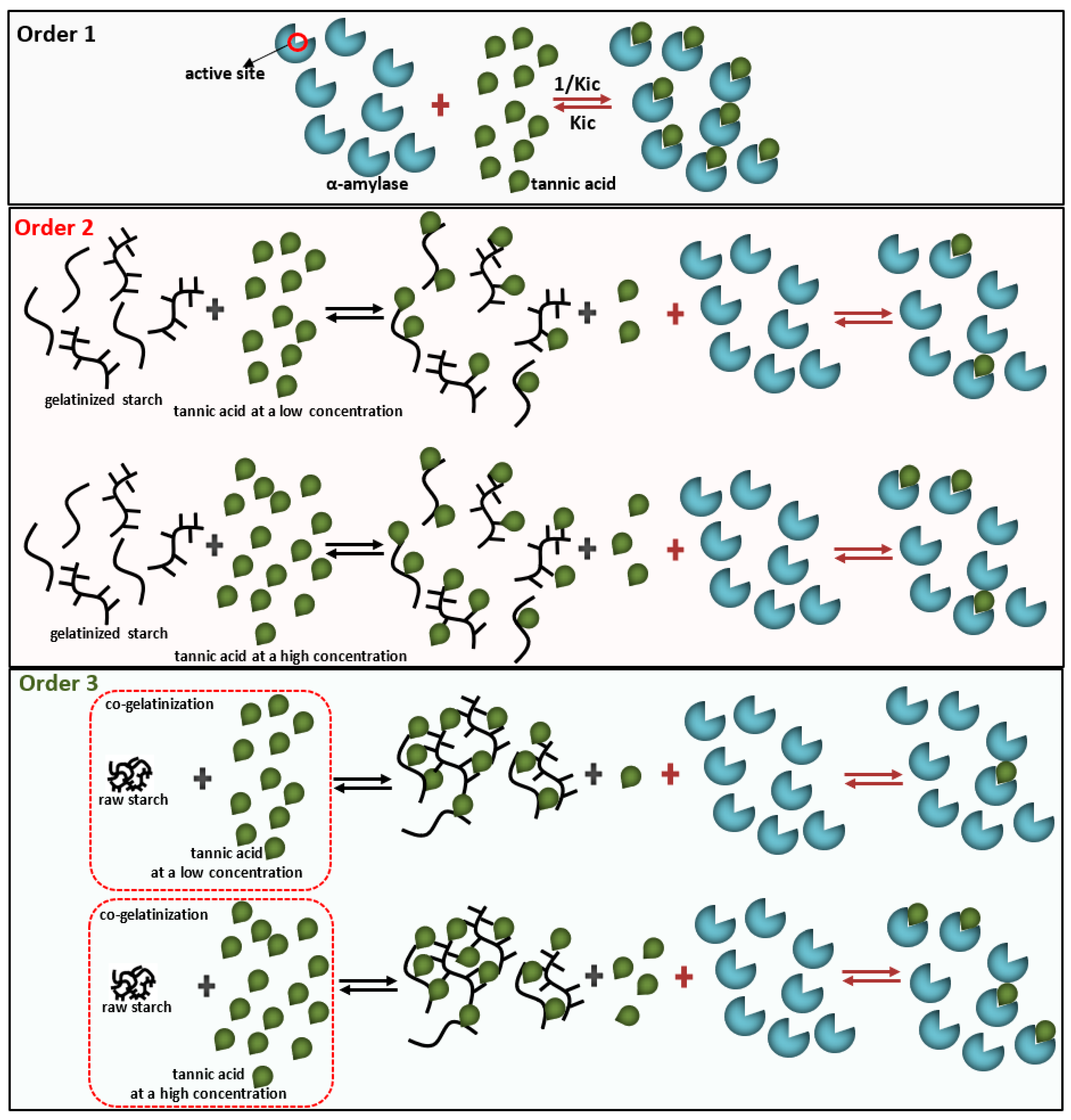
2.2. Three Mixing Orders of Tannic Acid, α-Amylase, and Starch
2.3. α-Amylase Inhibition of TA
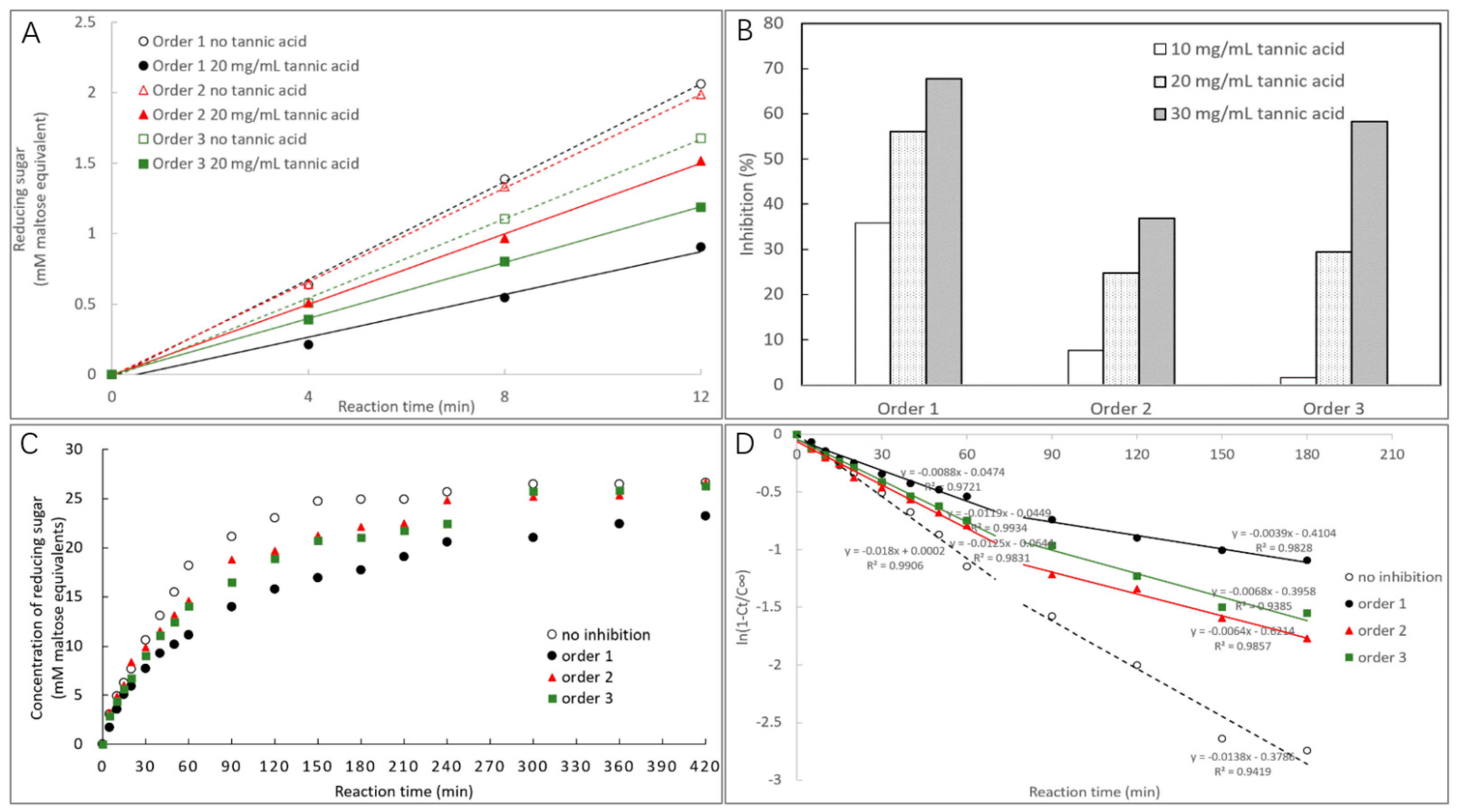
2.3.1. α-Amylase Inhibition Characterized by Starch Digestion
2.3.2. Time Course of Starch Digestion
2.4. Mechanism in α-Amylase Inhibition of TA
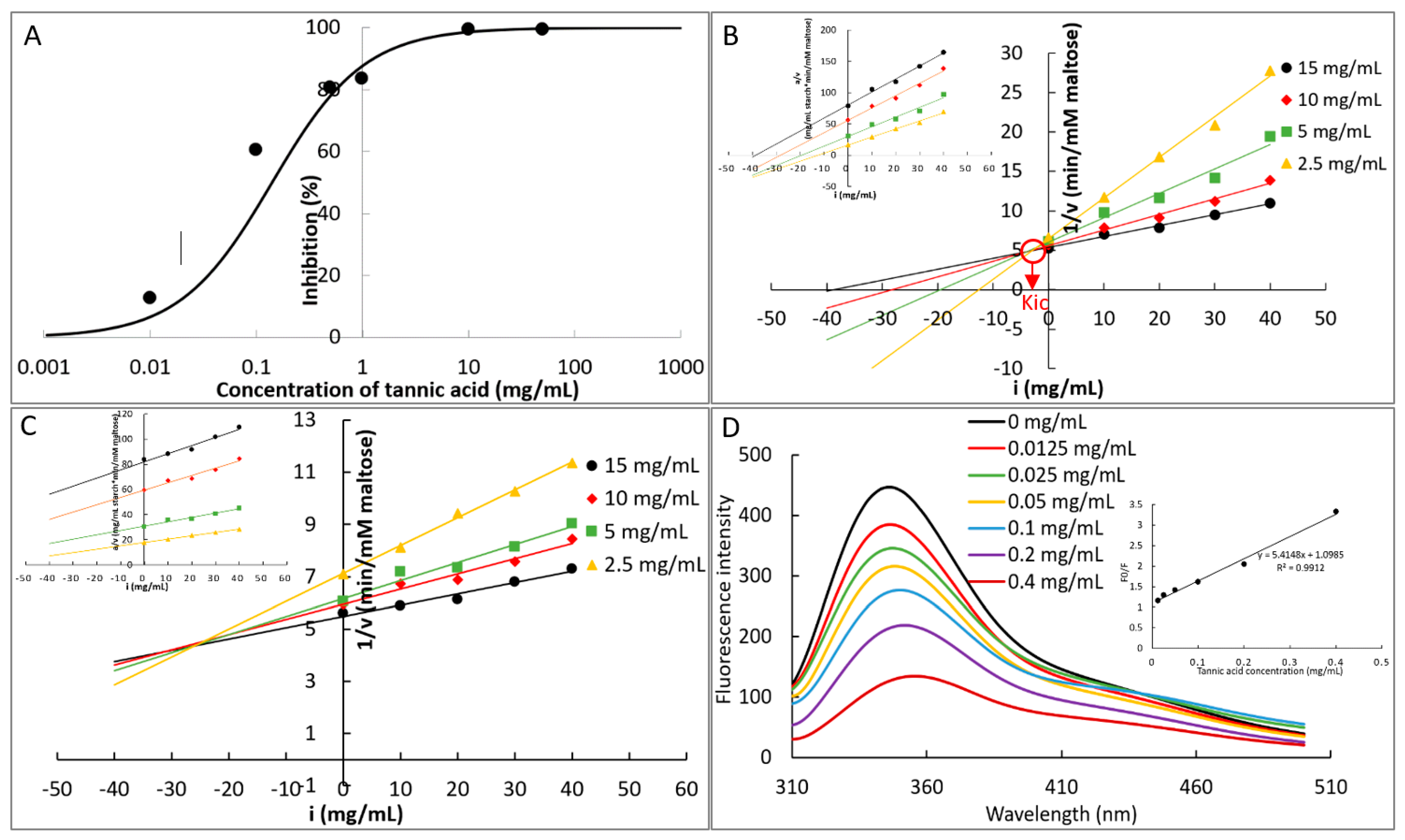
2.4.1. IC50 Value
2.4.2. Inhibition Kinetics
2.4.3. Fluorescence Quenching
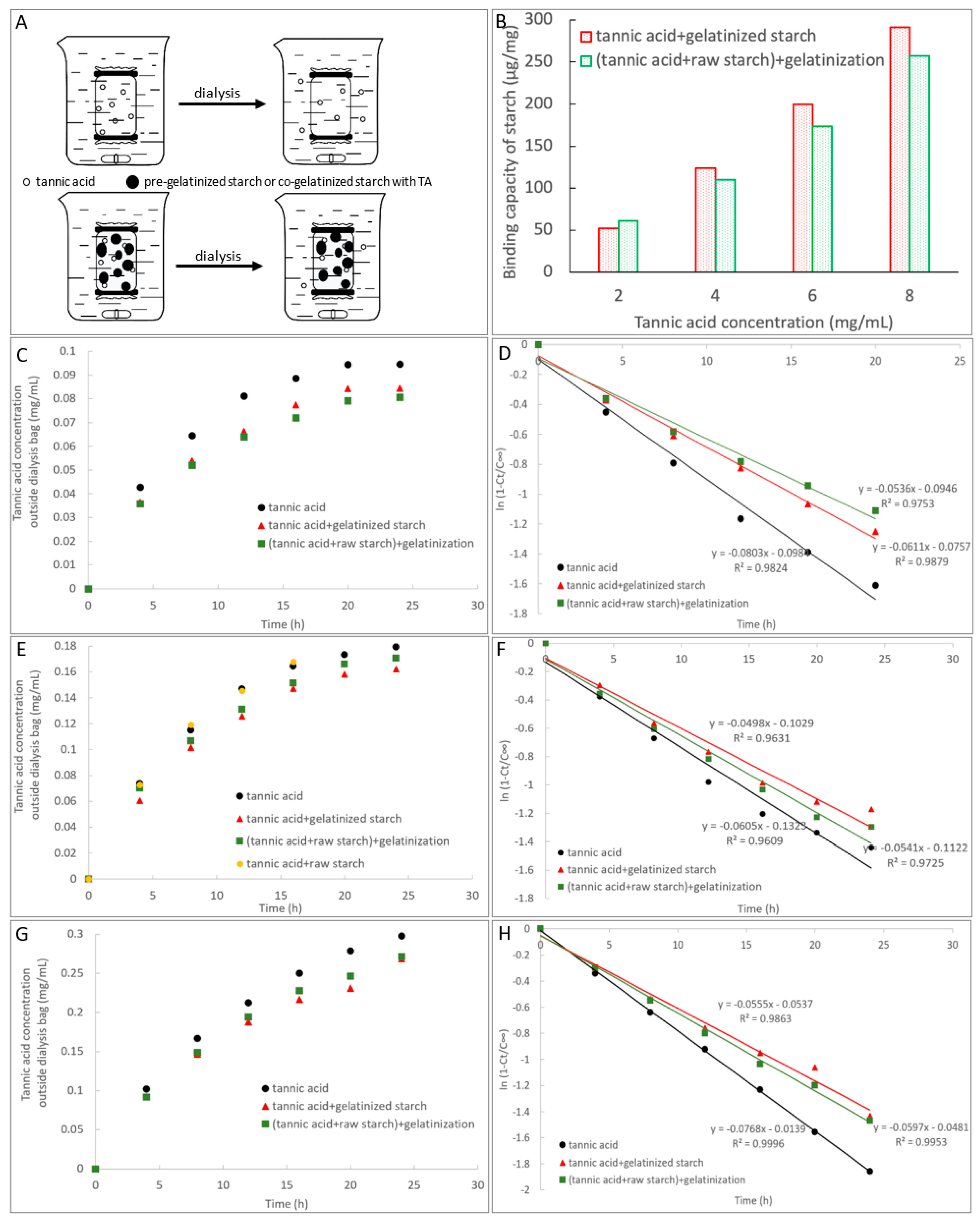
2.5. Adsorption of TA with Starch
2.6. Binding Interactions between TA and Starch
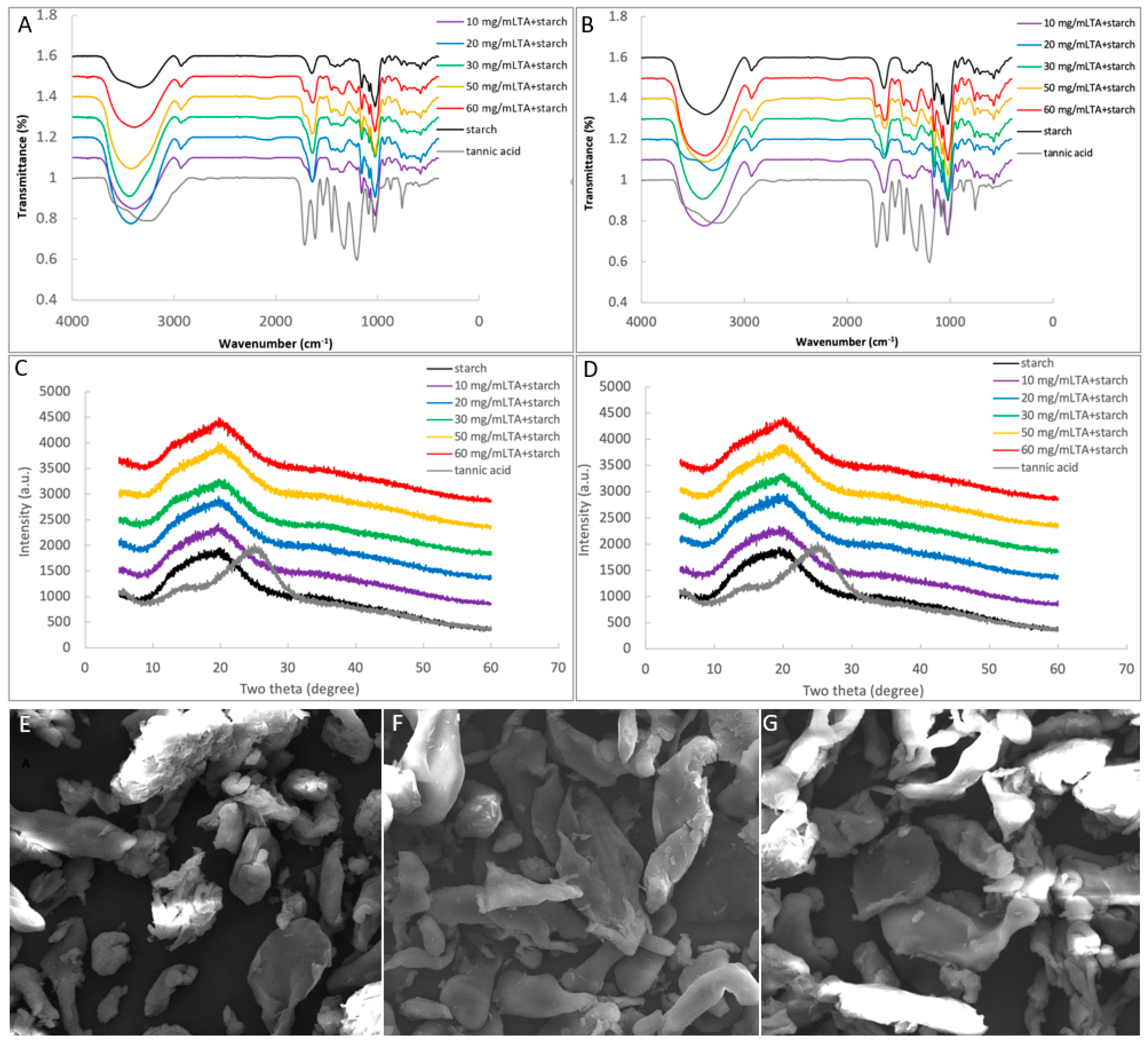
2.6.1. FTIR Analysis
2.6.2. X-ray Diffraction
2.6.3. SEM Observation
2.7. Statistical Analysis
3. Results
3.1. α-Amylase Inhibition of TA
3.2. The Mechanism in α-Amylase Inhibition of TA
3.3. Adsorption of TA with Starch
3.4. Binding Interactions between TA and Starch
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, F.J.; Zhang, B.; Waltzer, G.; Gidley, M.J.; Dhital, S. The interplay of α-amylase and amyloglucosidase activities on the digestion of starch in in vitro enzymic systems. Carbohydr. Polym. 2015, 117, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of α-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trends Food Sci. Technol. 2020, 104, 190–207. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Han, L.; Zhang, S.; Duan, X.; Sun, L.; Wang, M. Both Galloyl Moiety Number and Molecular Flexibility Are Important to Alpha-amylase Inhibition by Galloyl-based Polyphenols. Food Funct. 2020, 11, 3838–3850. [Google Scholar] [CrossRef]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for Controlling Starch Digestion: Structural Requirements for Inhibiting Human α-Amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A Review on Structure–Activity Relationship of Dietary Polyphenols Inhibiting α-Amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Warren, F.J.; Gidley, M.J. Soluble polysaccharides reduce binding and inhibitory activity of tea polyphenols against porcine pancreatic α-amylase. Food Hydrocoll. 2018, 79, 63–70. [Google Scholar] [CrossRef]
- Soares, S.I.; Goncalves, R.M.; Fernandes, I.; Mateus, N.; de Freitas, V. Mechanistic approach by which polysaccharides inhibit α-amylase/procyanidin aggregation. J. Agric. Food Chem. 2009, 57, 4352–4358. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, W.; Meng, Y.; Yang, X.; Yuan, L.; Guo, Y. Interactions between polyphenols in thinned young apples and porcine pancreatic α-amylase: Inhibition, detail kinetics and fluorescence quenching. Food Chem. 2016, 208, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, L.; Li, K.; He, J.; Xin, H.; Wang, Q. Effect of gelatinization processing on the antioxidant, digestion, and physicochemical properties of wheat starch enhanced with tannic acid. LWT 2020, 125, 109228. [Google Scholar] [CrossRef]
- Lou, W.; Chen, Y.; Ma, H.; Liang, G.; Liu, B. Antioxidant and α-amylase inhibitory activities of tannic acid. J. Food Sci. Technol. 2018, 55, 3640–3646. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Iyer, V.; Flores, F.P.; Donhowe, E.; Kong, F. Microencapsulation of tannic acid for oral administration to inhibit carbohydrate digestion in the gastrointestinal tract. Food Funct. 2013, 4, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Warren, F.J.; Netzel, G.; Gidley, M.J. 3 or 3′-Galloyl substitution plays an important role in association of catechins and theaflavins with porcine pancreatic α-amylase: The kinetics of inhibition of α-amylase by tea polyphenols. J. Funct. Foods 2016, 26, 144–156. [Google Scholar] [CrossRef]
- Butterworth, P.J.; Warren, F.J.; Grassby, T.; Patel, H.; Ellis, P.R. Analysis of starch amylolysis using plots for first-order kinetics. Carbohydr. Polym. 2012, 87, 2189–2197. [Google Scholar] [CrossRef]
- Edwards, C.H.; Warren, F.J.; Milligan, P.J.; Butterworth, P.J.; Ellis, P.R. A novel method for classifying starch digestion by modelling the amylolysis of plant foods using first-order enzyme kinetic principles. Food Funct. 2014, 5, 2751–2758. [Google Scholar] [CrossRef]
- Zou, W.; Sissons, M.; Gidley, M.J.; Gilbert, R.G.; Warren, F.J. Combined techniques for characterising pasta structure reveals how the gluten network slows enzymic digestion rate. Food Chem. 2015, 188, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.A.; Kugel, J.F.; Kugel, J.K. Binding and Kinetics for Molecular Biologists; CSHL Press: New York, NY, USA, 2007. [Google Scholar]
- Cornish-Bowden, A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors (Short Communication). Biochem. J. Mol. Asp. 1974, 137, 143–144. [Google Scholar] [CrossRef]
- Butterworth, P.J. The use of Dixon plots to study enzyme inhibition. Biochim. Biophys. Acta 1972, 289, 251–253. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Filser, J.G.; Bolt, H.M. Pharmacokinetics of halogenated ethylenes in rats. Arch. Toxicol. 1978, 42, 123–136. [Google Scholar] [CrossRef]
- Rowland, M.; Benet, L.Z.; Graham, G.G. Clearance concepts in pharmacokinetics. J. Pharmacokinet. Biopharm. 1973, 1, 123–136. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H.; Sun, L.; Liu, N.; Wang, J.; Huang, Y.; Wang, F.; Cao, J.; Fan, R.; Zhang, X. The effects of dielectric barrier discharge plasma on physicochemical and digestion properties of starch. Int. J. Biol. Macromol. 2019, 138, 819–830. [Google Scholar] [CrossRef]
- Soares, S.; Mateus, N.; de Freitas, V. Interaction of Different Polyphenols with Bovine Serum Albumin (BSA) and Human Salivary α-Amylase (HSA) by Fluorescence Quenching. J. Agric. Food Chem. 2007, 55, 6726–6735. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H.; Sun, L.; Cao, J.; Yang, J.; Lu, M.; Wang, M. Rheological, thermal and in vitro digestibility properties on complex of plasma modified Tartary buckwheat starches with quercetin. Food Hydrocoll. 2021, 110, 106209. [Google Scholar] [CrossRef]
- Pasquali, C.L.; Herrera, H. Pyrolysis of lignin and IR analysis of residues. Thermochim. Acta 1997, 293, 39–46. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR–ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, J.; Li, B. Multiple steps and critical behaviors of the binding of tannic acid to wheat starch: Effect of the concentration of wheat starch and the mass ratio of tannic acid to wheat starch. Food Hydrocoll. 2019, 94, 174–182. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, S.; Lin, H.; Chen, L.; Qin, S.; Wu, W.; Zheng, B.; Guo, Z. Physicochemical properties and digestion of the lotus seed starch-green tea polyphenol complex under ultrasound-microwave synergistic interaction. Ultrason. Sonochem. 2019, 52, 50–61. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R. Cold plasma treatment of dairy proteins in relation to functionality enhancement. Trends Food Sci. Technol. 2020, 102, 30–36. [Google Scholar] [CrossRef]
- Moosavi, M.H.; Khani, M.R.; Shokri, B.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Modifications of protein-based films using cold plasma. Int. J. Biol. Macromol. 2020, 142, 769–777. [Google Scholar] [CrossRef]
- Dzyaloshinskii, I.E.; Lifshitz, E.M.; Pitaevskii, L.P. The general theory of van der Waals forces. Adv. Phys. 1961, 10, 165–209. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Yang, X.; Wang, X.; Guo, C.; Sun, L.; Guo, Y. Modulation of gelatinized wheat starch digestion and fermentation profiles by young apple polyphenols in vitro. Food Funct. 2021, 12, 1983–1995. [Google Scholar] [CrossRef]
- Warren, F.J.; Royall, P.G.; Gaisford, S.; Butterworth, P.J.; Ellis, P.R. Binding interactions of α-amylase with starch granules: The influence of supramolecular structure and surface area. Carbohydr. Polym. 2011, 86, 1038–1047. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, J.; Luo, S.J.; Liu, C.M.; Liu, W. Effect of food additives on starch retrogradation: A review. Starch Stärke 2015, 67, 69–78. [Google Scholar] [CrossRef]
- Han, X.; Zhang, M.; Zhang, R.; Huang, L.; Jia, X.; Huang, F.; Liu, L. Physicochemical interactions between rice starch and different polyphenols and structural characterization of their complexes. LWT 2020, 125, 109227. [Google Scholar] [CrossRef]
| Methods | Mixing Orders | Constants Characterizing α-Amylase Inhibition of TA | |
|---|---|---|---|
| IC50 value | (TA + α-amylase) + ‘DQ starch’ | IC50 value (mg/mL) | 0.140 |
| Inhibition effect | Order 1 | Inhibition (10, 20, and 30 mg/mL TA, %) | 35.82 a, 56.06 a, and 67.67 a |
| Order 2 | 7.58 b, 24.76 c, and 36.87 c | ||
| Order 3 | 1.56 c, 29.45 b, and 58.18 b | ||
| Logarithm of slope (LOS) analysis | No inhibition | Digestion rate constant (k1 and k2, min−1) | 0.0180 a and 0.0138 a |
| Order 1 | 0.0088 d and 0.0039 c | ||
| Order 2 | 0.0125 b and 0.0064 b | ||
| Order 3 | 0.0119 bc and 0.0068 b | ||
| Inhibition kinetics | Order 1 | Competitive inhibition constant and its reciprocal (Kic and 1/Kic, mg/mL and mL/mg) | 3.454 b and 0.290 a |
| Order 2 | 25.912 a and 0.038 b | ||
| Fluorescence quenching | TA + α-amylase | Fluorescence quenching constant (KFQ, M−1) Bimolecular quenching constant (M−1s−1) | 9.209 × 103 3.1 × 1012 |
| Gelatinization Procedures | Constants Characterizing Binding/Adsorption of TA with Starch | ||||
|---|---|---|---|---|---|
| Dialysis | TA | Transport rate constant (kt, min−1) | 0.0803 a (2 mg/mL TA) | 0.0605 a (4 mg/mL TA) | 0.0768 a (6 mg/mL TA) |
| TA + gelatinized starch | 0.0611 b (2 mg/mL TA) | 0.0498 c (4 mg/mL TA) | 0.0555 c (6 mg/mL TA) | ||
| (TA + raw starch) + gelatinization | 0.0536 c (2 mg/mL TA) | 0.0541 b (4 mg/mL TA) | 0.0597 b (6 mg/mL TA) | ||
| FTIR | Gelatinized starch | R-1047 cm−1/1022 cm−1 | 0.797 a (control for TA + gelatinized starch), 0.745 a (control for (TA + raw starch) + gelatinization) | ||
| TA + gelatinized starch | 0.757 ab (10 mg/mL TA) | 0.804 a (30 mg/mL TA) | 0.774 a (50 mg/mL TA) | ||
| (TA + raw starch) + gelatinization | 0.731 a (10 mg/mL TA) | 0.727 a (50 mg/mL TA) | 0.727 a (60 mg/mL TA) | ||
| XRD | Gelatinized starch | Crystallinity (%) | 16.32 a (control for TA + gelatinized starch), 16.25 a (control for (TA + raw starch) + gelatinization) | ||
| TA + gelatinized starch | 16.17 a (10 mg/mL TA) | 15.76 ab (30 mg/mL TA) | 15.16 ab (50 mg/mL TA) | ||
| (TA + raw starch) + gelatinization | 15.95 a (10 mg/mL TA) | 15.67 a (30 mg/mL TA) | 15.39 a (50 mg/mL TA) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, S.; Bai, F.; Cao, J.; Sun, L. The Physical Adsorption of Gelatinized Starch with Tannic Acid Decreases the Inhibitory Activity of the Polyphenol against α-Amylase. Foods 2021, 10, 1233. https://doi.org/10.3390/foods10061233
Wang Y, Li S, Bai F, Cao J, Sun L. The Physical Adsorption of Gelatinized Starch with Tannic Acid Decreases the Inhibitory Activity of the Polyphenol against α-Amylase. Foods. 2021; 10(6):1233. https://doi.org/10.3390/foods10061233
Chicago/Turabian StyleWang, Yueyi, Shuangshuang Li, Fangting Bai, Junwei Cao, and Lijun Sun. 2021. "The Physical Adsorption of Gelatinized Starch with Tannic Acid Decreases the Inhibitory Activity of the Polyphenol against α-Amylase" Foods 10, no. 6: 1233. https://doi.org/10.3390/foods10061233
APA StyleWang, Y., Li, S., Bai, F., Cao, J., & Sun, L. (2021). The Physical Adsorption of Gelatinized Starch with Tannic Acid Decreases the Inhibitory Activity of the Polyphenol against α-Amylase. Foods, 10(6), 1233. https://doi.org/10.3390/foods10061233