1. Introduction
Type 2 diabetes mellitus (T2DM) and hypertension are the most shared comorbidities in coronavirus-infected patients [
1]. In the current COVID-19 pandemic context and according to some papers [
1,
2], including those from the Centers for Disease Control and Prevention (CDC), patients with type 2 diabetes mellitus and the metabolic syndrome could suffer with an up to ten-times higher risk of dying when they contract COVID-19 [
3].
T2DM is a universal disease affecting the populations of developed and developing nations. Moreover, T2DM is the most common endocrine disease with indirect relation to several other disorders. It is expected that more than 300 million persons worldwide will suffer from T2DM in 2025 [
4]. A genetic susceptibility to the disease exists that is promoted by environmental reasons, for example an unhealthful eating behavior, with obesity being one of the greatest important risk reasons. T2DM is caused by an irregularity of the carbohydrate metabolism, which is directly connected to down insulin levels in blood [
5,
6].
Recently, natural food components and natural anti-hyperglycemic mixtures, which have advantages on human health, have received additional interest from the scientific society [
7]. For example, the ethanolic extract of Kigelia Africana indicated the existence of bioactive compounds like phenolic acids and iridoids with beneficial activity in insulin resistance and diabetes, and other bioactive compounds in Kigelia Africana presented protective effects for patients with testis cancer against the damages caused by chemotherapy [
8].
Some experiments were performed on the anti-hyperglycemic impacts of Mediterranean foods and plants [
9,
10,
11]. The anti-hyperglycemic effect was analyzed in directive to show effective, safe, and natural components for pharmaceutical applications and food diets [
7]. The saponin compounds and the oil from many plants exhibit an anti-hyperglycemic action that can be used against diabetes and its long-term complications [
12]. No study has been reported yet on the in-vitro and in-vivo anti-hyperglycemic activities of
Argania spinosa (saponins and oil).
A study has proposed that the dietary intake of edible oils, for example, olive oil, containing phenolic compounds, can decrease chronic inflammatory disease progress [
7].
Another work, about the structure of some extra virgin oils formed by cold pressing (macadamia, pequi, avocado, palm, coconut, and olive), suggested important levels of natural antioxidants marked some of the considered oils which could be categorized by a better product safety, and by good health properties [
13]. The beneficial effects of extra virgin olive oil (EVOO) on human health are related to its high nutritional value, and its distinctive composition in fatty acids and minor elements, like phytosterols and squalene, polyphenols, aldehydes, pigments, minerals, and vitamins [
14].
Different biological properties generally recognized by traditional medicine have obtained several scientific endorsements. This is particularly the situation for the traditional utilization of almond oil to reduce hypertension and blood cholesterol level [
15,
16,
17], to cure skin diseases like chicken pox pustules and juvenile acne, or for the more beneficial activities of saponin derivatives especially their anti-microbial, and lipolytic properties [
18]. The antioxidant properties of saponins from A. spinosa and their capability to potentate the antioxidant activity of vitamin E were also demonstrated [
19].
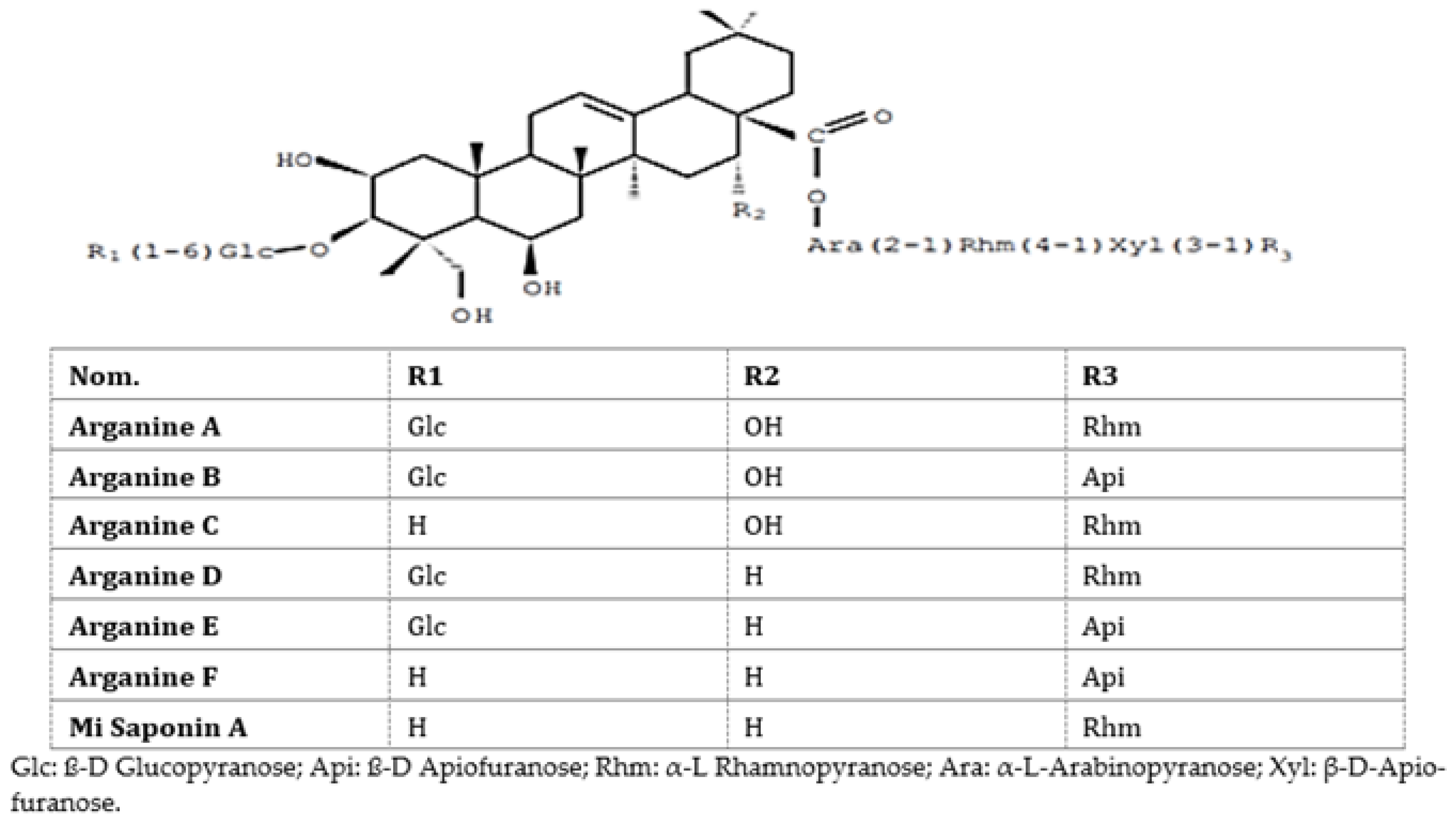
Earlier, our team evaluated the toxicity and pharmacological activities of saponins of A. spinosa cakes in mice and rats [
20]. Argan cake saponins (
Figure 1) were found not toxic orally (lethal dose; DL 1300 mg/kg per os) and showed at 50 mg/kg per os a peripheric analgesic action equivalent to acetyl salicylic acid (200 mg/kg per os). A total safety was acquired with 500 mg/kg per os. Anti-inflammatory experiments were performed in vivo utilizing oedema due to carrageenan or experimental trauma in mice. There was a reduction in the foot inflammation at 10 mg/kg per os. At doses of 50 to 100 mg/kg per os, the anti-inflammatory action was comparable to that of indomethacin at 10 to 20 mg/kg per os. The chemical structures of
Argania spinosa saponins are presented in
Figure 1.
Many chemical analyses discovered that Argan oil is principally well stable in relation to its fatty acid composition [
22,
23,
24,
25].
We consequently studied the anti-hyperglycemic effect of Argan seeds by researching the actions of saponin extracts using α-glucosidase and α-amylase assays as well as an in-vivo model of alloxan-induced diabetic mice. In particular, we evaluated the ability of Argan extracts to rise the inhibitory properties on digestive enzymes (α-amylase and α-glucosidase). The saponin extracts had an activity with an antidiabetic potential.
The specific chemical profile of the Argan fruit extracts, namely cake and Argan oil, could be the reason of a possible anti-hyperglycemic action. The chemical composition and bioactive molecules were discussed. This experiment presents and discusses the first study about the in-vitro and in-vivo antidiabetic potential of Argania spinosa saponins cake extracts and oil.
4. Discussion
The regular consumption of Argan oil could protect the human body from cancer and heart diseases [
46]. The quality and purity of the hand pressed Argan oil were tested and investigated measuring 35 chemical parameters (
Table 2).
The Argan oil has a specific chemical composition, which contributes to its uniqueness and its therapeutic effects. Its sensory profile and chemical composition lead to the classification of it in different Argan oil categories, including extra virgin, virgin, and lampante oil as described in the Moroccan Official Guidelines [
47]. The oil category and freshness is certified based on parameters that include index of acidity (0.3%), peroxide value (PV, 2.3 meq O
2/kg), and UV absorbances coefficients K232 (1.38) and K270 (0.27). From those parameter values, the applied oil is classified as extra virgin [
47].
The fatty acids, sterols, and tocopherol contents of the oil are within the extra virgin category limits [
47]. Previous studies conducted on Argan oil determined some factors that could influence its chemical composition, such as processing and extraction practices [
48], roasting processes [
49], ripeness and/or post-harvests of the Argan fruits [
50] and geographical origin [
24]. The extraction process of Argan oil (mechanical or traditional pressing) significantly influences the total polyphenol contents [
7]. In addition, Argan oil shows the highest total polyphenol content in comparison to other vegetable oils [
51]. Hand pressed Argan oil was reported to have a higher polyphenol content than mechanically pressed [
7]. Moreover, the phenolic profile of the individual compounds varied significantly between both oil extractions (mechanical vs. hand processed) [
40,
52,
53,
54].
The hand pressed Argan oil showed a good and balanced chemical profile which needs more advanced investigations.
Numerous plant-resulting products are rich in bioactive polyphenols, of which some present a powerful anti-hyperglycemic property or health-promoting properties [
7].
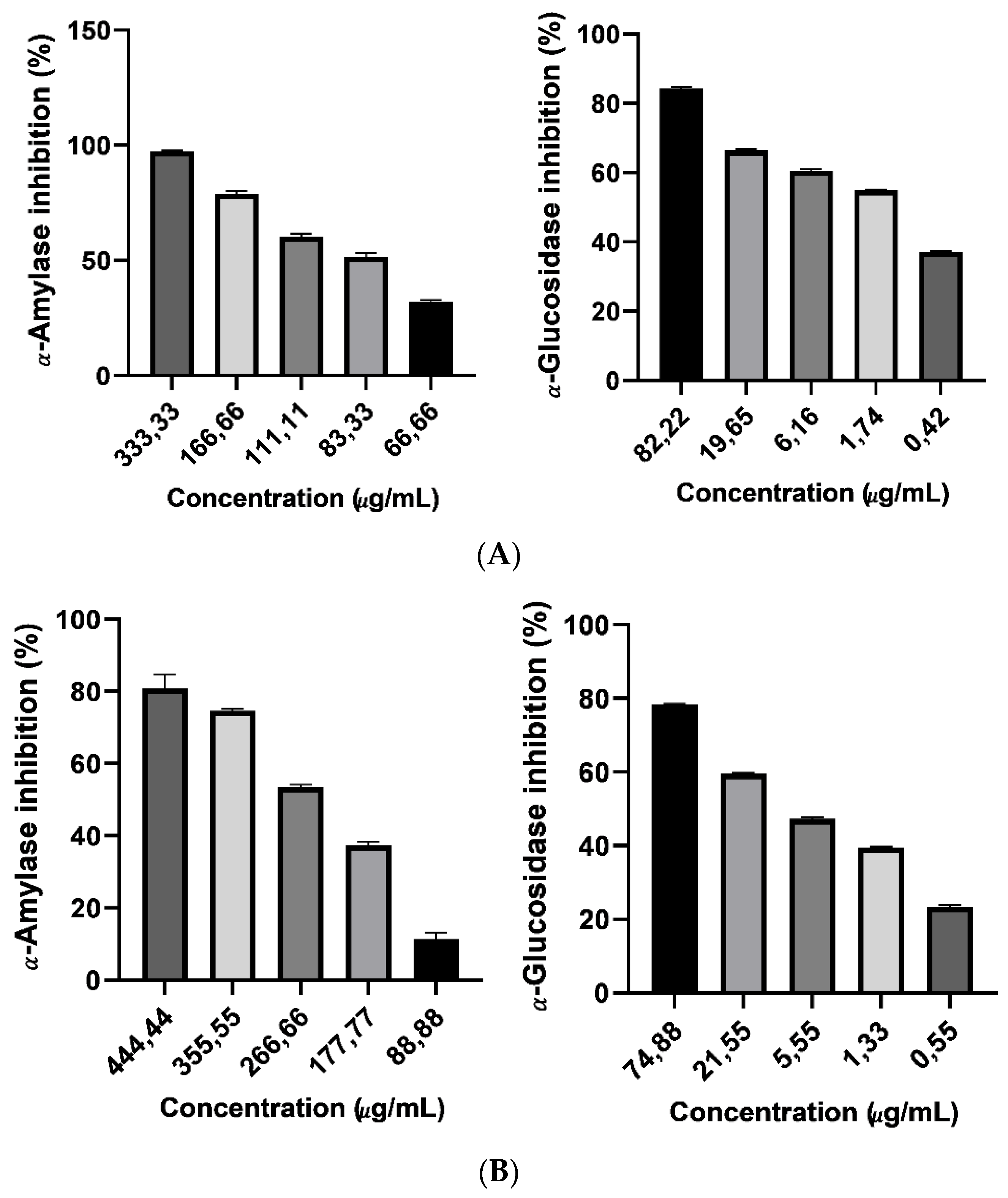
This study informs about the inhibitory kinetics of the Argan saponin cake extract and Argan oil anti-key enzymes (α-amylase and α-glucosidas) associated to hyperglycemia. Compared to the positive control, the in-vitro test showed a modest inhibitory activity. Considering the safe profile of Argan saponin cake extract, it can be of importance to consider an extract formulation for inhibition of (α-amylase and α-glucosidas) key enzymes in the small bowel. Such extracts may show an anti-hyperglycemic activity and be utilized as a capsulated formulation. The existence of various compounds in the extract, e.g., Arganin A, B, C, D, E, and F and Mi-Saponin A [
20], results in a varied form of inhibition. In therapeutic strategies, many bioactive compounds together could be much more active than the individual compounds.
Inducing diabetes in an animal model by alloxan provides knowledge regarding the pathologic mechanism of diabetes, and is also used to screen treatments for diabetes and diabetes-associated problems. In this study, the potential hypoglycemic activities of Argan saponin cake extract and Argan oil treatments were studied using alloxan-induced diabetic mice.
In reality, the interaction between deficiencies in insulin emission and insulin effect give the heterogeneous character of type 2 diabetes. Many body systems are damaged because of the insufficiency resulting from improved concentrations of blood glucose [
54]. Thus, for good diabetes management, there is a requirement to control postprandial blood glucose. The inhibition of key digestives is one of the approaches for glucose management [
55], which gives a considerable decrease of the post-prandial blood glucose [
56]. Some studies examined natural nutritional sources as choices with negligible side impacts and minimal therapy fees, despite the availability of glucosidase inhibitors [
57]. Regardless of the efficacy of acarbose as an anti-hyperglycemic treatment with the inhibitory effect of glucosidase, food choices are required because of the side effect of acarbose [
58]. Many studies about functional foods have been conducted [
59]. A research paper described the amylase-inhibitory activities of pulp, leaf, and seed extracts of two Argan varieties [
60].Yet, no previous study has revealed the kinetics and concentrations of this inhibition. The reported studies did not particularly examine the Argan cake saponin extract, they only focused on entire sets of phenolic compounds. The Argan anti-enzymatic activity is possibly partly associated to its cake saponin content. As we approved a nutritional therapy with (analgesic, antiradical, anti-inflammatory, and antidiabetic) bioactivities [
23], the safe Argan cake saponin extracts were selected for in-vitro and in-vivo activity studies. The capacity to decrease the post-prandial rise of blood glucose levels was demonstrated by the inhibitory activity of the extract and Argan oil against the enzymes α-amylase and α-glucosidase. Our conclusion is in accord with previous articles that confirmed that some extracts of bioactive medicinal plants have more extra powerful α-glucosidase inhibitory activities than potent synthetic inhibitors [
34,
61]. Saponin compounds were reported to exhibit a glucosidase and amylase inhibitory effect [
62,
63,
64,
65]. The findings are also linked with a report concerning the insulin-sensitizing activities of
Argania spinosa seed extracts that showed an insulin-sensitizing activity in the saponin-rich press cake fractions, and this gives support to the traditional utilization of Argan almonds against T2DM [
66].
Recently, we have reported the in-vivo anti-inflammatory activity and the bioactive compounds’ profile of the polyphenolic extract from edible Argan oil acquired by two extraction techniques [
7], who have completed previous studies, i.e., on the in-vivo anti-inflammatory activity of argan oil [
27], the in-vivo acute and chronic toxicity of saponins from
Argania spinosa [
20], and the in-vivo anti-inflammatory and analgesic actions of
Argania spinosa saponins [
26].
Alloxan at 150 mg/kg damages the pancreatic β-cells. More quantities of glucose in the blood result of the insufficient secretion of insulin [
42]. The hypoglycemic activities of Argan oil, 3 mL/kg per os, was shown to have the ability to increase the hepatic glycogen levels in diabetic hypertensive rats [
67].
Moreover, Argan oil considerably decreased the quantity of absorbed glucose in a perfused jejunum segment relative to controls rats [
68].
Another study about insulin-sensitizing seed extracts of
Argania spinosa demonstrated that a saponin cake subfraction enhanced insulin-induced protein kinase B activation [
66].
Previous work demonstrated an anti-inflammatory effect, like that of indomethacin that occurred within one month in animals treated by 100 mg /kg per os of Argan cake saponins [
20], and it was reported that the hypoglycemic effect might be associated to improve transportation of the glucose into adipose, which is stimulated via the phosphatidyl-inositol-3-kinase pathway [
66]. Manifestly, many papers described the hypoglycemic effects of the triterpenic type saponins. Matsuda and Yoshikawa [
69] have described that with the presence of olean-12-ene 3 and 28-acylated bidesmoside have been the composition requirements for hypoglycemic action. There is a reduction of the transmission of glucose from the stomach to the small intestine by these saponins [
70,
71], a reduction of the glucose transfer at the brush border of the intestinal lining [
72,
73], and a decrease of serum glucose levels in glucose-loaded mice.
The reduction of the body weight in diabetic mics was attached to the loss in muscle mass [
41]. The muscle weight and the kidneys’ rejection capability determined the blood creatinine concentration, but the rise of mice blood creatinine may be explained by the loss of mass detected in untreated animals [
35,
63]. Alternatively, for identifying toxic activities associated to therapy with compounds in mice, the concentration of creatinine is regularly judged to be a clinical factor [
74]. The diabetic animals cured with Argan cake saponins and Argan oil were observed for the period of four weeks, and a considerable decrease in creatinine concentration was confirmed. Hence, two suggestions can be possible: Argan cake saponins and Argan oil reduced creatine catabolism and phosphocreatine catabolism in animal muscles or increased renal variations in diabetic animals.
Then again, the evolution in urinary quantity, food and water intake in diabetic mice is similar to the polyphagia and polydipsia detected in diabetic patients [
75]. Argan therapy reduced diabetic signs. After a therapy phase, the Argan saponin cake capacity to inhibit key enzymes could be responsible for the reduction of glucose levels in serum. Argan cake saponin therapy decreased the total cholesterol and serum TG in an alloxan-induced diabetic animal. As a result, the regulation of blood lipid abnormalities and the decrease of probability of atherosclerosis can be produced by Argan intake therapy. Accordingly, for better liver function, the ALAT and ASAT are responsible markers [
76]. The progress in ALAT and ASAT actions in plasma, in the alloxan model of diabetes, shows the liver necrosis and the alloxan hepatotoxic action [
77]. Argan cake saponins and Argan oil therapies demonstrated their hepatoprotective impact, and decreased theses enzymes concentrations in plasma associated to the DC and therefore avoided the liver destruction.
Metformin decreases glucose creation via the suppression of gluconeogenesis in the liver. Furthermore, it enhances the insulin control by endogenous glucose production and reduces the intestinal glucose absorption to a less significant level.
In this study, the safety of pancreatic β-cells seemed more related with the bioactive compounds from the Argan saponin cake than from Argan oil. In alloxan-induced diabetic mice, Argan saponin cake therapy reduced plasma glucose concentration, polydipsia, hyperphagia, and body mass. Argan saponin cake extract and Argan oil intakes also showed an improvement of glucose tolerance. Furthermore, Argan cake intake avoided hypercholesterolemia and atherosclerosis in the diabetic animals.
In summary, the results encourage the theory that Argan cake saponins could successfully improve the symptoms of diabetes and regulate glucose metabolism. As a consequence, Argan cake intake is good for both patients with T2DM and healthy subjects.
This study confirmed the natural nutritional benefit of Argan oil and Argan cake saponins as a food and investigated the effect of Argan on selected digestive enzymes; the anti-enzymatic activity is possibly partly associated to its cake saponin content. We approved a nutritional therapy with anti-hyperglycemic bioactivity.
Focusing on their pharmacological and agri-food valorization, the present research sought and compared the inhibition of digestive enzymes of Argan cake saponins and traditional Argan oil. However, various individual elements of Argania spinosa seeds might be reliable to the consequences noted in this experiment, and antagonism or synergy between components is also probable. Thus, we can consider the possibility of introducing argan products in the food compliment formation.