Inhibitory Effect of Catechin-Rich Açaí Seed Extract on LPS-Stimulated RAW 264.7 Cells and Carrageenan-Induced Paw Edema
Abstract
1. Introduction
2. Materials and Methods
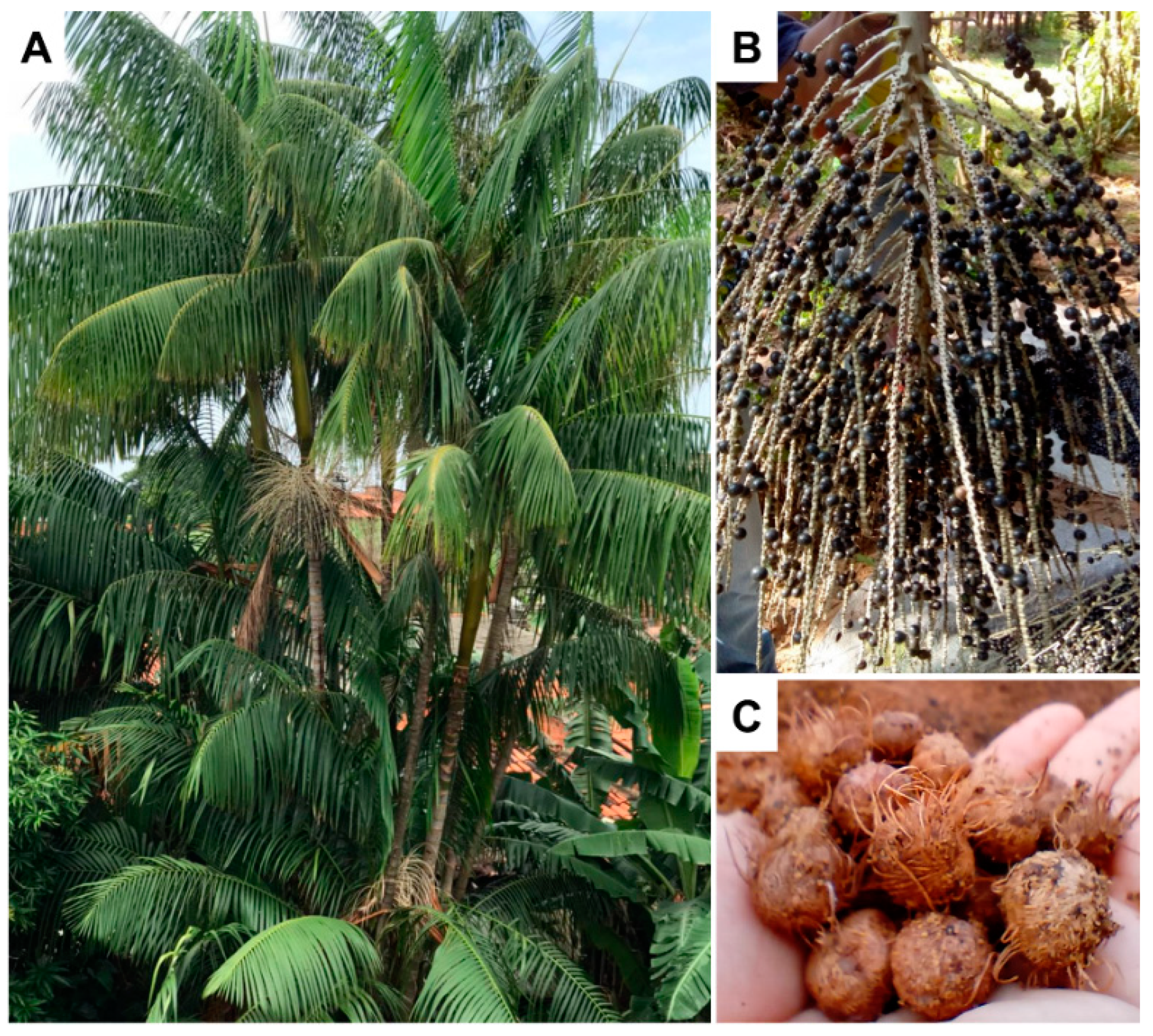
2.1. Plant Material
2.2. Obtaining of Ethyl Acetate Extract from E. oleracea Seed
2.3. Thin Layer Chromatography (TLC) Analysis
2.4. Analysis by High-Performance Liquid Chromatography Coupled to Diode-Array Detection and Mass Spectrometry (HPLC-DAD-MS)
2.5. Quantification of Endotoxins
2.6. Cell Culture
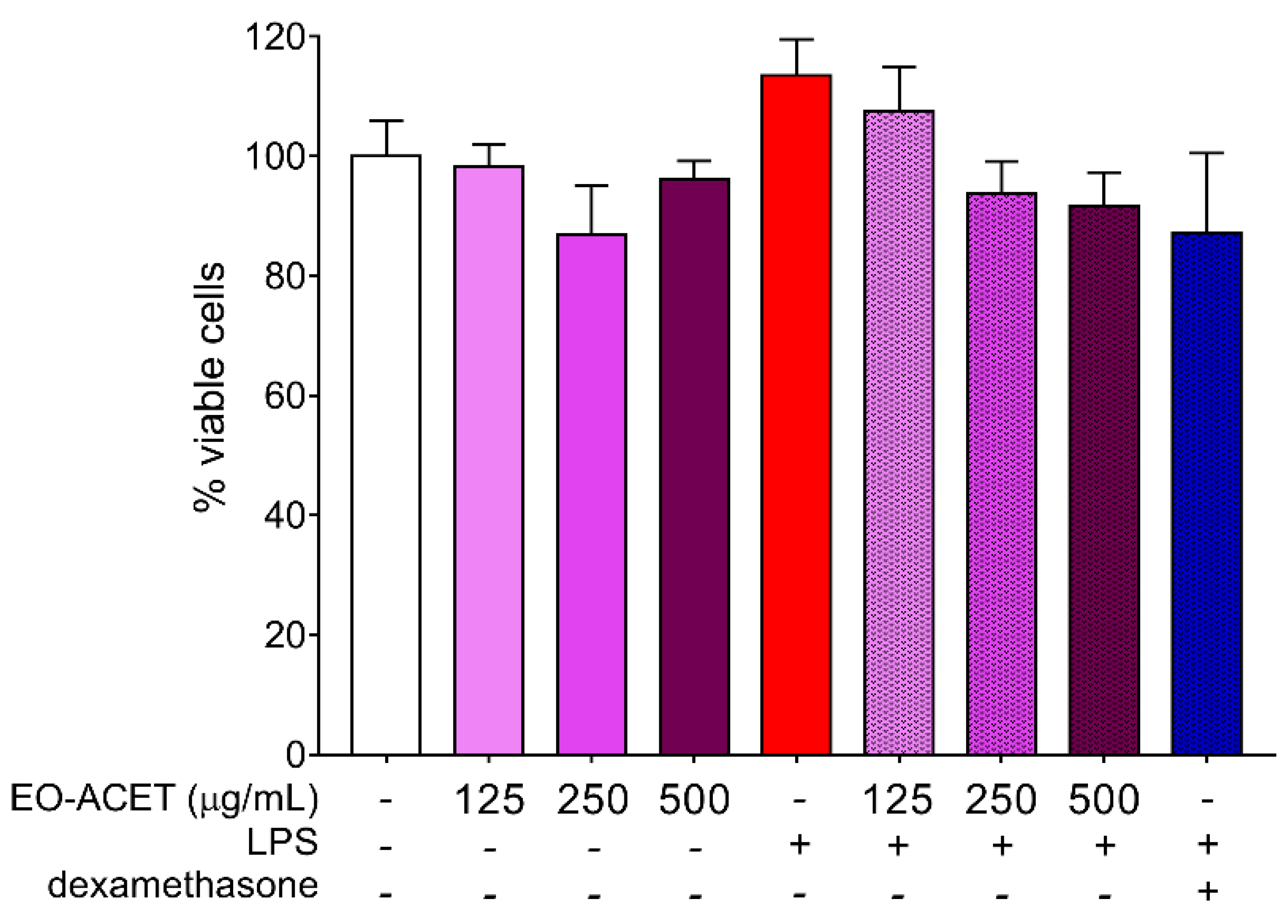
2.7. Cytotoxicity Assay
2.8. EO-ACET Treatment in RAW 264.7 Macrophages Stimulated with LPS
2.9. Nitrite and Cytokines Quantification
2.10. Animals and Ethical Statement
2.11. Paw Edema Induced by λ-Carrageenan
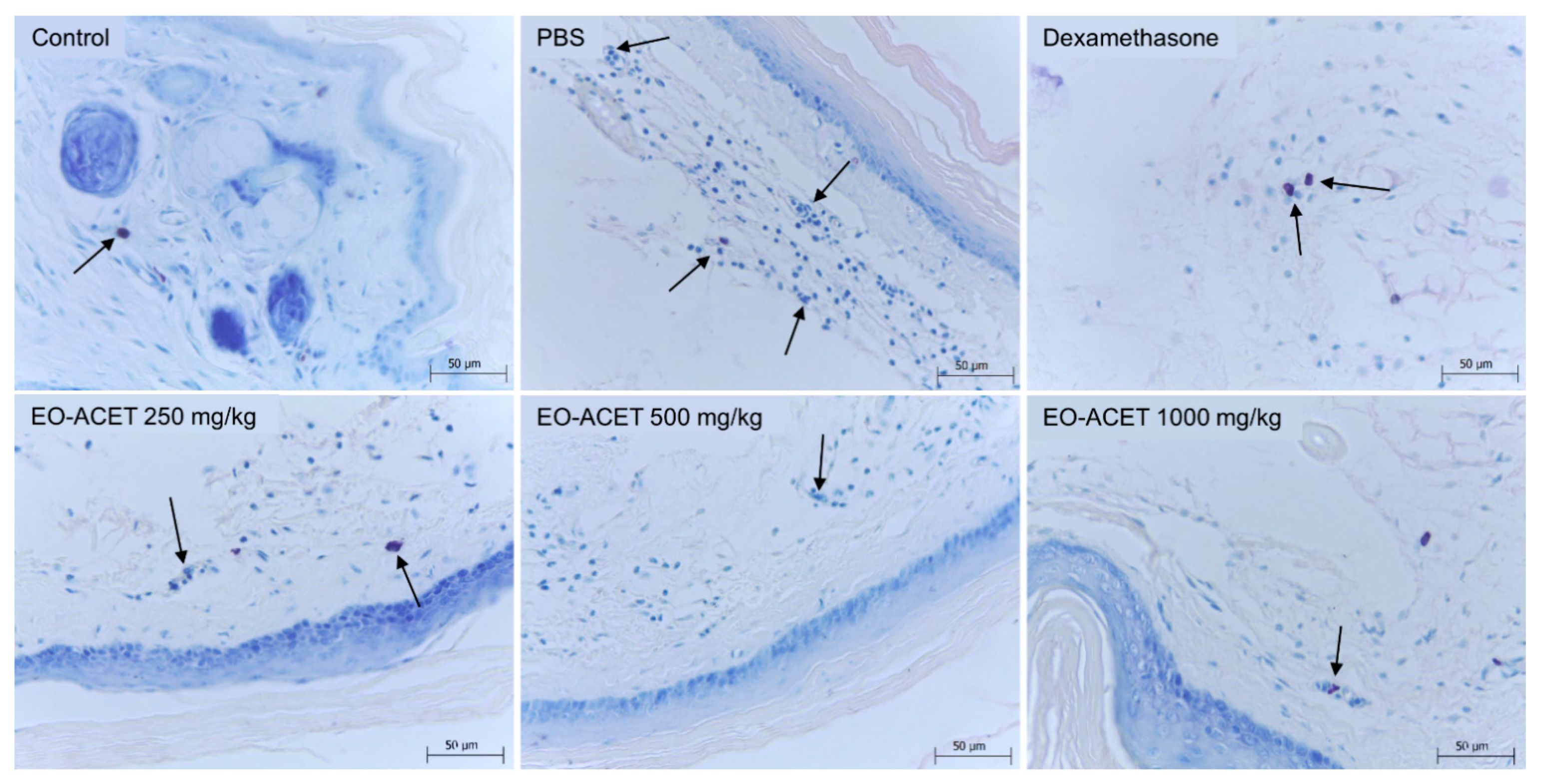
2.12. Histology Analysis
2.13. Statistical Analysis
3. Results
3.1. Chemical Characterization of E. oleracea Seed Extracts
3.2. EO-ACET Is Endotoxin-Free and Not Displayed Cytotoxicity
3.3. EO-ACET Reduced Levels of Pro-Inflammatory Markers in RAW 264.7 Cells Stimulated with LPS
3.4. EO-ACET Inhibited Paw Edema Induced by λ-Carrageenan
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rapaka, R.S.; Coates, P.M. Dietary supplements and related products: A brief summary. Life Sci. 2006, 78, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Mulabagal, V.; Keller, W.J.; Calderón, A.I. Quantitative analysis of anthocyanins in Euterpe oleracea (açaí) dietary supplement raw materials and capsules by Q-TOF liquid chromatography/mass spectrometry. Pharm. Biol. 2012, 50, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Di Stefano, V.; Lauria, A.; Pitonzo, R.; Gentile, C. Vaccinium macrocarpon (Cranberry)-Based Dietary Supplements: Variation in Mass Uniformity, Proanthocyanidin Dosage and Anthocyanin Profile Demonstrates Quality Control Standard Needed. Nutrients 2020, 12, 992. [Google Scholar] [CrossRef] [PubMed]
- Helkar, P.B.; Sahoo, A.K.; Patil, N.J. Review: Food Industry By-Products used as a Functional Food Ingredients. Int. J. Waste Resour. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B. Antioxidant and Potentially Anti-Inflammatory Activity of Anthocyanin Fractions from Pomace Obtained from Enzymatically Treated Raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef]
- Petruk, G.; Illiano, A.; Del Giudice, R.; Raiola, A.; Amoresano, A.; Rigano, M.M.; Piccoli, R.; Monti, D.M. Malvidin and cyanidin derivatives from açai fruit (Euterpe oleracea Mart.) counteract UV-A-induced oxidative stress in immortalized fibroblasts. J. Photochem. Photobiol. B Biol. 2017, 172. [Google Scholar] [CrossRef]
- Aguiar, M.O.; Mendonça, M.S.d. Morfo-anatomia da semente de Euterpe precatoria Mart. (Palmae). Rev. Bras. Sementes 2003, 25, 37–42. [Google Scholar] [CrossRef]
- Martins, C.C.; Bovi, M.L.A.; Nakagawa, J.; Machado, C.G. Secagem e armazenamento de sementes de juçara. Rev. Árvore 2009, 33, 635–642. [Google Scholar] [CrossRef][Green Version]
- Reis, R.C.d.C. Palmeiras (Arecaceae) das restingas do Estado do Rio de Janeiro, Brasil. Acta Bot. Bras. 2006, 20, 501–512. [Google Scholar] [CrossRef]
- de Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; Converti, A.; Neves de Lima, Á.A. The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules 2020, 10, 813. [Google Scholar] [CrossRef]
- de Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; Kawashima Pacheco, S.Y.; Silva, S.S.D.; Barbosa, E.G.; Pereira, R.R.; Costa, R.M.R.; Silva Junior, J.O.C.; da Silva Ferreira, M.A.; de Almeida, J.C.; et al. Development and Evaluation of Antimicrobial and Modulatory Activity of Inclusion Complex of Euterpe oleracea Mart Oil and β-Cyclodextrin or HP-β-Cyclodextrin. Int. J. Mol. Sci. 2020, 21, 942. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Talcott, S.T.; Safe, S.; Mertens-Talcott, S. Absorption and biological activity of phytochemical-rich extracts from açai (Euterpe oleracea Mart.) pulp and oil in vitro. J. Agric. Food Chem. 2008, 56. [Google Scholar] [CrossRef]
- Cedrim, P.C.A.S.; Barros, E.M.A.; Nascimento, T.G. Propriedades antioxidantes do açaí (Euterpe oleracea) na síndrome metabólica. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Carvalho, C.A.; Ferreira Ferreira da Silveira, T.; Mattietto, R.A.; Padilha de Oliveira, M.D.; Godoy, H.T. Chemical composition and antioxidant capacity of açaí (Euterpe oleracea) genotypes and commercial pulps. J. Sci. Food Agric. 2017, 97. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.K.; Pereira, L.F.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, N.K.S.; Almeida, M.R.S.; Pontes, F.M.M.; Barcelos, M.P.; de Paula da Silva, C.H.T.; Rosa, J.M.C.; Cruz, R.A.S.; da Silva Hage-Melim, L.I. Antioxidant Effect of Flavonoids Present in Euterpe oleracea Martius and Neurodegenerative Diseases: A Literature Review. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.S.; Ferreira, T.S.; Lopes, A.A.; Pires, K.M.; Nesi, R.T.; Resende, A.C.; Souza, P.J.; Silva, A.J.; Borges, R.M.; Porto, L.C.; et al. Effects of Euterpe oleracea Mart. (AÇAÍ) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomed. Int. J. Phytother. Phytopharm. 2012, 19. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.K.; Cadoná, F.C.; Assmann, C.E.; Andreazza, A.C.; Duarte, M.M.M.F.; Branco, C.S.; Zhou, X.; de Souza, D.V.; Ribeiro, E.E.; da Cruz, I.B.M. Açaí (Euterpe oleracea Mart.) has anti-inflammatory potential through NLRP3-inflammasome modulation. J. Funct. Foods 2019, 56, 364–371. [Google Scholar] [CrossRef]
- Melo, P.S.; Massarioli, A.P.; Lazarini, J.G.; Soares, J.C.; Franchin, M.; Rosalen, P.L.; Alencar, S.M. Simulated gastrointestinal digestion of Brazilian açaí seeds affects the content of flavan-3-ol derivatives, and their antioxidant and anti-inflammatory activities. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Lee, H.A.; Song, Y.R.; Park, M.H.; Chung, H.Y.; Na, H.S.; Chung, J. Catechin ameliorates Porphyromonas gingivalis-induced inflammation via the regulation of TLR2/4 and inflammasome signaling. J. Periodontol. 2020, 91. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Levy, R.M. The combination of catechin, baicalin and β-caryophyllene potentially suppresses the production of inflammatory cytokines in mouse macrophages in vitro. Exp. Ther. Med. 2019, 17. [Google Scholar] [CrossRef]
- Li, T.; Li, F.; Liu, X.; Liu, J.; Li, D. Synergistic anti-inflammatory effects of quercetin and catechin via inhibiting activation of TLR4-MyD88-mediated NF-κB and MAPK signaling pathways. Phytother. Res. PTR 2019, 33. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Souza, F.; de Souza, C.a.S.; Taniwaki, N.N.; Silva, J.J.; de Oliveira, R.M.; Abreu-Silva, A.L.; Calabrese, K.a.S. Morinda citrifolia Linn. fruit (Noni) juice induces an increase in NO production and death of Leishmania amazonensis amastigotes in peritoneal macrophages from BALB/c. Nitric Oxide 2016, 58, 51–58. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65. [Google Scholar] [CrossRef]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Calabrese, K.D.S.; Abreu-Silva, A.L.; Marinho, S.C.; Mouchrek, A.N.; Filho, V.E.M.; Almeida-Souza, F. Aniba rosaeodora (Var. amazonica Ducke) Essential Oil: Chemical Composition, Antibacterial, Antioxidant and Antitrypanosomal Activity. Antibiotics 2020, 10, 24. [Google Scholar] [CrossRef]
- Mondêgo-Oliveira, R.; de Sá Sousa, J.C.; Moragas-Tellis, C.J.; de Souza, P.V.R.; Dos Santos Chagas, M.D.S.; Behrens, M.D.; Jesús Hardoim, D.; Taniwaki, N.N.; Chometon, T.Q.; Bertho, A.L.; et al. Vernonia brasiliana (L.) Druce induces ultrastructural changes and apoptosis-like death of Leishmania infantum promastigotes. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 133. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Almeida-Souza, F.; Silva, V.D.D.; Silva, G.X.; Taniwaki, N.N.; Hardoim, D.J.; Buarque, C.D.; Abreu-Silva, A.L.; Calabrese, K.D.S. 1,4-Disubstituted-1,2,3-Triazole Compounds Induce Ultrastructural Alterations in Leishmania amazonensis Promastigote: An in Vitro Antileishmanial and in Silico Pharmacokinetic Study. Int. J. Mol. Sci. 2020, 21, 6839. [Google Scholar] [CrossRef]
- Oliveira, I.S.S.; Colares, A.V.; Cardoso, F.O.; Tellis, C.J.M.; Chagas, M.S.S.; Behrens, M.D.; Calabrese, K.S.; Almeida-Souza, F.; Abreu-Silva, A.L. Vernonia Polysphaera Baker: Anti-inflammatory Activity in Vivo and Inhibitory Effect in LPS-stimulated RAW 264.7 Cells. PLoS ONE 2019, 14, e0225275. [Google Scholar] [CrossRef]
- Freitas, D.D.S.; Morgado-Díaz, J.A.; Gehren, A.S.; Vidal, F.C.B.; Fernandes, R.M.T.; Romão, W.; Tose, L.V.; Frazão, F.N.S.; Costa, M.C.P.; Silva, D.F.; et al. Cytotoxic analysis and chemical characterization of fractions of the hydroalcoholic extract of the Euterpe oleracea Mart. seed in the MCF-7 cell line. J. Pharm. Pharmacol. 2017, 69. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.B.; Lichtenthäler, R.; Zimmermann, B.F.; Papagiannopoulos, M.; Fabricius, H.; Marx, F.; Maia, J.G.; Almeida, O. Total oxidant scavenging capacity of Euterpe oleracea Mart. (açaí) seeds and identification of their polyphenolic compounds. J. Agric. Food Chem. 2006, 54. [Google Scholar] [CrossRef]
- Barros, L.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Santos, E.A.; Regis, W.C.B.; Ferreira, I.C.F.R. The powerful in vitro bioactivity of Euterpe oleracea Mart. seeds and related phenolic compounds. Ind. Crop. Prod. 2015, 76, 318–322. [Google Scholar] [CrossRef]
- Sakurai, K.; Shen, C.; Shiraishi, I.; Inamura, N.; Hisatsune, T. Consumption of Oleic Acid on the Preservation of Cognitive Functions in Japanese Elderly Individuals. Nutrients 2021, 13, 284. [Google Scholar] [CrossRef]
- Romualdo, G.R.; de Souza, I.P.; de Souza, L.V.; Prata, G.B.; Fraga-Silva, T.F.C.; Sartori, A.; Borguini, R.G.; Santiago, M.C.P.A.; Fernandes, A.A.H.; Cogliati, B.; et al. Beneficial effects of anthocyanin-rich peels of Myrtaceae fruits on chemically-induced liver fibrosis and carcinogenesis in mice. Food Res. Int. 2021, 139. [Google Scholar] [CrossRef] [PubMed]
- De Souza, F.G.; de Araújo, F.F.; de Paulo Farias, D.; Zanotto, A.W.; Neri-Numa, I.A.; Pastore, G.M. Brazilian fruits of Arecaceae family: An overview of some representatives with promising food, therapeutic and industrial applications. Food Res. Int. 2020, 138. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Oyarzún, P.; Cornejo, P.; Gómez-Alonso, S.; Ruiz, A. Influence of Profiles and Concentrations of Phenolic Compounds in the Coloration and Antioxidant Properties of Gaultheria poeppigii Fruits from Southern Chile. Plant Foods Hum. Nutr. 2020, 75. [Google Scholar] [CrossRef]
- Katsimbri, P.; Korakas, E.; Kountouri, A.; Ikonomidis, I.; Tsougos, E.; Vlachos, D.; Papadavid, E.; Raptis, A.; Lambadiari, V. The Effect of Antioxidant and Anti-Inflammatory Capacity of Diet on Psoriasis and Psoriatic Arthritis Phenotype: Nutrition as Therapeutic Tool? Antioxidants 2021, 10, 157. [Google Scholar] [CrossRef]
- Vega-Ruiz, Y.C.; Hayano-Kanashiro, C.; Gámez-Meza, N.; Medina-Juárez, L.A. Determination of Chemical Constituents and Antioxidant Activities of Leaves and Stems from Jatropha cinerea (Ortega) Müll. Arg and Jatropha cordata (Ortega) Müll. Arg. Plants 2021, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Gao, Y.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Licursi, M.; Skora, N.C.; Whelan, J.; Villa, B.P.; Straub, K.D. ‘Antioxidant’ Berries, Anthocyanins, Resveratrol and Rosmarinic Acid Oxidize Hydrogen Sulfide to Polysulfides and Thiosulfate: A Novel Mechanism Underlying Their Biological Actions. Free Radic. Biol. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Niyukuri, J.; Raiti, J.; Ntakarutimana, V.; Hafidi, A. Lipid composition and antioxidant activities of some underused wild plants seeds from Burundi. Food Sci. Nutr. 2020, 9. [Google Scholar] [CrossRef]
- Sattar, A.A.; Abate, W.; Fejer, G.; Bradley, G.; Jackson, S.K. Evaluation of the proinflammatory effects of contaminated bathing water. J. Toxicol. Environ. Health Part A 2019, 82. [Google Scholar] [CrossRef]
- Seeley, J.J.; Ghosh, S. Molecular mechanisms of innate memory and tolerance to LPS. J. Leukoc. Biol. 2017, 101. [Google Scholar] [CrossRef]
- Sheu, M.-J.; Deng, J.-S.; Huang, M.-H.; Liao, J.-C.; Wu, C.-H.; Huang, S.-S.; Huang, G.-J. Antioxidant and anti-inflammatory properties of Dichondra repens Forst. and its reference compounds. Food Chem. 2012, 132, 1010–1018. [Google Scholar] [CrossRef]
- Bredt, D.S. Endogenous Nitric Oxide Synthesis: Biological Functions and Pathophysiology. Free Radic. Res. 1999, 31. [Google Scholar] [CrossRef] [PubMed]
- Pyrillou, K.; Burzynski, L.C.; Clarke, M.C.H. Alternative Pathways of IL-1 Activation, and Its Role in Health and Disease. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Silveira, L.S.; Antunes, B.M.; Minari, A.L.; Dos Santos, R.V.; Neto, J.C.; Lira, F.S. Macrophage Polarization: Implications on Metabolic Diseases and the Role of Exercise. Crit. Rev. Eukaryot. Gene Expr. 2016, 26. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19. [Google Scholar] [CrossRef]
- Soares, E.R.; Monteiro, E.B.; de Bem, G.F.; Inada, K.O.P.; Torres, A.G.; Perrone, D.; Soulage, C.O.; Monteiro, M.C.; Resende, A.C.; Moura-Nunes, N.; et al. Up-regulation of Nrf2-antioxidant signaling by Açaí (Euterpe oleracea Mart.) extract prevents oxidative stress in human endothelial cells. J. Funct. Foods 2017, 37, 107–115. [Google Scholar] [CrossRef]
- Eze, F.I.; Uzor, P.F.; Ikechukwu, P.; Obi, B.C.; Osadebe, P.O. In vitro and In vivo Models for Anti-inflammation: An Evaluative Review. INNOSC Theranostics Pharmacol. Sci. 2019, 2, 13. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Romão, M.H.; de Bem, G.F.; Santos, I.B.; de Andrade Soares, R.; Ognibene, D.T.; de Moura, R.S.; da Costa, C.A.; Resende, Â.C. Açaí (Euterpe oleracea Mart.) seed extract protects against hepatic steatosis and fibrosis in high-fat diet-fed mice: Role of local renin-angiotensin system, oxidative stress and inflammation. J. Funct. Foods 2020, 65, 103726. [Google Scholar] [CrossRef]
- Heger, K.; Fierens, K.; Vahl, J.C.; Aszodi, A.; Peschke, K.; Schenten, D.; Hammad, H.; Beyaert, R.; Saur, D.; van Loo, G.; et al. A20-deficient mast cells exacerbate inflammatory responses in vivo. PLoS Biol 2014, 12, e1001762. [Google Scholar] [CrossRef] [PubMed]
- El Ansari, Y.S.; Kanagaratham, C.; Oettgen, H.C. Mast Cells as Regulators of Adaptive Immune Responses in Food Allergy. Yale J. Biol. Med. 2020, 93, 711. [Google Scholar] [PubMed]
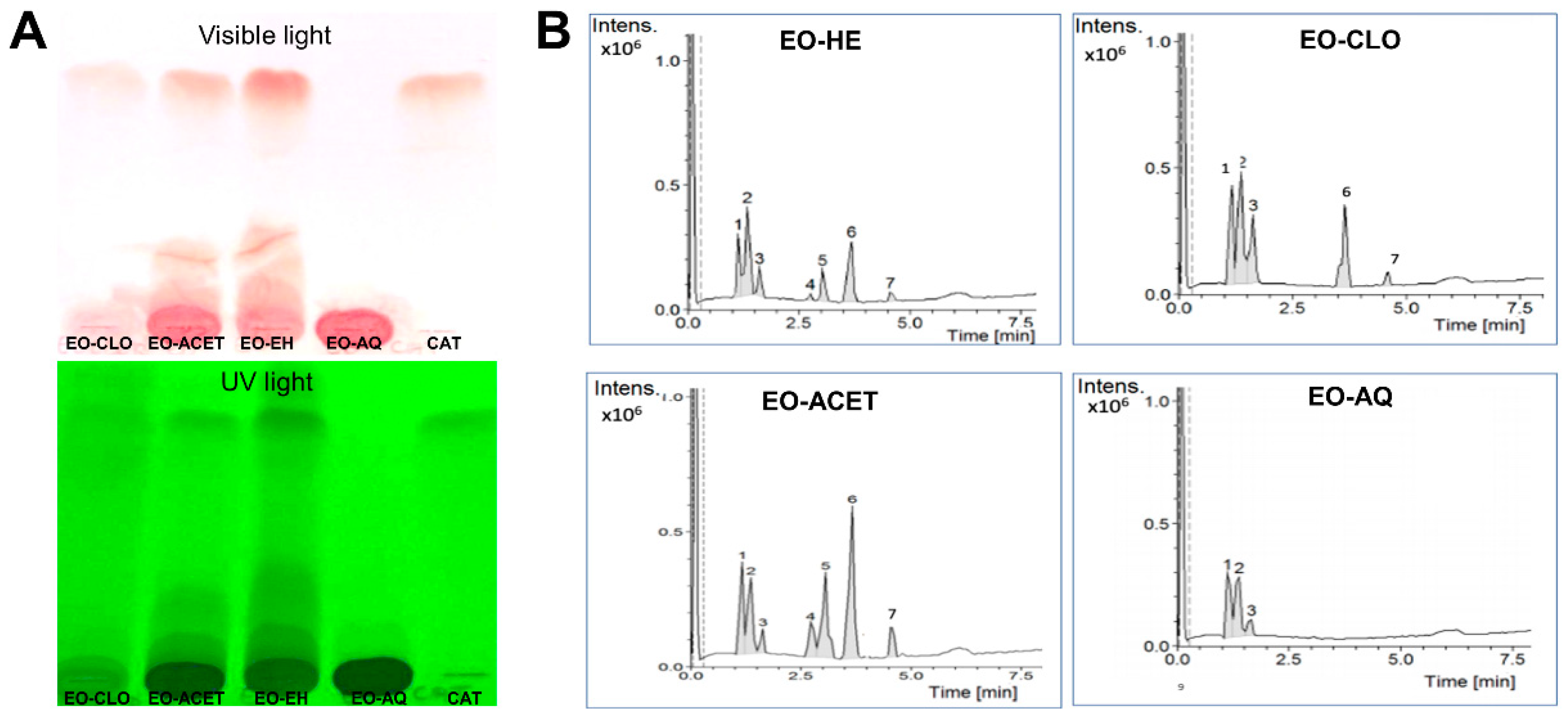



| Peak | RT 1 | m/z (M+H+) | EO-HE 2 | EO-CLO 3 | EO-ACET 4 | EO-AQ 5 | Identification 6 |
|---|---|---|---|---|---|---|---|
| 1 | 1.1 | 203.0528 | 16.07 | 25.22 | 17.06 | 43.07 | Not identified |
| 2 | 1.3 | 231.0845 | 33.87 | 32.31 | 15.32 | 46.07 | Not identified |
| 3 | 1.6 | 231.0841 | 9.61 | 18.06 | 3.65 | 10.84 | Not identified |
| 4 | 2.8 | 867.2142 | 1.70 | - | 7.66 | - | trimeric procyanidins |
| 5 | 3.0 | 579.1488 | 10.23 | - | 19.62 | - | pelargonidin-3-rutinoside |
| 6 | 3.7 | 291.0860 | 25.24 | 21.40 | 30.91 | - | Catechin |
| 7 | 4.6 | 291.0855 | 3.25 | 2.98 | 5.75 | - | Epicatechin |
| Treatment | λ-Carragenan | Dose (mg/kg) | Administration Time (% Edema Inhibition) | |||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | |||
| PBS | - | - | 0.04 ± 0.055 | 0.02 ± 0.045 | 0.02 ± 0.045 | – 1 |
| + | - | 0.22 ± 0.084 | 0.32 ± 0.134 | 0.48 ± 0.084 | 0.56 ± 0.055 | |
| EO-ACET | + | 250 | 0.20 ± 0.100 | 0.25 ± 0.129 | 0.36 ± 0.089 (25.00) | 0.46 ± 0.089 (17.85) |
| + | 500 | 0.22 ± 0.096 | 0.25 ± 0.058 | 0.34 ± 0.114 (29.16) | 0.36 ± 0.114 (35.71) * | |
| + | 1000 | 0.20 ± 0.100 | 0.25 ± 0.058 | 0.32 ± 0.084 (33.33) | 0.34 ± 0.055 (39.29) ** | |
| Dexamethanose | + | 5 | 0.18 ± 0.084 | 0.20 ± 0.100 | 0.20 ± 0.071 (58.33) **** | 0.20 ± 0.071 (64.28) **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, G.S.; Teles, A.M.; Moragas-Tellis, C.J.; Chagas, M.d.S.d.S.; Behrens, M.D.; Moreira, W.F.d.F.; Abreu-Silva, A.L.; Calabrese, K.d.S.; Nascimento, M.d.D.S.B.; Almeida-Souza, F. Inhibitory Effect of Catechin-Rich Açaí Seed Extract on LPS-Stimulated RAW 264.7 Cells and Carrageenan-Induced Paw Edema. Foods 2021, 10, 1014. https://doi.org/10.3390/foods10051014
Xavier GS, Teles AM, Moragas-Tellis CJ, Chagas MdSdS, Behrens MD, Moreira WFdF, Abreu-Silva AL, Calabrese KdS, Nascimento MdDSB, Almeida-Souza F. Inhibitory Effect of Catechin-Rich Açaí Seed Extract on LPS-Stimulated RAW 264.7 Cells and Carrageenan-Induced Paw Edema. Foods. 2021; 10(5):1014. https://doi.org/10.3390/foods10051014
Chicago/Turabian StyleXavier, Gabriel Silva, Amanda Mara Teles, Carla Junqueira Moragas-Tellis, Maria do Socorro dos Santos Chagas, Maria Dutra Behrens, Wendel Fragoso de Freitas Moreira, Ana Lucia Abreu-Silva, Kátia da Silva Calabrese, Maria do Desterro Soares Brandão Nascimento, and Fernando Almeida-Souza. 2021. "Inhibitory Effect of Catechin-Rich Açaí Seed Extract on LPS-Stimulated RAW 264.7 Cells and Carrageenan-Induced Paw Edema" Foods 10, no. 5: 1014. https://doi.org/10.3390/foods10051014
APA StyleXavier, G. S., Teles, A. M., Moragas-Tellis, C. J., Chagas, M. d. S. d. S., Behrens, M. D., Moreira, W. F. d. F., Abreu-Silva, A. L., Calabrese, K. d. S., Nascimento, M. d. D. S. B., & Almeida-Souza, F. (2021). Inhibitory Effect of Catechin-Rich Açaí Seed Extract on LPS-Stimulated RAW 264.7 Cells and Carrageenan-Induced Paw Edema. Foods, 10(5), 1014. https://doi.org/10.3390/foods10051014








