Effects of the Reactive Moiety of Phenolipids on Their Antioxidant Efficiency in Model Emulsified Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Phenolipids
2.2.1. Synthesis of Caffeates and Hydroxycinnamic Esters
2.2.2. Synthesis of Tyrosol and Hydroxytyrosol Esters
2.2.3. Synthesis of Dihydrocaffeates
2.3. Preparation of Emulsions
2.4. Reactivity of the Different Phenols
2.4.1. DPPH Radical Scavenging Efficiency
2.4.2. Cyclic Voltammetry
2.4.3. Ferric-Reducing Antioxidant Potential (FRAP) Assay
2.5. Determining the Partition Constants and Distribution of Polyphenols in Intact Olive Oil-in-Water Emulsions: Application of the Pseudophase Kinetic Model
2.6. Antioxidant Efficiency of Phenolipids in Emulsions
2.7. Statistical Analysis.
3. Results and Discussion
3.1. Reactivity of Phenolic Compounds
3.2. Determining the Partition Constants and Distribution of Polyphenols in Intact Olive Oil-in-Water Emulsions: Application of the Pseudophase Kinetic Model
3.3. Antioxidant Activity of Phenolic Compounds in Olive Oil-in-Water Emulsions
3.4. Pearson Correlation and Stepwise Linear Regression Analysis (SLRA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Lipid Oxidation; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 085709792X. [Google Scholar]
- Waraho, T.; McClements, D.J.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Akoh, C.C. Food Lipids: Chemistry, Nutrition and Biotechnology; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1498744877. [Google Scholar]
- Ferreira, I.; Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Modulating the interfacial concentration of gallates to improve the oxidative stability of fish oil-in-water emulsions. Food Res. Int. 2018, 112. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. A direct correlation between the antioxidant efficiencies of caffeic acid and its alkyl esters and their concentrations in the interfacial region of olive oil emulsions. the pseudophase model interpretation of the “cut-off” effect. Food Chem. 2015, 175, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Chain Length Affects Antioxidant Properties of Chlorogenate Esters in Emulsion: The Cutoff Theory Behind the Polar Paradox. J. Agric. Food Chem. 2009, 57, 11335–11342. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.-D.M.; Durand, E.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; Jacobsen, C. Antioxidant Properties and Efficacies of Synthesized Alkyl Caffeates, Ferulates, and Coumarates. J. Agric. Food Chem. 2014, 62, 12553–12562. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Relationship between Hydrophobicity and Antioxidant Ability of “Phenolipids” in Emulsion: A Parabolic Effect of the Chain Length of Rosmarinate Esters. J. Agric. Food Chem. 2010, 58, 2869–2876. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Physical evidence that the variations in the efficiency of homologous series of antioxidants in emulsions are a result of differences in their distribution. J. Sci. Food Agric. 2017, 97, 564–571. [Google Scholar] [CrossRef]
- Almeida, J.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. Interfacial Concentrations of Hydroxytyrosol and Its Lipophilic Esters in Intact Olive Oil-in-Water Emulsions: Effects of Antioxidant Hydrophobicity, Surfactant Concentration, and the Oil-to-Water Ratio on the Oxidative Stability of the Emulsions. J. Agric. Food Chem. 2016, 64, 5274–5283. [Google Scholar] [CrossRef]
- Meireles, M.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Monteiro, L.S. Control of antioxidant efficiency of chlorogenates in emulsions: Modulation of antioxidant interfacial concentrations. J. Sci. Food Agric. 2019, 99, 3917–3925. [Google Scholar] [CrossRef]
- Silva, R.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Partitioning and antioxidative effect of protocatechuates in soybean oil emulsions: Relevance of emulsifier concentration. Eur. J. Lipid Sci. Technol. 2017, 119, 1600274. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Costa, M.; Bravo-Díaz, C.; Paiva-Martins, F. Distribution and antioxidant efficiency of resveratrol in stripped corn oil emulsions. Antioxidants 2014, 3, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Bravo-Díaz, C.; Costa, M.; Paiva-Martins, F. Distribution of catechol in emulsions. J. Phys. Org. Chem. 2014, 27, 290. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo Díaz, C.; Paiva Martins, F.; Romsted, L.S. Maxima in antioxidant distributions and efficiencies with increasing hydrophobicity of gallic acid and its alkyl esters. The pseudophase model interpretation of the ″Cut-off effect. J. Agric. Food Chem. 2013, 61, 6533. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Bravo-Díaz, C.; Vicente, A.A.; Monteiro, L.S.; Paiva-Martins, F. Influence of AO chain length, droplet size and oil to water ratio on the distribution and on the activity of gallates in fish oil-in-water emulsified systems: Emulsion and nanoemulsion comparison. Food Chem. 2020, 310, 125716. [Google Scholar] [CrossRef]
- Lisete-Torres, P.; Losada-Barreiro, S.; Albuquerque, H.; Sánchez-Paz, V.; Paiva-Martins, F.; Bravo-Díaz, C. Distribution of Hydroxytyrosol and Hydroxytyrosol Acetate in Olive Oil Emulsions and Their Antioxidant Efficiency. J. Agric. Food Chem. 2012, 60, 7318–7325. [Google Scholar] [CrossRef] [PubMed]
- Gunaseelan, K.; Romsted, L.S.; Gallego, M.J.P.; Gonzalez-Romero, E.; Bravo-Diaz, C. Determining α-Tocopherol Distributions between the Oil, Water, and Interfacial Regions of Macroemulsions: Novel Applications of Electroanalytical Chemistry and the Pseudophase Kinetic Model. Adv. Colloid Interface Sci. 2006, 123, 303. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 15–27. ISBN 0076-6879. [Google Scholar]
- Bravo-Díaz, C.; Romsted, L.S.; Liu, C.; Losada-Barreiro, S.; Pastoriza-Gallego, M.J.; Gao, X.; Gu, Q.; Krishnan, G.; Sánchez-Paz, V.; Zhang, Y.; et al. To Model Chemical Reactivity in Heterogeneous Emulsions, Think Homogeneous Microemulsions. Langmuir 2015, 31, 8961–8979. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Bravo-Díaz, C.; Monteiro, L.S.; Paiva-Martins, F. Interfacial Concentrations of Hydroxytyrosol Derivatives in Fish Oil-in-Water Emulsions and Nanoemulsions and Its Influence on Their Lipid Oxidation: Droplet Size Effects. Foods 2020, 9, 1897. [Google Scholar] [CrossRef]
- Yildizdas, H.Y.; Poyraz, B.; Atli, G.; Sertdemir, Y.; Mert, K.; Ozlu, F.; Satar, M. Effects of two different lipid emulsions on antioxidant status, lipid peroxidation and parenteral nutrition-related cholestasis in premature babies, a randomized-controlled study. Pediatr. Neonatol. 2019, 60, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Mollica, F.; Lucarini, M.; Passerini, C.; Carati, C.; Pavoni, S.; Bonoldi, L.; Amorati, R. Effect of Antioxidants on High-Temperature Stability of Renewable Bio-Oils Revealed by an Innovative Method for the Determination of Kinetic Parameters of Oxidative Reactions. Antioxidants 2020, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Silva, F.A.M.; Borges, F.; Guimarães, C.; Lima, J.L.F.C.; Matos, C.; Reis, S. Phenolic Acids and Derivatives: Studies on the Relationship among Structure, Radical Scavenging Activity, and Physicochemical Parameters. J. Agric. Food Chem. 2000, 48, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Siquet, C.; Paiva-Martins, F.; Lima, J.L.F.C.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy-and trihydroxyphenolic acids-A structure–activity relationship study. Free Radic. Res. 2006, 40, 433–442. [Google Scholar] [CrossRef]
- Galato, D.; Ckless, K.; Susin, M.F.; Giacomelli, C.; Ribeiro-do-Valle, R.M.; Spinelli, A. Antioxidant capacity of phenolic and related compounds: Correlation among electrochemical, visible spectroscopy methods and structure–antioxidant activity. Redox Rep. 2001, 6, 243–250. [Google Scholar] [CrossRef]
- Blasco, A.J.; González Crevillén, A.; González, M.C.; Escarpa, A. Direct electrochemical sensing and detection of natural antioxidants and antioxidant capacity in vitro systems. Electroanalysis 2007, 19, 2275–2286. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Vinqvist, M.R. Phenols as Antioxidants. In The Chemistry of Phenols; Rappoport, Z., Ed.; Patai Series: The Chemistry of Functional Groups; John Wiley & Sons, Ltd.: Chichester, UK, 2003; pp. 839–908. [Google Scholar]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Dangles, O.; Dufour, C.; Tonnelé, C.; Trouillas, P. The Physical Chemistry of Polyphenols. Recent Adv. Polyphen. Res. 2017, 1–35. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Rene, A.; Abasq, M.-L.; Hauchard, D.; Hapiot, P. How do phenolic compounds react toward superoxide ion? A simple electrochemical method for evaluating antioxidant capacity. Anal. Chem. 2010, 82, 8703–8710. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Siquet, C.; Orrù, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A. Lipophilic phenolic antioxidants: Correlation between antioxidant profile, partition coefficients and redox properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Pourali, S.; Saso, L.; Borges, F.; Firuzi, O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-Transfer Reaction of Cinnamic Acids and Their Methyl Esters with the DPPH• Radical in Alcoholic Solutions. J. Org. Chem. 2004, 69, 2309. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Tsimidou, M.Z.; Vafiadis, A.P.; Bakalbassis, E.G. Structure−DPPH• scavenging activity relationships: Parallel study of catechol and guaiacol acid derivatives. J. Agric. Food Chem. 2006, 54, 5763–5768. [Google Scholar] [CrossRef]
- Dunford, H.B. Free radicals in iron-containing systems. Free Radic. Biol. Med. 1987, 3, 405–421. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Gordon, M.H. Effects of pH and ferric ions on the antioxidant activity of olive polyphenols in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2002, 79, 571–576. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Gordon, M.H. Interactions of Ferric Ions with Olive Oil Phenolic Compounds. J. Agric. Food Chem. 2005, 53, 2704–2709. [Google Scholar] [CrossRef]
- Trujillo, M.; Gallardo, E.; Madrona, A.; Bravo, L.; Sarria, B.; Gonzalez-Correa, J.A.; Mateos, R.; Espartero, J.L. Synthesis and antioxidant activity of nitrohydroxytyrosol and its acyl derivatives. J. Agric. Food Chem. 2014, 62, 10297–10303. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Optimizing the efficiency of antioxidants in emulsions by lipophilization: Tuning interfacial concentrations. RSC Adv. 2016, 6, 91483–91493. [Google Scholar] [CrossRef]




| Epa (V vs. Ag/AgCl) | EC50 a (mol AO/mol DPPH) | Emulsion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | R | pH 7.4 | pH 3.65 | pH 3.65 + Tween 20 | 5 min | 60 min | FRAP Value (μM) | |||
 | TY | H | 0.645 ± 0.010 a | 0.798 ± 0.015 a | - | 21 ± 2 a | 20 ± 2 a | 54.7 ± 2.5 a | - | - |
| TY8 | -CO-(CH2)6CH3 | 0.561 ± 0.008 b | 0.763 ± 0.005 a | - | 23 ± 2 a | 23 ± 3 a | 19.6 ± 3.6 b | - | - | |
| TY16 | -CO-(CH2)14CH3 | 0.583 ± 0.004 b | 0.783 ± 0.014 a | - | 23 ± 4 a | 23 ± 3 a | 12.5 ± 1.8 c | - | - | |
 | HT | H | 0.217 ± 0.002 c | 0.411 ± 0.011 b | 0.410 ± 0.010 a | 0.323 ± 0.005 b | 0.258 ± 0.004 b | 1523 ± 19 d | 53 ± 7 | - |
| HT8 | -CO-(CH2)6CH3 | 0.235 ± 0.003 c | 0.419 ± 0.006 b | 0.392 ± 0.009 a | 0.295 ± 0.005 b | 0.243 ± 0.003 b | 1193 ± 10 e | - | 296 ± 85 | |
| HT16 | -CO-(CH2)14CH3 | 0.222 ± 0.002 c | 0.403 ± 0.020 b | 0.391 ± 0.010 a | 0.331 ± 0.011 b | 0.268 ± 0.007 b | 653 ± 4 f | - | 52 ± 5 | |
 | HCA | H | 0.612 ± 0.006 d | 0.805 ± 0.005 c | - | 23 ± 2 a | 21 ± 2 a | 59.9 ± 4.2 a | - | - |
| HCA8 | -(CH2)7CH3 | 0.579 ± 0.003 e | 0.760 ± 0.003 d | - | 24 ± 2 a | 22 ± 3 a | 56.4 ± 1.7 a | - | 39 ± 2 | |
| HCA16 | -(CH2)15CH3 | 0.566 ± 0.010 e | 0.787 ± 0.018 d | - | 24 ± 3 a | 23 ± 3 a | 54.1 ± 1.7 a | - | 36 ± 3 | |
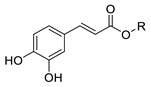 | CA | H | 0.265 ± 0.001 f | 0.394 ± 0.018 e | 0.411 ± 0.009 a | 0.330 ± 0.005 b | 0.344 ± 0.005 c | 1765 ± 12 g | 204 ± 16 | - |
| CA8 | -(CH2)7CH3 | 0.263 ± 0.003 f | 0.379 ± 0.008 e | 0.387 ± 0.010 a | 0.293 ± 0.012 b | 0.199 ± 0.005 d | 1617 ± 24 h | 502 ± 32 | ||
| CA16 | -(CH2)15CH3 | 0.260 ± 0.001 f | 0.378 ± 0.018 e | 0.386 ± 0.008 a | 0.317 ± 0.003 b | 0.196 ± 0.002 d | 1078 ± 15 i | 376 ± 35 | ||
 | DCA | H | 0.157 ± 0.004 g | 0.344 ± 0.007 f | - | 0.204 ± 0.003 c | 0.149 ± 0.006 e | 2921 ± 59 j | 58 ± 13 | - |
| DCA8 | -(CH2)7CH3 | 0.155 ± 0.003 g | 0.339 ± 0.017 f | - | 0.266 ± 0.005 d | 0.267 ± 0.008 b | 1613 ± 24 h | - | 368 ± 29 | |
| DCA16 | -(CH2)15CH3 | 0.150 ± 0.008 g | 0.349 ± 0.026 f | - | 0.268 ± 0.006 d | 0.270 ± 0.004 b | 1513 ± 21 d | - | 220 ± 54 | |
| (AOI) | (AOw) | (AOO) | Epa | EC50 | FRAP | ||
|---|---|---|---|---|---|---|---|
| pH = 3.65 | 5 min | 60 min | |||||
| Oxidative Stability | 0.820 ** | −0.470 * | −0.184 | −0.677 ** | −0.654 * | −0.654 * | −0.434 * |
| Model | Predictors | R | Cum.R2 | Part.R2 |
|---|---|---|---|---|
| 1 | (AOI) | 0.820 | 0.672 | 0.672 |
| 2 | (AOI) Epa | 0.847 | 0.717 | 0.044 |
| 3 | (AOI) Epa (AOw) | 0.905 | 0.819 | 0.103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.; Losada-Barreiro, S.; Magalhães, J.; Monteiro, L.S.; Bravo-Díaz, C.; Paiva-Martins, F. Effects of the Reactive Moiety of Phenolipids on Their Antioxidant Efficiency in Model Emulsified Systems. Foods 2021, 10, 1028. https://doi.org/10.3390/foods10051028
Costa M, Losada-Barreiro S, Magalhães J, Monteiro LS, Bravo-Díaz C, Paiva-Martins F. Effects of the Reactive Moiety of Phenolipids on Their Antioxidant Efficiency in Model Emulsified Systems. Foods. 2021; 10(5):1028. https://doi.org/10.3390/foods10051028
Chicago/Turabian StyleCosta, Marlene, Sonia Losada-Barreiro, Júlia Magalhães, Luís S. Monteiro, Carlos Bravo-Díaz, and Fátima Paiva-Martins. 2021. "Effects of the Reactive Moiety of Phenolipids on Their Antioxidant Efficiency in Model Emulsified Systems" Foods 10, no. 5: 1028. https://doi.org/10.3390/foods10051028
APA StyleCosta, M., Losada-Barreiro, S., Magalhães, J., Monteiro, L. S., Bravo-Díaz, C., & Paiva-Martins, F. (2021). Effects of the Reactive Moiety of Phenolipids on Their Antioxidant Efficiency in Model Emulsified Systems. Foods, 10(5), 1028. https://doi.org/10.3390/foods10051028






