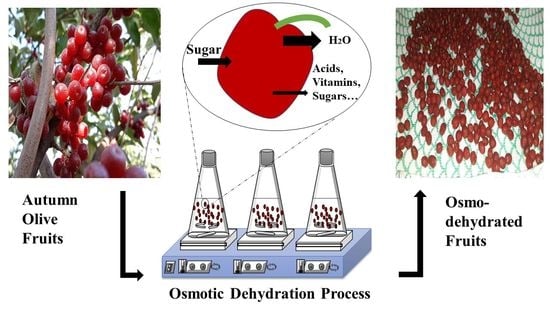
Optimization of Osmotic Dehydration of Autumn Olive Berries Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Chemical
2.2. Physico-Chemical Analyses
2.3. Water Loss, Solute Gain, and Weight Reduction
2.4. Osmotic Dehydration (OD)
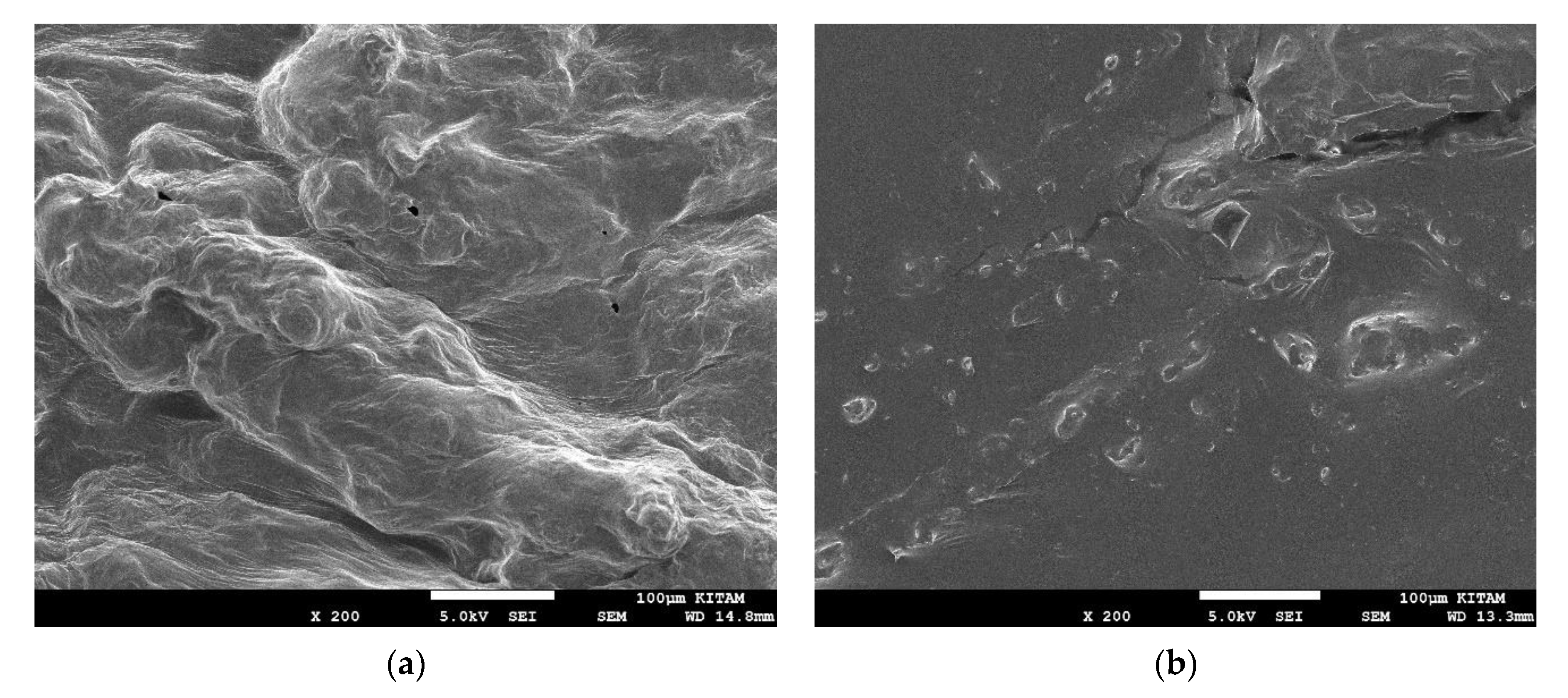
2.5. Scanning Electron Microscope (SEM)
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Characteristic and Antioxidant Activity of Autumn Berries
| Fruit Properties (Present Studies) Mean ± S. D | Fruits Properties (Previous Studies) | Reference | |
|---|---|---|---|
| Color parameters: L* | 31.53 ± 1.20 a | NF | - |
| a* | 6.10 ± 1.23 a | NF | - |
| b* | 4.43 ± 0.44 a | NF | - |
| Number of fruits/Weight (50 g of berries) | 259 ± 6 b | 17.54–18.40 g/100 berries | [6] |
| 16.41–22.80 g/100 berries | [27] | ||
| Length mm | 6.19 ± 0.64 c | 6.99 ± 0.13 mm | [28] |
| 7.1–8.7 mm | [27] | ||
| Width mm | 6.40 ± 0.72 c | 3.78–4.28 mm | [6] |
| 4.8–6.7 mm | [27] | ||
| Density (ρ) g/cm3 | 1.038 ± 0.010 d | NF | - |
| Water activity (aw) | 0.955 ± 0.007 d | NF | - |
| Moisture content (M.C%. FW) | 77.24 ± 0.41 e | 78.49–81.71% | [5] |
| 71.4 ± 1.8% | [6] | ||
| Soluble solids content (SSC: °Brix) | 14.80 ± 0.27 f | 12.3–15.4 | [29] |
| 9.03–11.76 | [6] | ||
| 11–17 | [30] | ||
| pH | 3.07 ± 0.03 f | 3.1–4.0 | [29] |
| 3.30–3.90 | [6] | ||
| 4.5 ± 0.1 | [5] | ||
| Titratable acidity T.A% (malic Acid%) | 1.20 ± 0.05 g | 0.79–1.29 (TA%) FW | [31] |
| 2.02–6.88 (Malic. A) mg/100 g FW | [31] | ||
| 26.59 ± 1.63 mg/g FW (total acids) | [28] | ||
| 2.20–2.94% FW | [6] | ||
| 3.1 ± 0.1% FW | [5] | ||
| Lycopene mg/100 g FW | 20.47 ± 3.18 g | 33.6–55.3 mg/100 g FW | [29] |
| 19.9 ± 3.2 (T. Carotenoids) mg/g DW | [5] | ||
| 30.58 to 46.23 mg/100 g FW | [31] | ||
| 30–50 mg/100 g FW | [30] | ||
| Vitamin C. mg A.A Eq/100 g | 7.17 ± 2.27 g | 7.65–10.10 mg/100 g FW | [6] |
| 27.8 ± 1.8 mg/100 g FW | [5] | ||
| 13.8–16.9 mg/100 g FW | [27] | ||
| Total phenols. mg G.A. Eq/100 g FW | 287 ± 40 g | 1399–1833 mg/kg FW | [29] |
| 5.56 mg/g DW | [32] | ||
| 168.9–258.1 mg G.A. Eq/100 g FW | [19] | ||
| 23.3 ± 2.0 mg/g DW | [5] | ||
| 16.3–20.0 mg G.A. Eq/g FW | [33] | ||
| 1700 mg Chlorogenic. A. Eq/kg FW | [30] | ||
| 190–275 mg GA Eq/100 g FW | [34] | ||
| Flavonoids mg-(-) Ep. Eq/100 g FW | 25.26 ± 3.78 g | 3.6 ± 0.1 mg/g DW | [5] |
| 1.5–3.8 mg quercetin. Eq/g FW | [33] | ||
| DPPH. mMol Trolox. Eq/100 g FW | 494 ± 48 g | IC50 = 0.13 ± 0.01 mmol of Trolox.Eq/g DW | [32] |
| IC50 = 2.42–5.37 mg of FW | [35] | ||
| IC50 = 45.40–49.00 µg/mL | [33] | ||
| FRAP. mMol. Trolox. Eq/100 g FW | 718 ± 43 g | NF | - |
| Total anthocyanins. mg cyanidin. Eq/100 g FW | ND | ND | [32] |
3.2. Optimization Process
3.2.1. Analysis of the Model
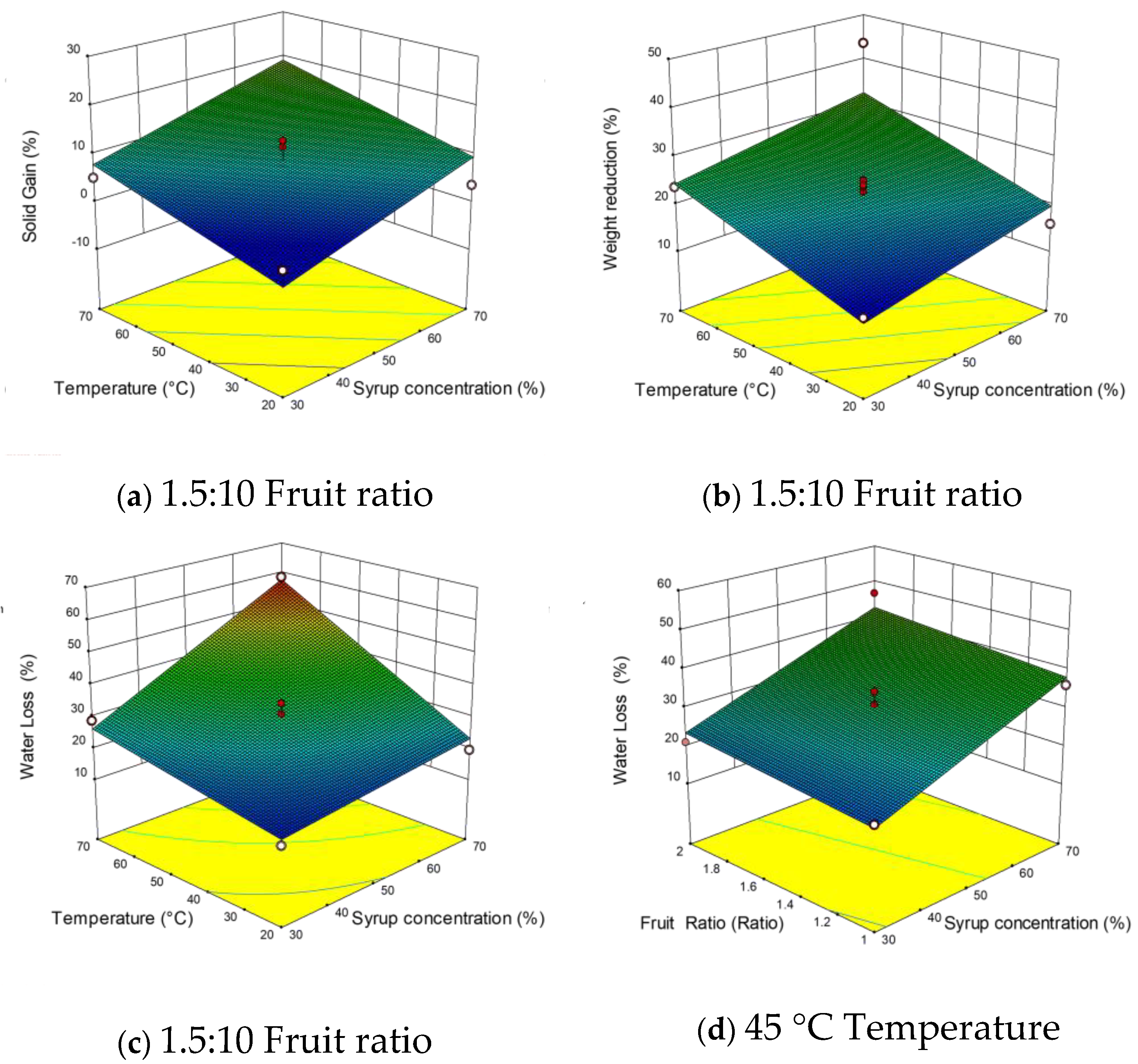
3.2.2. Effects of Independent Variables on Mass Transfer Responses (WL, WR, and SG)
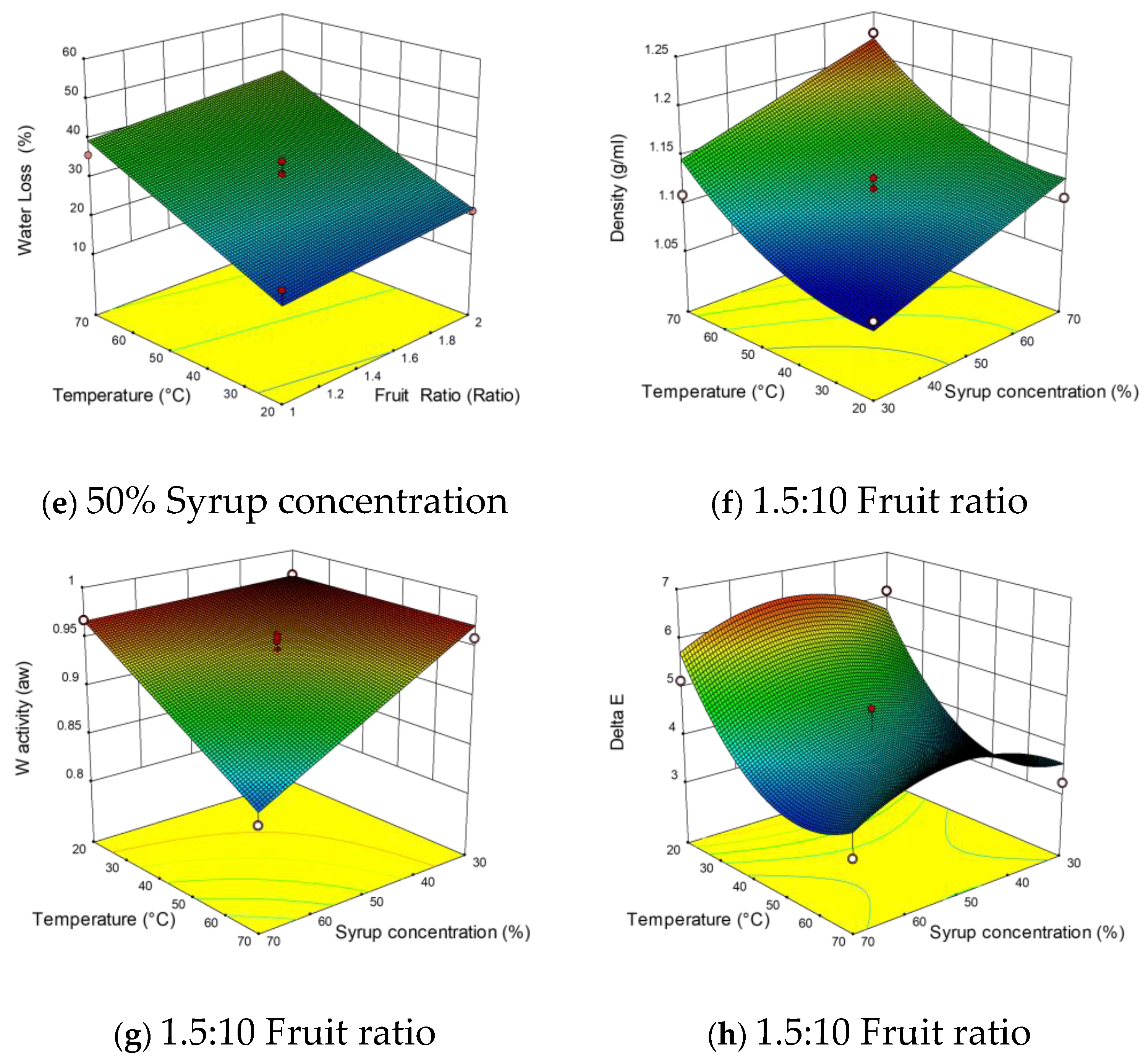
3.2.3. Effects of Independent Variables on Density
3.2.4. Effects of Independent Variables on ΔE
3.2.5. Effects of Independent Variables on Water Activity
3.2.6. Multi-Response of Optimization
3.3. Effects of Drying on Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Aldawoud, T.M.S.; Rizou, M.; Rowan, N.; Ibrahim, S. Food Ingredients and Active Compounds against the Coronavirus Disease (COVID-19) Pandemic: A Comprehensive Review. Foods 2020, 9, 1701. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Fordham, I.M.; Clevidence, B.A.; Wiley, E.R. Fruit of autumn olive: A rich source of lycopene. HortScience 2001, 36, 1136–1137. [Google Scholar] [CrossRef]
- Khattak, K.F. Free radical scavenging activity, phytochemical composition and nutrient analysis of Elaeagnus umbellata berry. J. Med Plants Res. 2012, 6, 5196–5203. [Google Scholar] [CrossRef]
- Hussain, I. Physiochemical and sensory Characteristics of Elaeagnus umbellata (Thunb) fruit from Rawalakot (Azad Kashmir) Pakistan. Afr. J. Food Sci. 2011, 2, 151–156. [Google Scholar]
- Fernandes, F.A.N.; Rodrigues, S.; Gaspareto, O.C.P.; Oliveira, E.L. Optimization of osmotic dehydration of papaya followed by air-drying. Food Res. Int. 2006, 39, 492–498. [Google Scholar] [CrossRef]
- Sagar, V.R. Recent advances in drying and dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2010, 47, 15–26. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Niranjan, K.; Knorr, D. Recent developments in osmotic dehydration: Methods to enhance mass transfer. Trends Food Sci. Technol. 2002, 13, 48–59. [Google Scholar] [CrossRef]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Lerici, C.R.; Pinnavaia, G.; Dalla, M.; Bartolucci, L. Osmotic dehydration of fruit: Influence of osmotic agents on drying behavior and product quality. J. Food Sci. 1985, 50, 1217–1219. [Google Scholar] [CrossRef]
- Torreggiani, D. Osmotic dehydration in fruit and vegetable processing. Food Res. Int. 1993, 26, 59–68. [Google Scholar] [CrossRef]
- Azuara, E.; Garciab, H.S.; Beristain, C.I. Effect of the centrifugal force on osmotic dehydration of potatoes and apples. Food Res. Int. 1996, 29, 195–199. [Google Scholar] [CrossRef]
- Bozkir, H.; Rayman, A.; Serdar, E.; Metin, G.; Baysal, T. Ultrasonics—Sonochemistry Influence of ultrasound and osmotic dehydration pre-treatments on drying and quality properties of persimmon fruit. Ultrason. Sonochem. 2019, 54, 135–141. [Google Scholar] [CrossRef]
- Stojanovic, J.; Silva, J.L. Influence of osmoconcentration, continuous high-frequency ultrasound and dehydration on properties and microstructure of rabbiteye blueberries. Dry. Technol. 2006, 24, 165–171. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, M.; Mujumdar, A.S.; Du, W.H.; Sun, J.C. Optimization of Osmotic Dehydration of Kiwifruit Optimization of Osmotic Dehydration of Kiwifruit. Dry. Technol. 2006, 24, 89–94. [Google Scholar] [CrossRef]
- Sadler, G.D.; Murphy, P.A. pH and titratable acidity. In Food Analysis; Springer: Boston, MA, USA, 2010; pp. 219–238. [Google Scholar]
- Fish, W.W.; Perkins-veazie, P.; Collins, J.K. A Quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I. Optimization and stabilization of the antioxidant properties from Alkanet (Alkanna tinctoria) with natural deep eutectic solvents. Arab. J. Chem. 2020, 13, 6437–6450. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I.; Aldawoud, T.M.S.; Galanakis, C.M. Recovery and stabilization of anthocyanins and phenolic antioxidants of roselle (Hibiscus sabdariffa L.) with hydrophilic deep eutectic solvents. Molecules 2020, 25, 3715. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 76, 70–76. [Google Scholar] [CrossRef]
- Yang, C.S.T.; Atallah, W.A. Effect of four drying methods on the quality of intermediate moisture lowbush blueberries dehydration time determination. J. Food Sci. 1985, 50, 1233–1237. [Google Scholar] [CrossRef]
- Jain, S.K.; Verma, R.C.; Murdia, L.K.; Jain, H.K.; Sharma, G.P. Optimization of process parameters for osmotic dehydration of papaya cubes. J. Food Sci. Technol. 2011, 48, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.D.; Sabir, S.M.; Zubair, M. Ecotypes diversity in autumn olive (Elaeagnus umbellata Thun): A single plant with multiple micronutrient genes. Chem. Ecol. 2006, 22, 509–521. [Google Scholar] [CrossRef]
- Wu, M.C.; Hu, H.T.; Yang, L.; Yang, L. Proteomic Analysis of up-accumulated proteins associated with fruit quality during autumn olive (Elaeagnus umbellata) fruit ripening. J. Agric. Food Chem. 2011, 59, 577–583. [Google Scholar] [CrossRef]
- Black, B.L.; Fordham, I.M.; Perkins-Veazie, P. Autumn berry (Elaeagnus umbellata): A potential cash crop. J. Am. Pomol. Soc. 2005, 59, 125–134. [Google Scholar]
- Pei, R.; Yu, M.; Bruno, R.; Bolling, B.W. Phenolic and tocopherol content of autumn olive (Elaeagnus umbellate) berries. J. Funct. Foods 2015, 16, 305–314. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bowman, L.; Ding, M. Variations in free radical scavenging capacity and antiproliferative activity among different genotypes of autumn olive (Elaeagnus umbellata). Planta Med. 2007, 53, 468–477. [Google Scholar] [CrossRef]
- Tiroutchelvame, D.; Sivakumar, V.; Maran, J.P. Mass transfer kinetics during osmotic dehydration of amla (Emblica officinalis L.) cubes in sugar solution. Chem. Ind. Chem. Eng. Q. 2015. [Google Scholar] [CrossRef]
- Ishaq, S.; Rathore, H.A.; Sabir, S.M.; Maroof, M.S. Antioxidant properties of Elaeagnus umbellata berry solvent extracts against lipid peroxidation in mice brain and liver tissues. Food Sci. Biotechnol. 2015, 24, 673–679. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Llorent-Martínez, E.J.; Castilho, P.C. Changes in the phenolic compositions of Elaeagnus umbellata and Sambucus lanceolata after in vitro gastrointestinal digestion and evaluation of their potential anti-diabetic properties. Food Res. Int. 2019, 122, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Fordham, I.M. Differences in chemical composition and antioxidant capacity among different genotypes of Autumn Olive (Elaeagnus umbellata Thunb.). Food Technol. Biotechnol. 2007, 45, 402–409. [Google Scholar]
- Nagarajan, J.; Prasad, N.K.; Ramakrishnan, N.R.; Raghunandan, M.E.; Galanakis, C.M.; Wei, O.C. A facile water-induced complexation of lycopene and pectin from pink guava byproduct: Extraction, characterization and kinetic studies. Food Chem. 2019, 296, 47–55. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and removal of phenolic compounds from olive mill wastewater. J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Mercali, G.D.; Marczak, L.D.F.; Tessaro, I.C.; Noreña, C.P.Z. Osmotic dehydration of bananas (Musa sapientum, shum.) in ternary aqueous solutions of sucrose and sodium chloride. J. Food Process Eng. 2012, 35, 149–165. [Google Scholar] [CrossRef]
- Barragán-Iglesias, J.; Rodríguez-Ramírez, J.; Sablani, S.S.; Méndez-Lagunas, L.L. Texture analysis of dried papaya (Carica papaya L. cv. Maradol) pretreated with calcium and osmotic dehydration. Dry. Technol. 2019, 37, 906–919. [Google Scholar] [CrossRef]
- Lazarides, H.N.; Katsanidis, E. Mass transfer kinetics during osmotic preconcentration aiming at minimal solid uptake. J. Food Eng. 1995, 25, 151–166. [Google Scholar] [CrossRef]
- Khoyi, M.R.; Hesari, J. Osmotic dehydration kinetics of apricot using sucrose solution. J. Food Eng. 2007, 78, 1355–1360. [Google Scholar] [CrossRef]
- Viberg, U.; Freuler, S.; Gekas, V.; Sjiiholm, I. Pre-treatment of strawberries effects and shrinkage. J. Food Eng. 1997, 35, 135–145. [Google Scholar] [CrossRef]
- Mavroudis, N.E. Osmotic dehydration of apples. Shrinkage phenomena and the significance of initial structure on mass lkansfer rates. J. Food Eng. 1998, 38, 101–123. [Google Scholar] [CrossRef]
- Mayor, L.; Moreira, R.; Sereno, A.M. Shrinkage, density, porosity and shape changes during dehydration of pumpkin (Cucurbita pepo L.) fruits. J. Food Eng. 2011, 103, 29–37. [Google Scholar] [CrossRef]
- Alam, M.S.; Amarjit, S.; Sawhney, B.K. Response surface optimization of osmotic dehydration process for aonla slices. J. Food Sci. Technol. 2010, 47, 47–54. [Google Scholar] [CrossRef]
- Grabowski, S.; Mujumdar, A.S.; Ramaswamy, H.S.; Strumillo, C. Osmo-convective drying of grapes. Dry. Technol. 1994, 12, 1211–1219. [Google Scholar] [CrossRef]
- Mandala, I.G.; Anagnostaras, E.F.; Oikonomou, C.K. Influence of osmotic dehydration conditions on apple air-drying kinetics and their quality characteristics. J. Food Sci. 2005, 69, 307–316. [Google Scholar] [CrossRef]
- Eren, I.; Kaymak-Ertekin, F. Optimization of osmotic dehydration of potato using response surface methodology. J. Food Eng. 2007, 79, 344–352. [Google Scholar] [CrossRef]
- Giraldo, G.; Talens, P.; Fito, P.; Chiralt, A. Influence of sucrose solution concentration on kinetics and yield during osmotic dehydration of mango. J. Food Eng. 2003, 58, 33–43. [Google Scholar] [CrossRef]
- Luchese, C.L.; Gurak, P.D.; Marczak, L.D.F. Osmotic dehydration of physalis (Physalis peruviana L.): Evaluation of water loss and sucrose incorporation and the quantification of carotenoids. LWT Food Sci. Technol. 2015, 63, 1128–1136. [Google Scholar] [CrossRef]
| Run | Factors | Responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | SG % | WR % | WL % | ρ g/cm3 | aw | ΔE | |
| 1 | 50 | 45 | 1.5:10 | 5.79 | 25.11 | 30.91 | 1.10 | 0.951 | 3.95 |
| 2 | 70 | 20 | 1.5:10 | 3.67 | 15.83 | 19.50 | 1.11 | 0.967 | 5.14 |
| 3 | 50 | 45 | 1.5:10 | 5.52 | 23.79 | 29.32 | 1.10 | 0.957 | 3.75 |
| 4 | 30 | 45 | 2:10 | 2.04 | 19.02 | 21.06 | 1.09 | 0.967 | 3.76 |
| 5 | 50 | 45 | 1.5:10 | 6.60 | 24.12 | 30.72 | 1.11 | 0.955 | 3.75 |
| 6 | 50 | 45 | 1.5:10 | 12.93 | 21.43 | 34.36 | 1.13 | 0.937 | 4.67 |
| 7 | 70 | 70 | 1.5:10 | 14.85 | 43.62 | 58.48 | 1.23 | 0.838 | 3.13 |
| 8 | 30 | 20 | 1.5:10 | 1.48 | 12.76 | 14.24 | 1.06 | 0.973 | 6.14 |
| 9 | 50 | 20 | 2:10 | 4.75 | 16.66 | 21.41 | 1.09 | 0.962 | 6.38 |
| 10 | 70 | 45 | 1:10 | 9.33 | 26.76 | 36.08 | 1.11 | 0.936 | 3.10 |
| 11 | 30 | 70 | 1.5. 10 | 5.15 | 23.70 | 28.85 | 1.11 | 0.958 | 3.23 |
| 12 | 50 | 70 | 2:10 | 14.58 | 27.60 | 42.18 | 1.21 | 0.918 | 4.18 |
| 13 | 50 | 20 | 1. 10 | 4.44 | 16.54 | 20.98 | 1.10 | 0.961 | 6.65 |
| 14 | 50 | 45 | 1.5:10 | 11.61 | 22.71 | 34.32 | 1.12 | 0.941 | 3.80 |
| 15 | 70 | 45 | 2:10 | 27.02 | 19.74 | 46.77 | 1.19 | 0.875 | 4.24 |
| 16 | 30 | 45 | 1:10 | 1.32 | 18.38 | 19.70 | 1.07 | 0.971 | 4.00 |
| 17 | 50 | 70 | 1:10 | 18.30 | 17.59 | 35.89 | 1.19 | 0.914 | 5.44 |
| SG | WR | WL | ρ | aw | ΔE | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CE | SS | p-Value | CE | SS | p-Value | CE | SS | p-Value | CE | SS | p-Value | CE | SS | p-Value | CE | SS | p-Value | |
| Model | 8.79 | 437.53 | 0.0029 | 22.08 | 450.15 | 0.0022 | 30.87 | 1928.89 | <0.0001 | 1.11 | 0.031 | <0.0001 | 0.94 | 0.018 | <0.0001 | 4.2 | 16.3 | <0.0001 |
| X1 | 5.61 | 251.79 | 0.0059 | 4.01 | 128.75 | 0.0326 | 9.62 | 740.62 | <0.0001 | 0.037 | 0.011 | 0.0004 | −0.032 | 7.95 × 10−3 | <0.0001 | - | - | - |
| X2 | 4.82 | 185.74 | 0.0146 | 6.34 | 321.4 | 0.0022 | 11.16 | 995.81 | <0.0001 | 0.047 | 0.018 | <0.0001 | −0.029 | 6.91 × 10−3 | - | 1.04 | 8.66 | 0.0002 |
| X3 | - | - | - | - | - | - | 2.34 | 43.98 | 0.0496 | - | - | - | - | - | - | - | - | - |
| X1 X2 | 6.09 | 148.48 | 0.0017 | - | 3.31 × 10−3 | 0.0001 | - | - | - | |||||||||
| X1 X3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| X2 X3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| X12 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | −0.7 | 2.08 | 0.0236 |
| X22 | - | - | - | - | - | - | - | - | - | 0.024 | 2.46 × 10−3 | 0.045 | −0.029 | 2.46 × 10−3 | 0.045 | 1.19 | 5.93 | 0.0008 |
| X32 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Residual | 335.04 | 335.04 | 110.65 | 6.49 × 10−3 | 3.08 × 10−3 | 4.11 | ||||||||||||
| Lack of Fit | 285.91 | 0.2156 | 312.54 | 0.0087 | 89.68 | 0.2414 | 5.98 × 10−3 | 0.3775 | 2.78 × 10−3 | 0.0933 | 3.49 | 0.1928 | ||||||
| Total | 772.57 | 770.66 | 2039.54 | 0.038 | 0.021 | 20.41 | ||||||||||||
| R2 | 0.5663 | 0.5841 | 0.9457 | 0.8281 | 0.855 | 0.7988 | ||||||||||||
| Adj-R2 | 0.5044 | 0.5247 | 0.9277 | 0.7884 | 0.8216 | 0.7524 | ||||||||||||
| Pred-R2 | 0.3178 | 0.2747 | 0.8621 | 0.6674 | 0.7374 | 0.637 | ||||||||||||
| CV% | 55.66 | 21.67 | 9.84 | 1.99 | 1.64 | 12.69 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghellam, M.; Zannou, O.; Pashazadeh, H.; Galanakis, C.M.; Aldawoud, T.M.S.; Ibrahim, S.A.; Koca, I. Optimization of Osmotic Dehydration of Autumn Olive Berries Using Response Surface Methodology. Foods 2021, 10, 1075. https://doi.org/10.3390/foods10051075
Ghellam M, Zannou O, Pashazadeh H, Galanakis CM, Aldawoud TMS, Ibrahim SA, Koca I. Optimization of Osmotic Dehydration of Autumn Olive Berries Using Response Surface Methodology. Foods. 2021; 10(5):1075. https://doi.org/10.3390/foods10051075
Chicago/Turabian StyleGhellam, Mohamed, Oscar Zannou, Hojjat Pashazadeh, Charis M. Galanakis, Turki M. S. Aldawoud, Salam A. Ibrahim, and Ilkay Koca. 2021. "Optimization of Osmotic Dehydration of Autumn Olive Berries Using Response Surface Methodology" Foods 10, no. 5: 1075. https://doi.org/10.3390/foods10051075
APA StyleGhellam, M., Zannou, O., Pashazadeh, H., Galanakis, C. M., Aldawoud, T. M. S., Ibrahim, S. A., & Koca, I. (2021). Optimization of Osmotic Dehydration of Autumn Olive Berries Using Response Surface Methodology. Foods, 10(5), 1075. https://doi.org/10.3390/foods10051075