A Fluorescent Detection for Paraquat Based on β-CDs-Enhanced Fluorescent Gold Nanoclusters
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
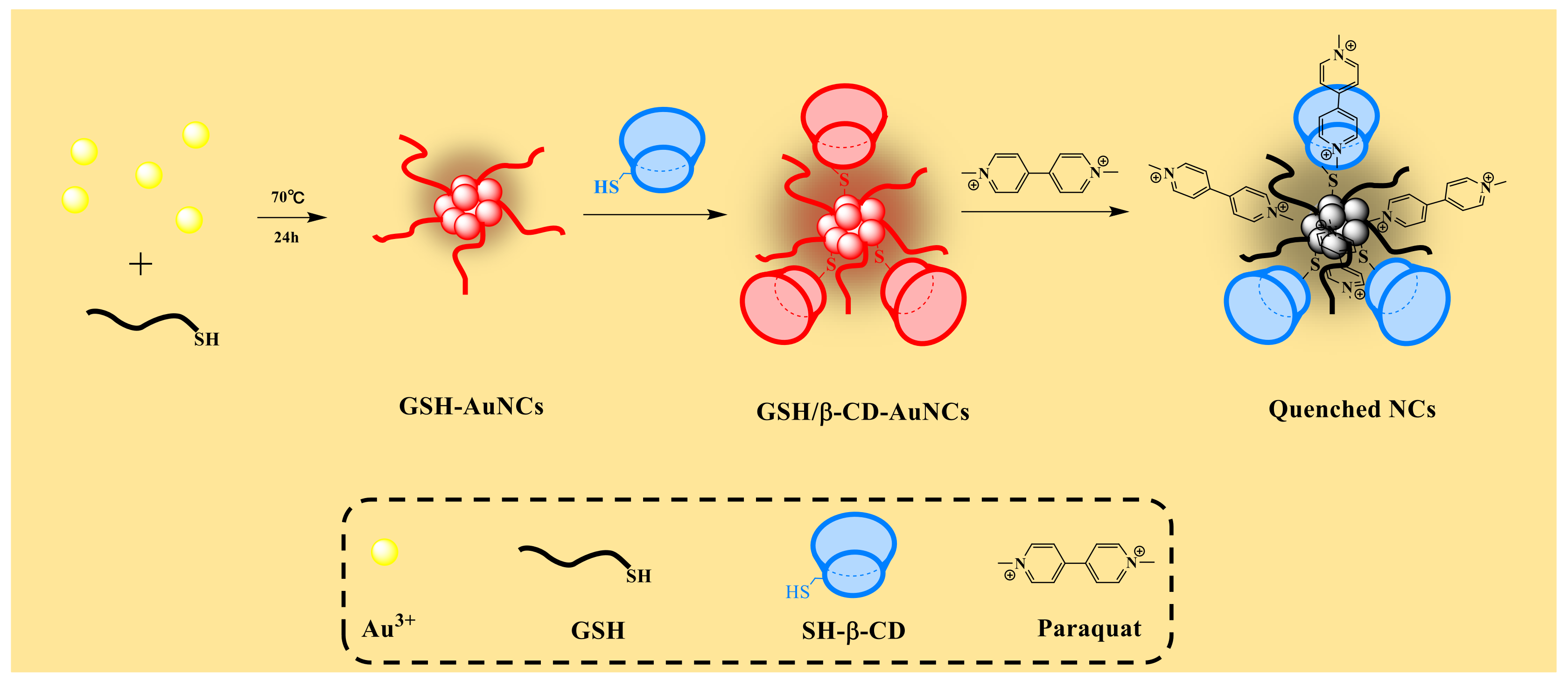
2.3. Synthesis of SH-β-CDs and GSH-Capped AuNCs
2.4. Fluorescent Detection of Paraquat
2.5. Validation of the Fluorescent Detection
3. Results and Discussion
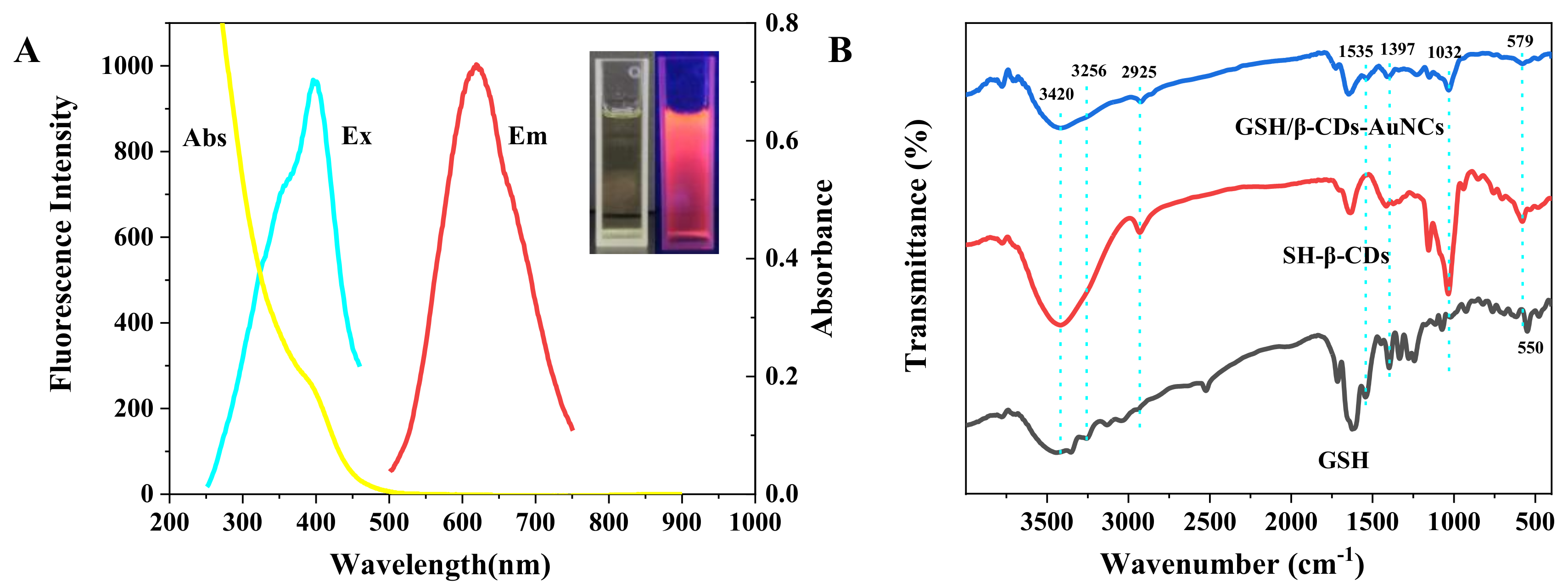
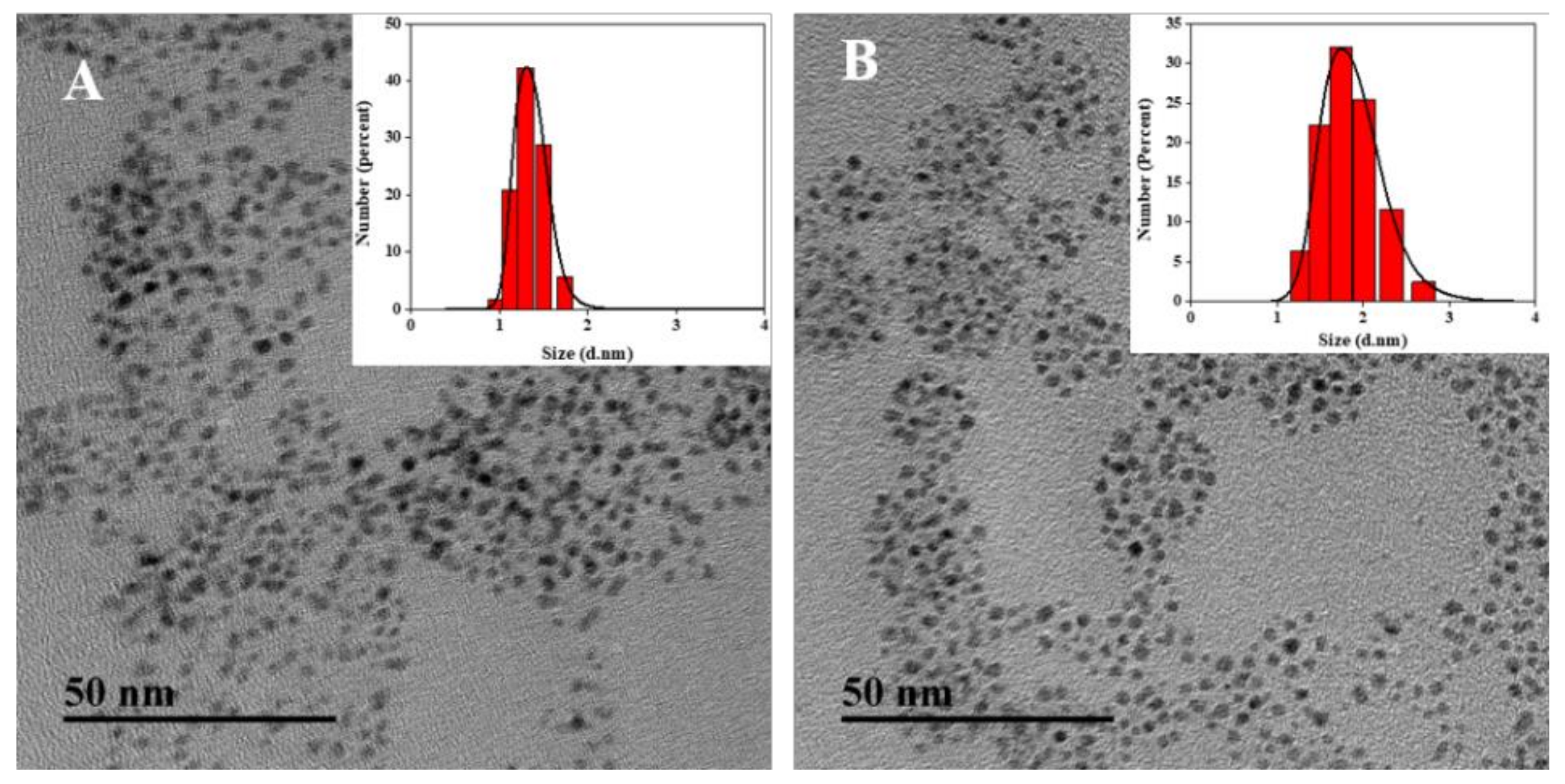
3.1. Properties of the GSH/β-CDs-AuNCs
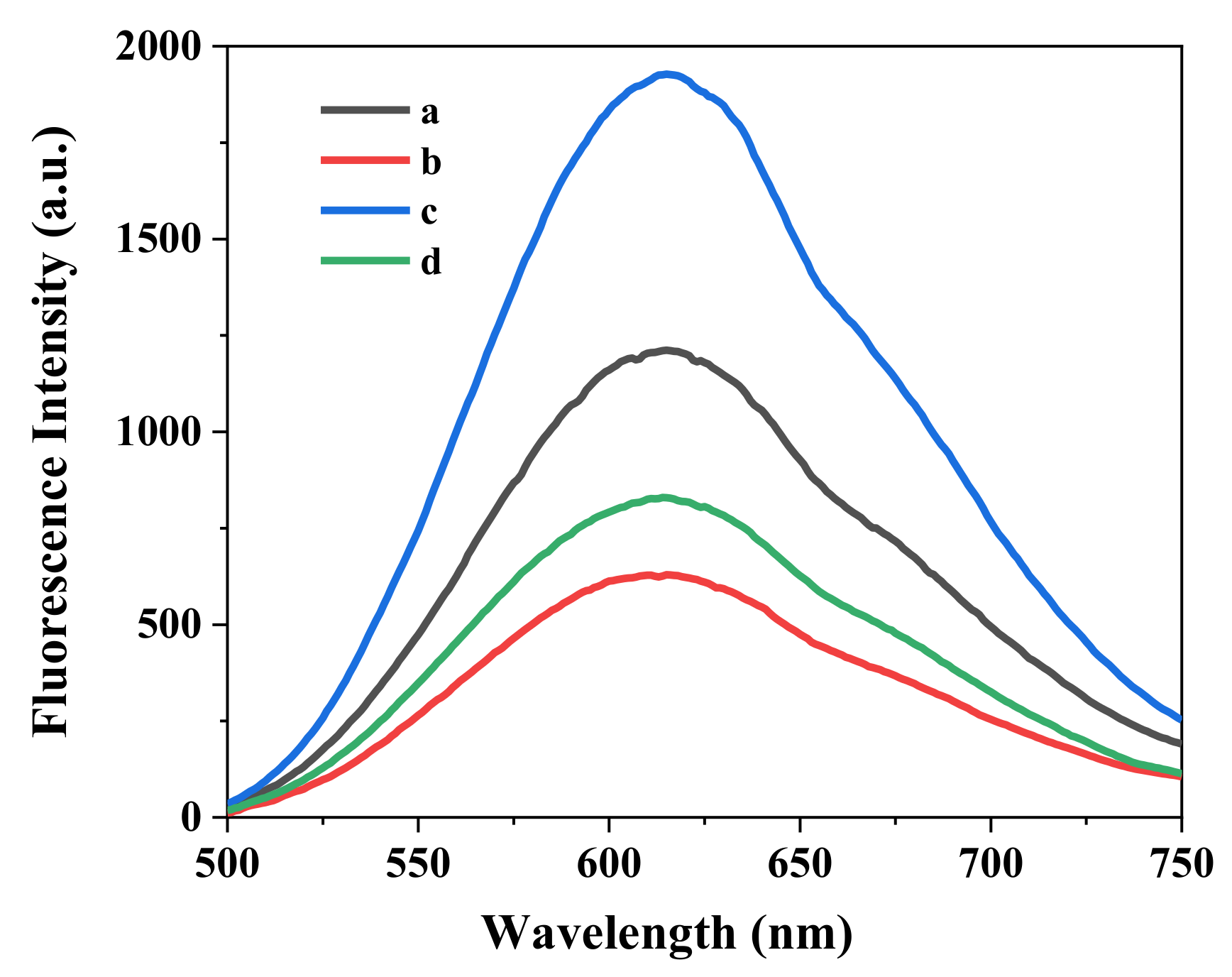
3.2. Mechanism of Sensing
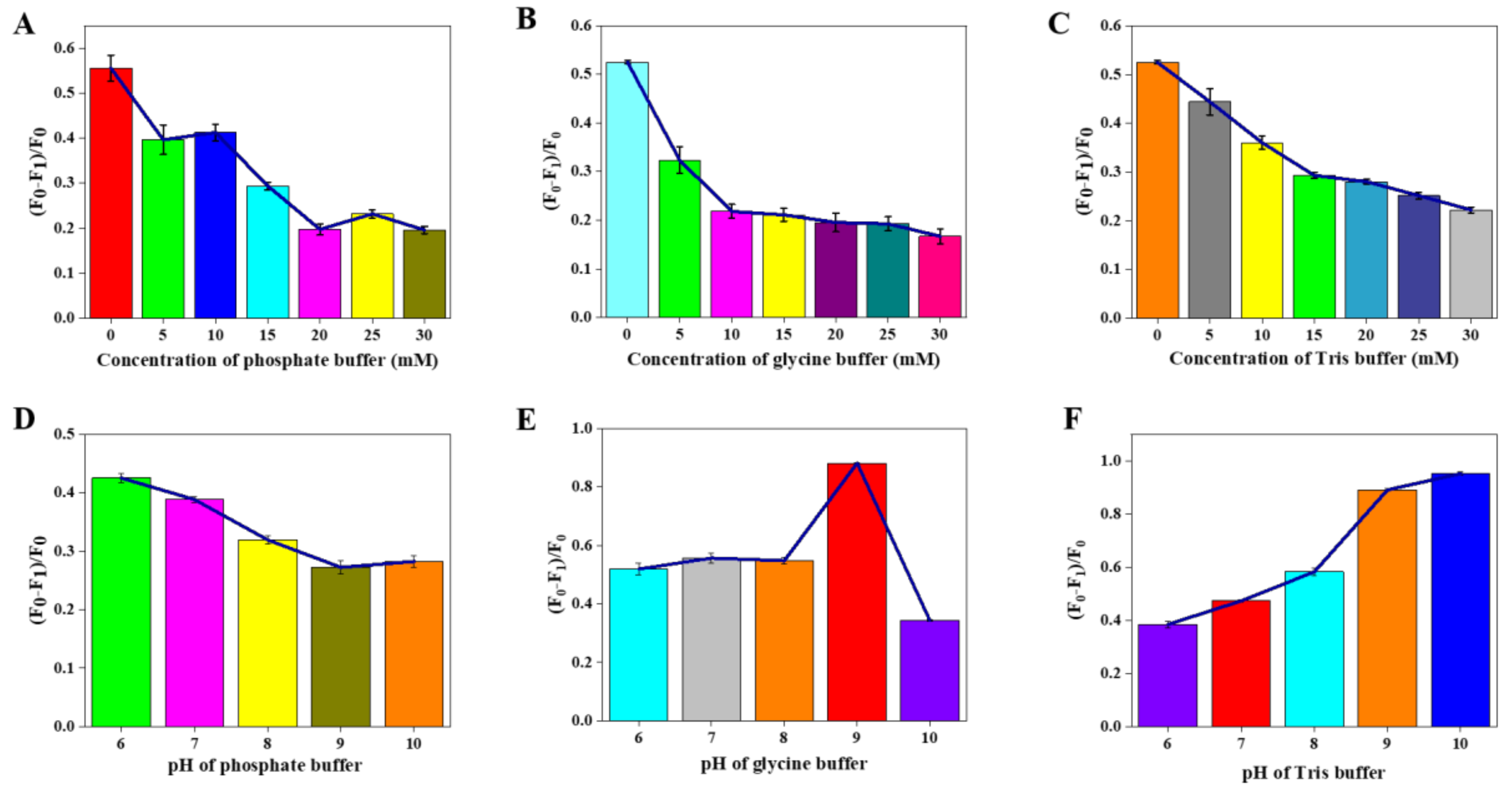
3.3. Optimization of Paraquat Sensing
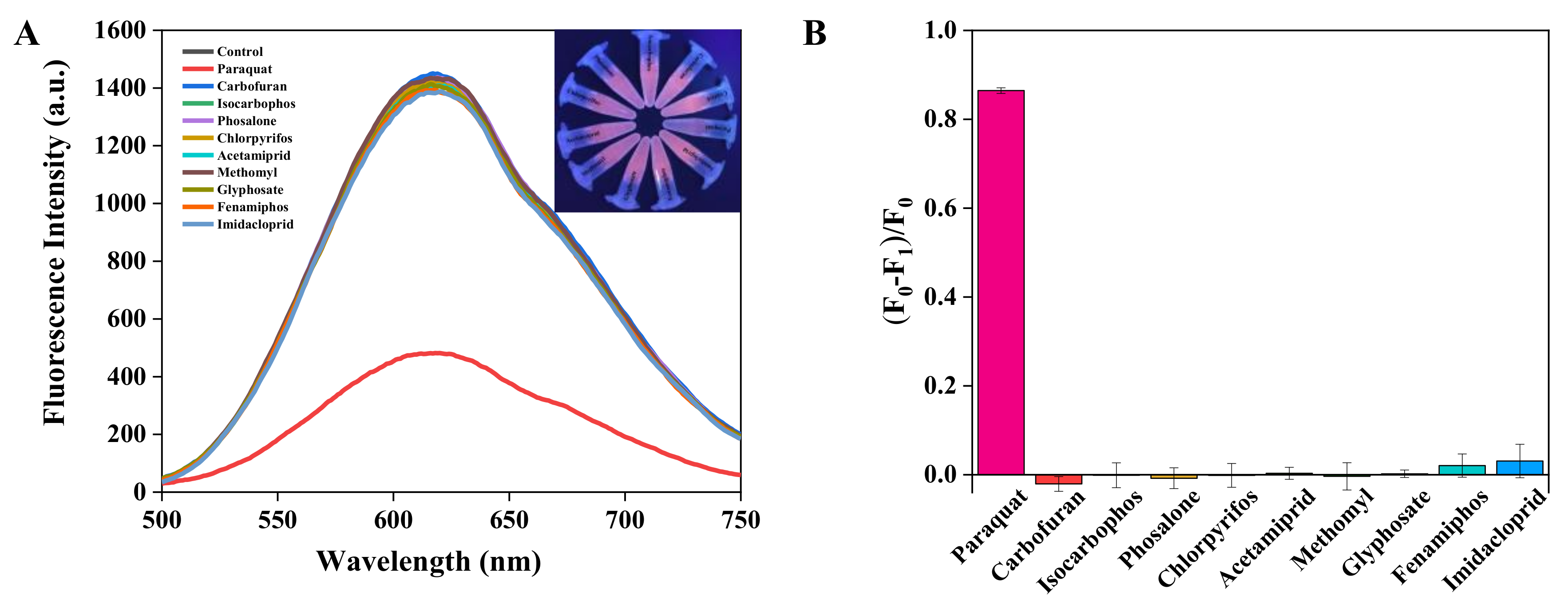
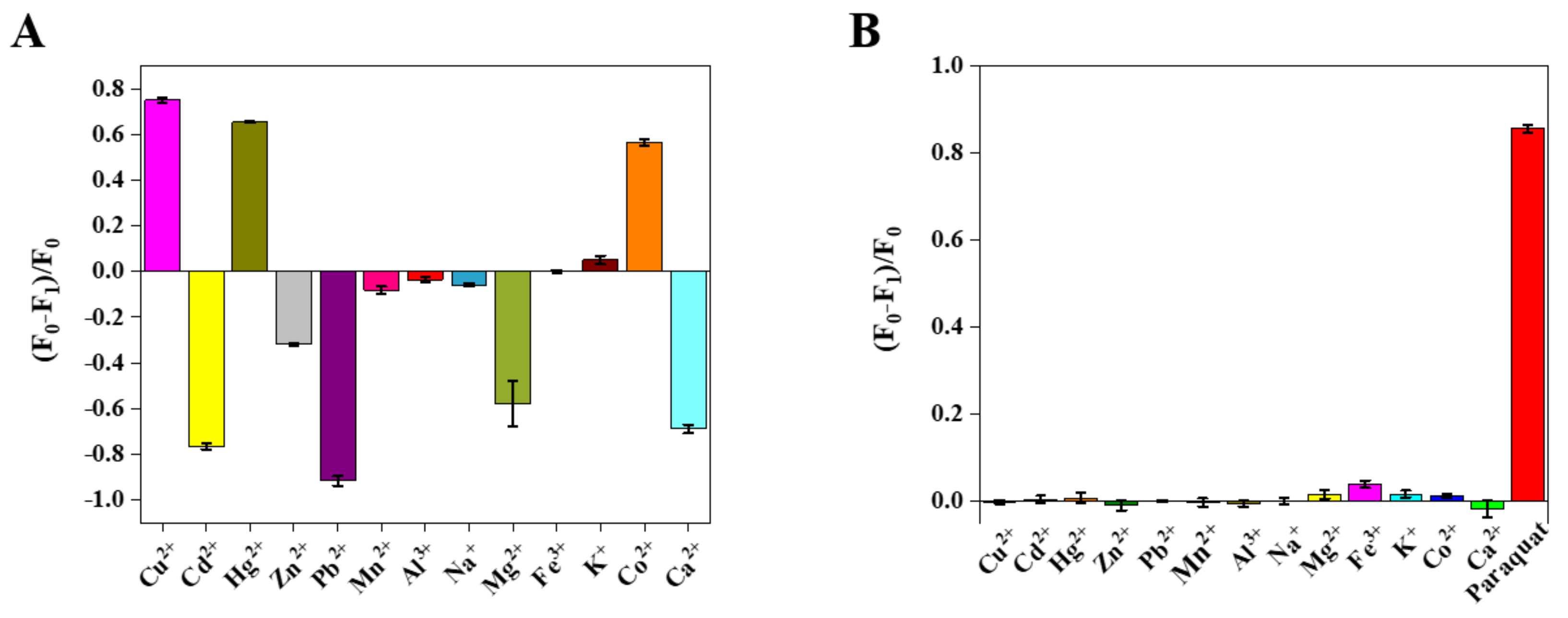
3.4. Interference Analysis
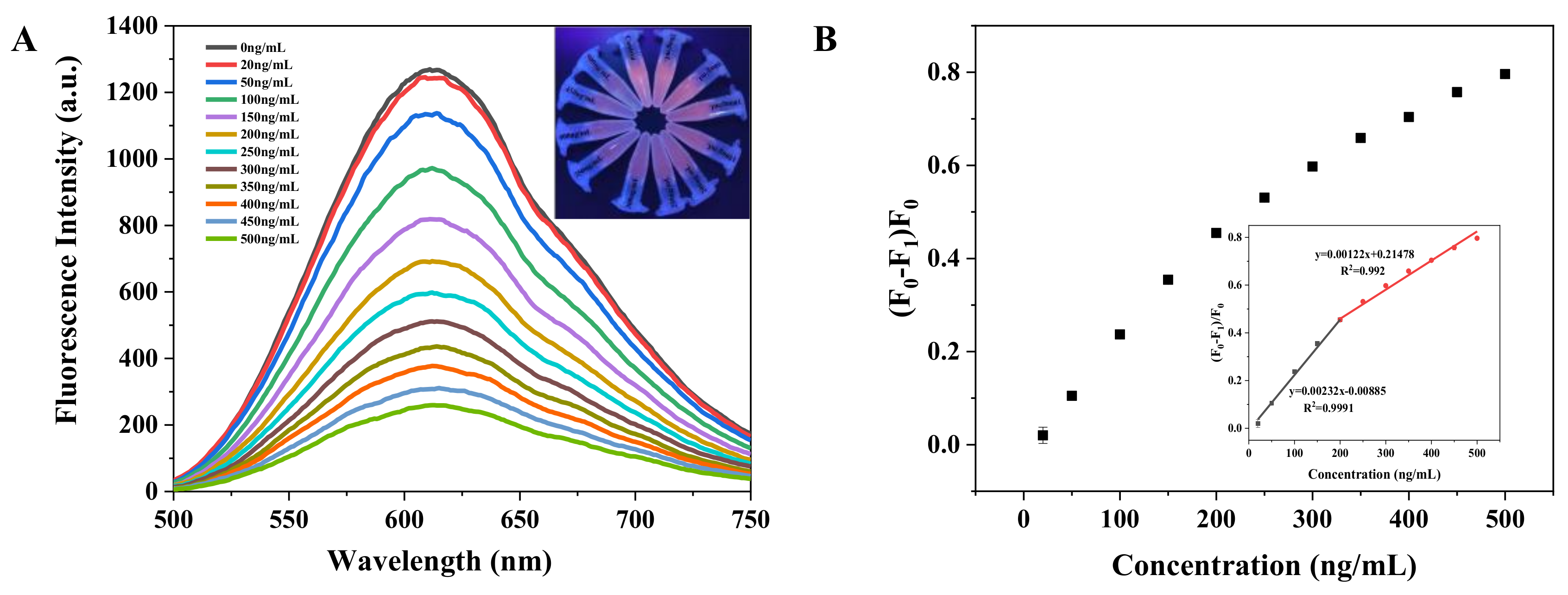
3.5. Analytical Performance Comparison between GSH-AuNCs, GSH/β-CDs-AuNCs, and Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Maity, S.; Bain, D.; Patra, A. An overview on the current understanding of the photophysical properties of metal nanoclusters and their potential applications. Nanoscale 2019, 11, 22685–22723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Xu, C.; Wang, X.; Liu, C.; Waterhouse, G.I.N.; Wang, Y.; Yin, H. Ultrasmall Au nanoclusters for biomedical and biosensing applications: A mini-review. Talanta 2019, 200, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-Y.; He, H.-X.; He, S.-B.; Zhang, X.-P.; Peng, H.-P.; Lin, Z.; Deng, H.-H.; Xia, X.-H.; Chen, W. Gold nanocluster-based fluorescence turn-off probe for sensing of doxorubicin by photoinduced electron transfer. Sens. Actuators B Chem. 2019, 296. [Google Scholar] [CrossRef]
- Zhang, P.; Jia, C.; Zhao, Y.; Luo, H.; Tan, X.; Ma, X.; Wang, Y. Detection of tiopronin in body fluids and pharmaceutical products using red-emissive DNA-stabilized silver nanoclusters as a fluorescent probe. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Qu, F.; Wang, B.; Li, K.; You, J.; Han, W. Copper nanoclusters@Al3+ complexes with strong and stable aggregation-induced emission for application in enzymatic determination of urea. Microchim. Acta 2020, 187. [Google Scholar] [CrossRef]
- Ao, H.; Qian, Z.; Zhu, Y.; Zhao, M.; Tang, C.; Huang, Y.; Feng, H.; Wang, A. A fluorometric biosensor based on functional Au/Ag nanoclusters for real-time monitoring of tyrosinase activity. Biosens. Bioelectron. 2016, 86, 542–547. [Google Scholar] [CrossRef]
- Shojaeifard, Z.; Heidari, N.; Hemmateenejad, B. Bimetallic AuCu nanoclusters-based florescent chemosensor for sensitive detection of Fe(3+) in environmental and biological systems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 209, 202–208. [Google Scholar] [CrossRef]
- Shellaiah, M.; Simon, T.; Thirumalaivasan, N.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-capped gold-copper nanoclusters for fluorometric determination and imaging of chromium(VI) and dopamine. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.; Kang, X.; Zhu, M. Customizing the Structure, Composition, and Properties of Alloy Nanoclusters by Metal Exchange. Acc. Chem. Res. 2018, 51, 2784–2792. [Google Scholar] [CrossRef]
- Qing, T.; Feng, B.; Zhang, P.; Zhang, K.; He, X.; Wang, K. Beyond native deoxyribonucleic acid, templating fluorescent nanomaterials for bioanalytical applications: A review. Anal. Chim. Acta 2020, 1105, 11–27. [Google Scholar] [CrossRef]
- Qing, T.; Zhang, K.; Qing, Z.; Wang, X.; Long, C.; Zhang, P.; Hu, H.; Feng, B. Recent progress in copper nanocluster-based fluorescent probing: A review. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, Y.; Ma, X.; Xia, M.; Li, S.; Zhang, Y. Thiolated DNA-templated silver nanoclusters with strong fluorescence emission and a long shelf-life. Nanoscale 2018, 10, 76–81. [Google Scholar] [CrossRef]
- Sun, J.; Jin, Y. Fluorescent Au nanoclusters: Recent progress and sensing applications. J. Mater. Chem. C 2014, 2, 8000–8011. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Wang, T.; Yun, Z.; Li, G.; Liu, J.; Jiang, G. Facile preparation of glutathione-stabilized gold nanoclusters for selective determination of chromium (III) and chromium (VI) in environmental water samples. Anal. Chim. Acta 2013, 770, 140–146. [Google Scholar] [CrossRef]
- Baig, M.M.F.; Chen, Y.C. Gold nanocluster-based fluorescence sensing probes for detection of dipicolinic acid. Analyst 2019, 144, 3289–3296. [Google Scholar] [CrossRef]
- Chen, L.Y.; Wang, C.W.; Yuan, Z.; Chang, H.T. Fluorescent gold nanoclusters: Recent advances in sensing and imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef]
- Ou, G.; Zhao, J.; Chen, P.; Xiong, C.; Dong, F.; Li, B.; Feng, X. Fabrication and application of noble metal nanoclusters as optical sensors for toxic metal ions. Anal. Bioanal. Chem. 2018, 410, 2485–2498. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, L.; Tian, X.; Li, Y.; Yang, C.; Nie, Y.; Zhou, Z. Ratiometric fluorescence detection of mercuric ions by sole intrinsic dual-emitting gold nanoclusters. Sens. Actuators B Chem. 2019, 278, 82–87. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, D.; Jin, R.; Li, H.; Yan, X.; Liu, F.; Sun, P.; Gao, Y.; Lu, G. On-site monitoring of thiram via aggregation-induced emission enhancement of gold nanoclusters based on electronic-eye platform. Sens. Actuators B Chem. 2019, 296. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Zhou, X.; Hu, J. A label-free turn-on-off fluorescent sensor for the sensitive detection of cysteine via blocking the Ag(+)-enhancing glutathione-capped gold nanoclusters. Talanta 2018, 179, 742–752. [Google Scholar] [CrossRef]
- Su, X.; Jiang, H.; Wang, X. Thiols-Induced Rapid Photoluminescent Enhancement of Glutathione-Capped Gold Nanoparticles for Intracellular Thiols Imaging Applications. Anal. Chem. 2015, 87, 10230–10236. [Google Scholar] [CrossRef]
- Xu, N.; Yuan, Y.; Lan, C.; Wei, W.; Meng, L.; Fan, L. A novel dual-emission fluorescent nanohybrid containing silica nanoparticles and gold nanoclusters for ratiometric determination of cysteine based on turn-on fluorescence strategy. New J. Chem. 2018, 42, 10092–10099. [Google Scholar] [CrossRef]
- You, J.G.; Tseng, W.L. Peptide-induced aggregation of glutathione-capped gold nanoclusters: A new strategy for designing aggregation-induced enhanced emission probes. Anal. Chim. Acta 2019, 1078, 101–111. [Google Scholar] [CrossRef]
- Li, D.; Chen, Z.; Mei, X. Fluorescence enhancement for noble metal nanoclusters. Adv. Colloid Interface Sci. 2017, 250, 25–39. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X.; Li, Z.; Li, J.; Ran, X.; Yang, L. Water-soluble amino pillar[5]arene functionalized gold nanoclusters as fluorescence probes for the sensitive determination of dopamine. Microchem. J. 2019, 150. [Google Scholar] [CrossRef]
- Rashidipour, N.; Karami-Mohajeri, S.; Mandegary, A.; Mohammadinejad, R.; Wong, A.; Mohit, M.; Salehi, J.; Ashrafizadeh, M.; Najafi, A.; Abiri, A. Where ferroptosis inhibitors and paraquat detoxification mechanisms intersect, exploring possible treatment strategies. Toxicology 2020, 433-434, 152407. [Google Scholar] [CrossRef]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef]
- Rosic, N.; Bradbury, J.; Lee, M.; Baltrotsky, K.; Grace, S. The impact of pesticides on local waterways: A scoping review and method for identifying pesticides in local usage. Environ. Sci. Policy 2020, 106, 12–21. [Google Scholar] [CrossRef]
- Camargo, E.R.; Zapiola, M.L.; Avila, L.A.d.; Garcia, M.A.; Plaza, G.; Gazziero, D.; Hoyos, V. Current situation regarding herbicide regulation and public perception in South America. Weed Sci. 2020, 68, 232–239. [Google Scholar] [CrossRef]
- Barron Cuenca, J.; Tirado, N.; Vikstrom, M.; Lindh, C.H.; Stenius, U.; Leander, K.; Berglund, M.; Dreij, K. Pesticide exposure among Bolivian farmers: Associations between worker protection and exposure biomarkers. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 730–742. [Google Scholar] [CrossRef]
- Nosratti, I.; Sabeti, P.; Chaghamirzaee, G.; Heidari, H. Weed problems, challenges, and opportunities in Iran. Crop Prot. 2020, 134. [Google Scholar] [CrossRef]
- Sha, O.; Cui, B.; Chen, X.; Liu, H.; Yao, J.; Zhu, Y. Separation and Determination of Paraquat and Diquat in Human Plasma and Urine by Magnetic Dispersive Solid Phase Extraction Coupled with High-Performance Liquid Chromatography. J. Anal. Methods Chem. 2020, 2020, 7359582. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhang, J.; He, Q.; Chen, X.; Jin, M. Fabrication of benzenesulfonic acid groups modified magnetic microspheres as an MSPE adsorbent for fast determination of paraquat and diquat in human urine combined with UPLC-HRMS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1136, 121880. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yang, H.; Li, Y.; Liu, A.; Wei, W.; Liu, S. Fluorescence sensor for organophosphorus pesticide detection based on the alkaline phosphatase-triggered reaction. Anal. Chim. Acta 2020, 1131, 102–108. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Zhou, X.; Yu, Y.; Liu, J.; Hu, N.; Wang, H.; Li, G.; Wu, Y. Recent progress in the construction of nanozyme-based biosensors and their applications to food safety assay. TrAC Trends Anal. Chem. 2019, 121. [Google Scholar] [CrossRef]
- Saberi, Z.; Rezaei, B.; Ensafi, A.A. Fluorometric label-free aptasensor for detection of the pesticide acetamiprid by using cationic carbon dots prepared with cetrimonium bromide. Mikrochim. Acta 2019, 186, 273. [Google Scholar] [CrossRef]
- Reynoso, E.; Torres, E.; Bettazzi, F.; Palchetti, I. Trends and Perspectives in Immunosensors for Determination of Currently-Used Pesticides: The Case of Glyphosate, Organophosphates, and Neonicotinoids. Biosensors 2019, 9, 20. [Google Scholar] [CrossRef]
- Sanabria Español, E.; Maldonado, M. Host–Guest Recognition of Pesticides by Calixarenes. Crit. Rev. Anal Chem. 2019, 49, 383–394. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, X.; Zou, T.; Wang, Z.; Zhao, R.; Hong, P.; Peng, S.; Zhang, X.; Wang, Y. A novel and sensitive ratiometric fluorescence assay for carbendazim based on N-doped carbon quantum dots and gold nanocluster nanohybrid. J. Hazard Mater. 2020, 386, 121958. [Google Scholar] [CrossRef]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From aggregation-induced emission of Au(I)-thiolate complexes to ultrabright Au(0)@Au(I)-thiolate core-shell nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef]
- Yang, H.; Lu, F.; Sun, Y.; Yuan, Z.; Lu, C. Fluorescent Gold Nanocluster-Based Sensor Array for Nitrophenol Isomer Discrimination via an Integration of Host-Guest Interaction and Inner Filter Effect. Anal. Chem. 2018, 90, 12846–12853. [Google Scholar] [CrossRef]
- Nsibande, S.A.; Forbes, P.B.C. Development of a quantum dot molecularly imprinted polymer sensor for fluorescence detection of atrazine. Luminescence 2019, 34, 480–488. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Shen, X.; Zhu, W.; Xu, L.; Zhou, X. Aggregation-induced emission from gold nanoclusters for use as a luminescence-enhanced nanosensor to detect trace amounts of silver ions. J. Colloid Interface Sci. 2016, 467, 90–96. [Google Scholar] [CrossRef]
- Peterson, M.D.; Jensen, S.C.; Weinberg, D.J.; Weiss, E.A. Mechanisms for adsorption of methyl viologen on CdS quantum dots. ACS Nano 2014, 8, 2826–2837. [Google Scholar] [CrossRef]
- Matylitsky, V.V.; Dworak, L.; Breus, V.V.; Basche, T.; Wachtveitl, J. Ultrafast charge separation in multiexcited CdSe quantum dots mediated by adsorbed electron acceptors. J. Am. Chem. Soc. 2009, 131, 2424–2425. [Google Scholar] [CrossRef]
- Scholz, F.; Dworak, L.; Matylitsky, V.V.; Wachtveitl, J. Ultrafast Electron Transfer from Photoexcited CdSe Quantum Dots to Methylviologen. Chemphyschem 2011, 12, 2255–2259. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Yang, X. Facile Synthesis of Glutathione-capped CdS Quantum Dots as a Fluorescence Sensor for Rapid Detection and Quantification of Paraquat. Anal. Sci. Inter. J. Jpn. Soc. Anal. Chem. 2015, 31, 1011–1017. [Google Scholar] [CrossRef][Green Version]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40. [Google Scholar] [CrossRef]
- Junthip, J. Water-insoluble cyclodextrin polymer crosslinked with citric acid for paraquat removal from water. J. Macromol. Sci. Part A 2019, 56, 555–563. [Google Scholar] [CrossRef]
- Zhai, W.; Wang, C.; Yu, P.; Wang, Y.; Mao, L. Single-Layer MnO2 Nanosheets Suppressed Fluorescence of 7-Hydroxycoumarin: Mechanistic Study and Application for Sensitive Sensing of Ascorbic Acid in Vivo. Anal. Chem. 2014, 86, 12206–12213. [Google Scholar] [CrossRef]
- Halawa, M.I.; Wu, F.; Fereja, T.H.; Lou, B.; Xu, G. One-pot green synthesis of supramolecular β-cyclodextrin functionalized gold nanoclusters and their application for highly selective and sensitive fluorescent detection of dopamine. Sens. Actuators B Chem. 2018, 254, 1017–1024. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; van den Hoek, I.A.F.; Renzetti, S. Sugar replacement with zwitterionic plasticizers like amino acids. Food Hydrocoll. 2020, 109. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Zhang, X.; Cao, H.; Huang, Y. Unconventional application of gold nanoclusters/Zn-MOF composite for fluorescence turn-on sensitive detection of zinc ion. Anal. Chim. Acta 2018, 1024, 145–152. [Google Scholar] [CrossRef]
- Luo, P.; Zheng, Y.; Qin, Z.; Li, C.; Jiang, H.; Wang, X. Fluorescence light up detection of aluminium ion and imaging in live cells based on the aggregation-induced emission enhancement of thiolated gold nanoclusters. Talanta 2019, 204, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Ungor, D.; Csapo, E.; Kismarton, B.; Juhasz, A.; Dekany, I. Nucleotide-directed syntheses of gold nanohybrid systems with structure-dependent optical features: Selective fluorescence sensing of Fe(3+) ions. Colloids Surf. B Biointerfaces 2017, 155, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Han, L. Displaying of acetylcholinesterase mutants on surface of yeast for ultra-trace fluorescence detection of organophosphate pesticides with gold nanoclusters. Biosens. Bioelectron. 2020, 148, 111825. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.A.; Insausti, M.; Ilari, R.; Zanini, G.P. Fluorescence enhancement novel green analytical method for paraquat herbicide quantification based on immobilization on clay. Analyst 2019, 144, 3357–3363. [Google Scholar] [CrossRef] [PubMed]
- Yuttakovit, S.; Santiwat, T.; Pratumyot, K.; Srikittiwanna, K.; Sukwattanasinitt, M.; Niamnont, N. A novel pyrenyl salicylic acid fluorophore for highly selective detection of paraquat in aqueous media. J. Photochem. Photobiol. A Chem. 2020, 397. [Google Scholar] [CrossRef]
- Qian, X.; Zhou, X.; Gao, W.; Li, J.; Ran, X.; Du, G.; Yang, L. One-step and green strategy for exfoliation and stabilization of graphene by phosphate pillar 6 arene and its application for fluorescence sensing of paraquat. Microchem. J. 2019, 150. [Google Scholar] [CrossRef]
- Du, F.; Sun, L.; Zen, Q.; Tan, W.; Cheng, Z.; Ruan, G.; Li, J. A highly sensitive and selective “on-off-on” fluorescent sensor based on nitrogen doped graphene quantum dots for the detection of Hg2+ and paraquat. Sens. Actuators B Chem. 2019, 288, 96–103. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Mungara, A.K.; Singhal, R.K.; Sonkeshariya, D.; Kailasa, S.K. One-step green synthetic approach for the preparation of multicolor emitting copper nanoclusters and their applications in chemical species sensing and bioimaging. Biosens. Bioelectron. 2016, 80, 243–248. [Google Scholar] [CrossRef]
- Tan, X.; Wu, Y.; Yu, S.; Zhang, T.; Tian, H.; He, S.; Zhao, A.; Chen, Y.; Gou, Q. The synthesis of water-soluble phosphate pillar 5 arenes functionalized graphene as a fluorescent probe for sensitive detection of paraquat. Talanta 2019, 195, 472–479. [Google Scholar] [CrossRef]
- Tu, J.; Xiao, L.; Jiang, Y.; He, Q.; Sun, S.; Xu, Y. Near-infrared fluorescent turn-on detection of paraquat using an assembly of squaraine and surfactants. Sens. Actuators B Chem. 2015, 215, 382–387. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, F.; Zhang, Z. A facile fluorescent “turn-off” method for sensing paraquat based on pyranine-paraquat interaction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 96–101. [Google Scholar] [CrossRef]

| Analytical Materials | Limit of Detection (LOD) | Linear Range | Ref. |
|---|---|---|---|
| Sodium montmorillonite clay | 0.37 µM | 2.0–8.0 µM | [57] |
| Fluorescent dye | 0.270 µM | 1.00–100 µM | [58] |
| PP6@graphene | 0.06 µM | 0.2–2 μM and 2–18μM | [59] |
| N-GQDs | 73.9 nM | 0.05–2.0 μg/mL | [60] |
| OVA-AuNCs | 49 nM | 0.2 to 1000 µM | [61] |
| PWP5-rGO | 3.5 nM | 0.01–2.0 and 2.0–50.0 µM | [62] |
| CdS QDs | 38.8 nM | 25–1500 ng/mL | [47] |
| Squaraines | 372 nM | 0–140 μM | [63] |
| Pyranine | 0.2 μM | 1.0–20.0 μM | [64] |
| GSH/β-CDs-AuNCs | 1.2 ng/mL (4.67nM) | 5.0–350 ng/mL | Our work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.-X.; Mao, M.-X.; Li, M.; Zhang, C.-Z.; Peng, C.-F.; Xu, J.-G.; Wei, X.-L. A Fluorescent Detection for Paraquat Based on β-CDs-Enhanced Fluorescent Gold Nanoclusters. Foods 2021, 10, 1178. https://doi.org/10.3390/foods10061178
Ren H-X, Mao M-X, Li M, Zhang C-Z, Peng C-F, Xu J-G, Wei X-L. A Fluorescent Detection for Paraquat Based on β-CDs-Enhanced Fluorescent Gold Nanoclusters. Foods. 2021; 10(6):1178. https://doi.org/10.3390/foods10061178
Chicago/Turabian StyleRen, Hong-Xin, Min-Xin Mao, Min Li, Cun-Zheng Zhang, Chi-Fang Peng, Jiang-Guo Xu, and Xin-Lin Wei. 2021. "A Fluorescent Detection for Paraquat Based on β-CDs-Enhanced Fluorescent Gold Nanoclusters" Foods 10, no. 6: 1178. https://doi.org/10.3390/foods10061178
APA StyleRen, H.-X., Mao, M.-X., Li, M., Zhang, C.-Z., Peng, C.-F., Xu, J.-G., & Wei, X.-L. (2021). A Fluorescent Detection for Paraquat Based on β-CDs-Enhanced Fluorescent Gold Nanoclusters. Foods, 10(6), 1178. https://doi.org/10.3390/foods10061178






