Health-Promoting Properties of Borage Seed Oil Fractionated by Supercritical Carbon Dioxide Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemicals
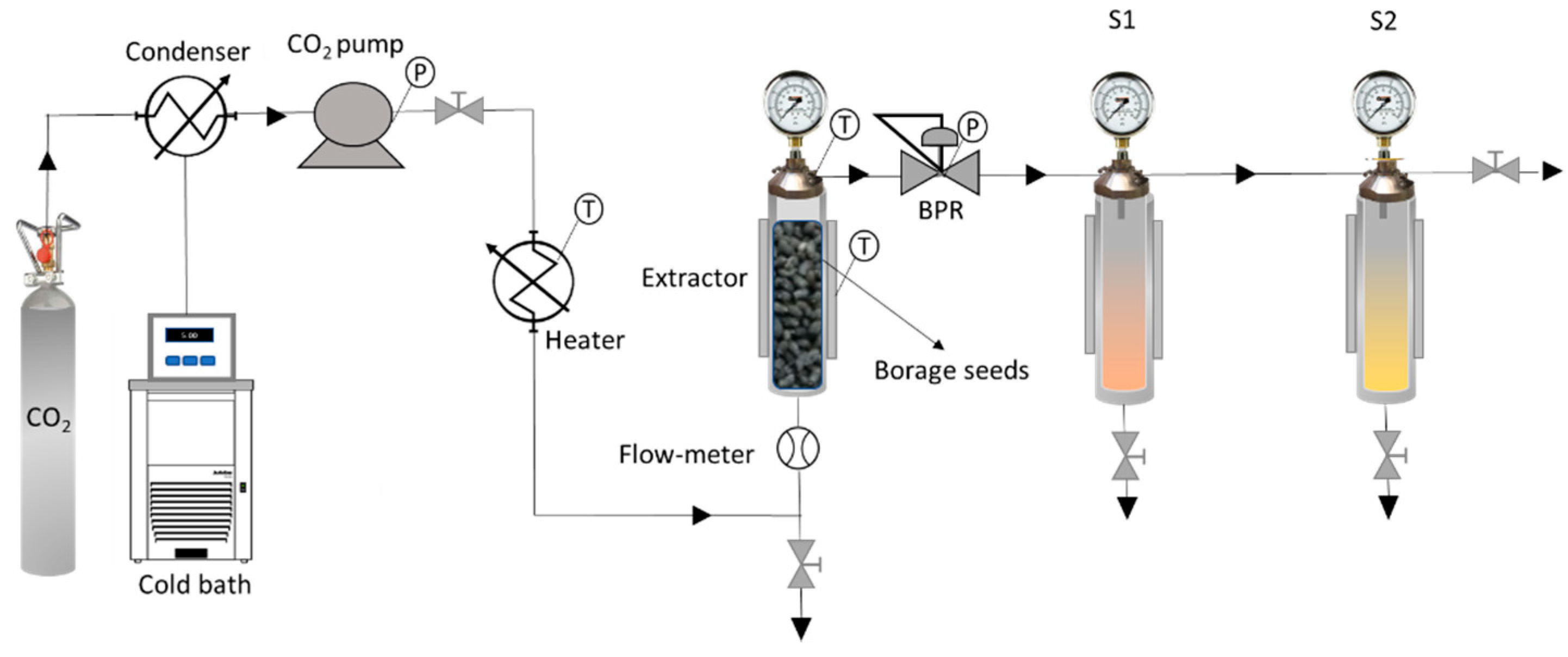
2.2. Extraction and Fractionation at High Pressure
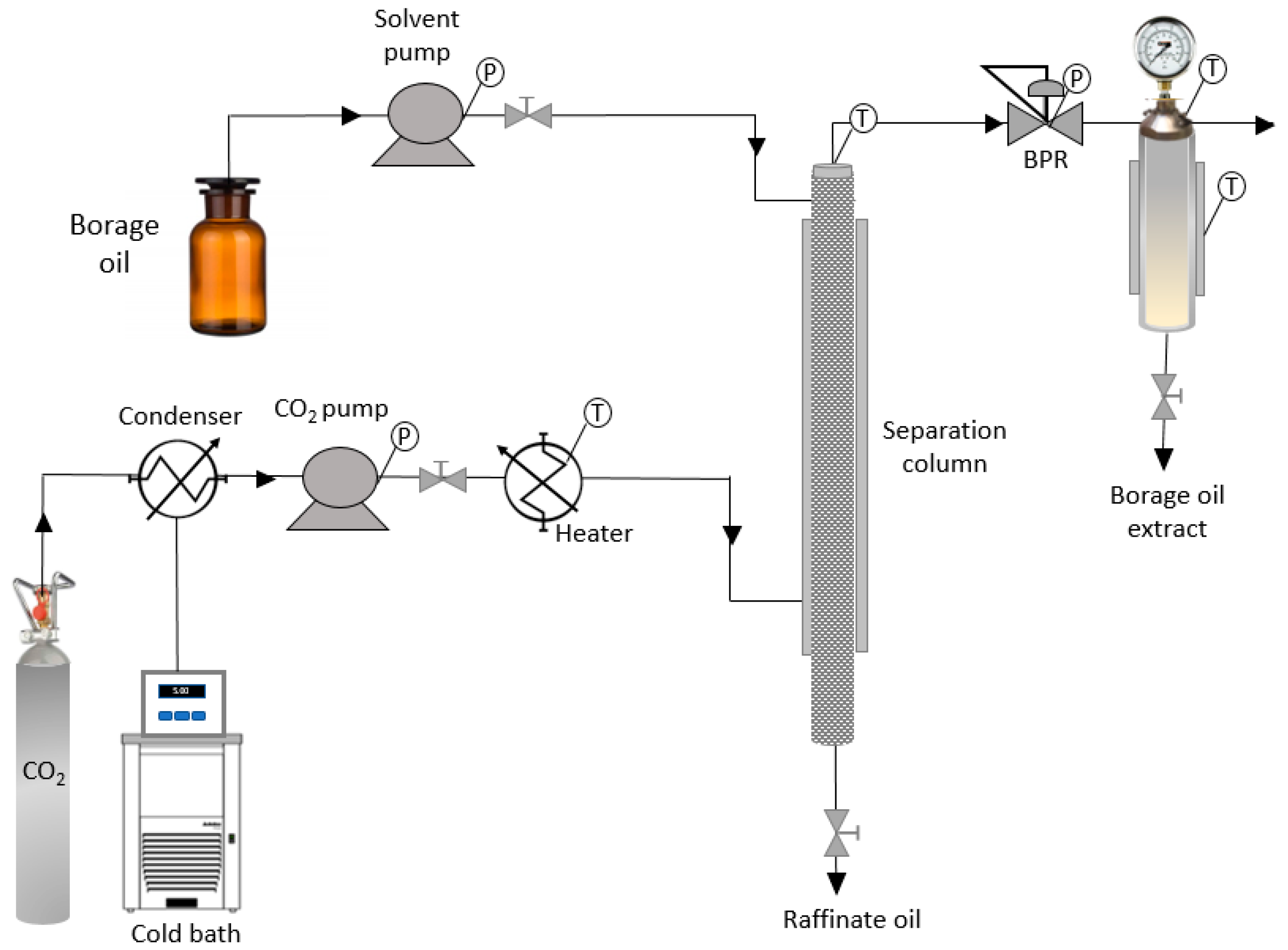
2.3. Supercritical Fluid Fractionation Column
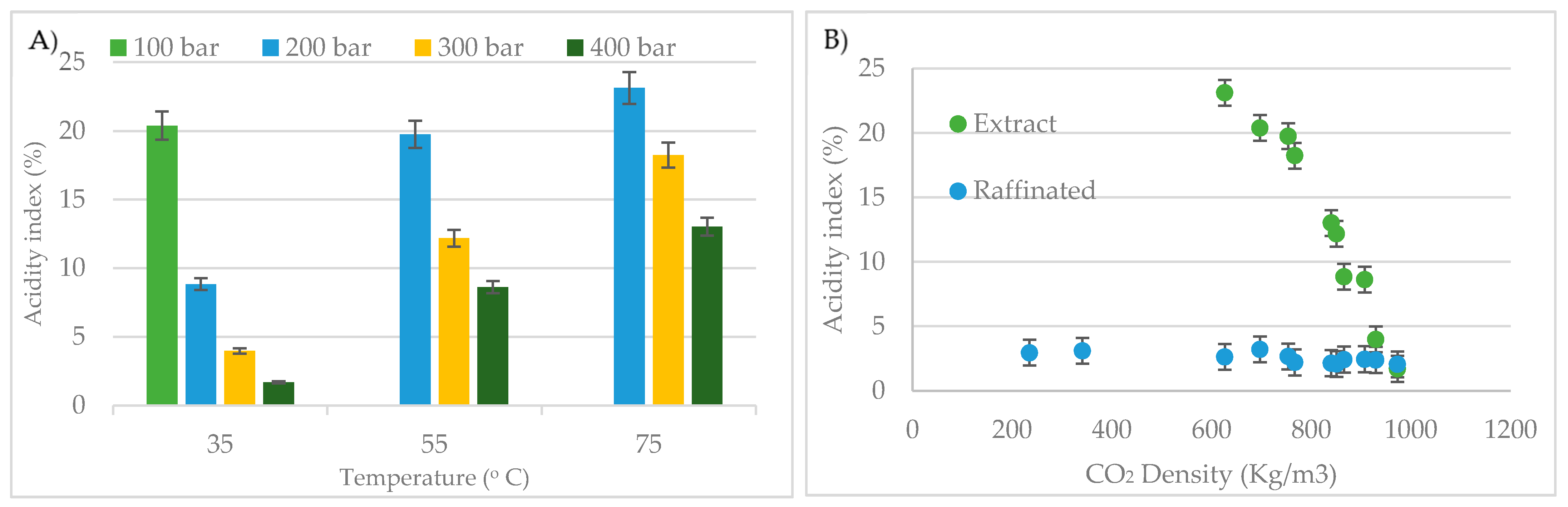
2.4. Acidity Index
2.5. Determining by DPPH the Antiradical Activities of the Potent Antioxidants
2.6. Fatty Acids Content
2.7. Cell Cultures
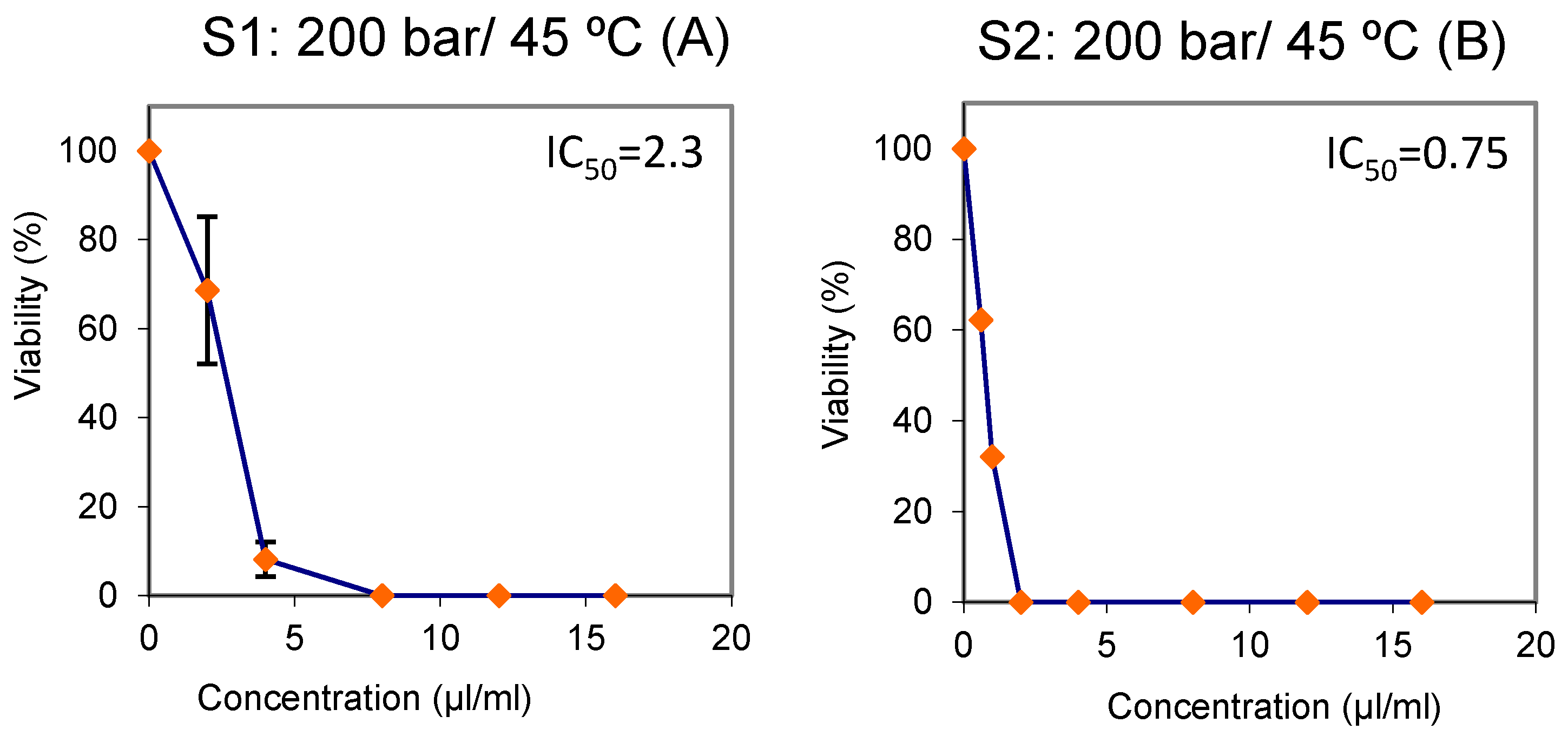
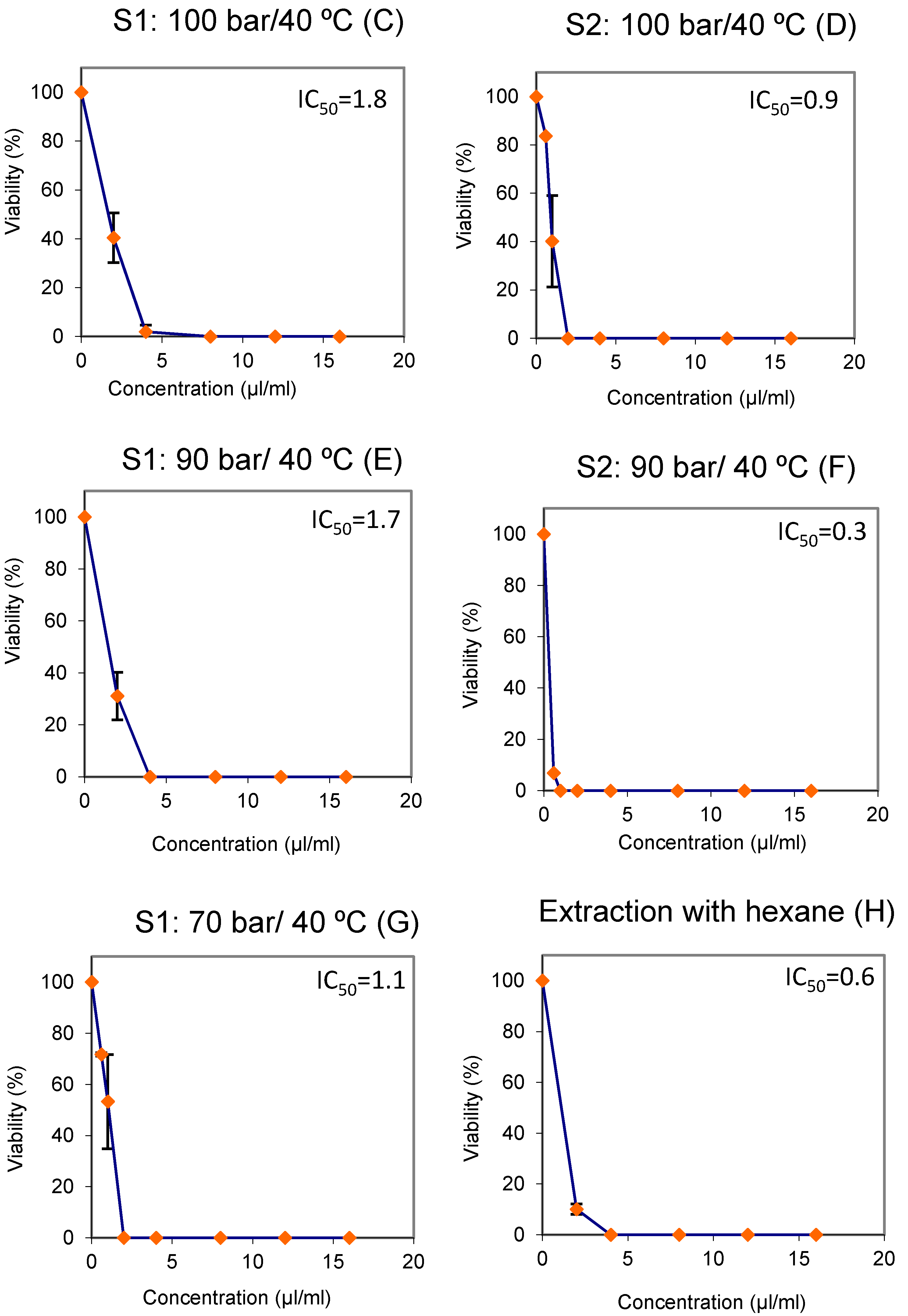
2.8. Cytotoxicity Assays
3. Results
3.1. Fractionation of the Extracts into Cyclone Separators
3.2. Countercurrent Fractionation of the Oil
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Temelli, F. Perspectives on Supercritical Fluid Processing of Fats and Oils. J. Supercrit. Fluids 2009, 47, 583–590. [Google Scholar] [CrossRef]
- Gerard, J. The History of Plants, 1597; Woodward, M., Ed.; Senate. Study Ltd.: London, UK, 1994; pp. 185–186. [Google Scholar]
- Janick, J.; Simon, J.E.; Quinn, J.; Beaubaire, N. Borage: A Source of Gamma Linolenic Acid. Herbs Spices Med. Plants Recent Adv. Bot. Hortic. Pharmacol. 1989, 4, 145–168. [Google Scholar]
- Gunstone, F.D. Gammar Linolenic Acid—Occurrence and Physical and Chemical Properties. Prog. Lipid Res. 1992, 31, 145–161. [Google Scholar] [CrossRef]
- Del Río-Celestino, M.; Font, R.; De Haro-Bailón, A. Distribution of Fatty Acids in Edible Organs and Seed Fractions of Borage (Borago officinalis L.). J. Sci. Food Agric. 2008, 88, 248–255. [Google Scholar] [CrossRef]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-Linolenic Acid, Dihommo-Gamma Linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. A Defect in Δ 6 and Δ 5 Desaturases May Be a Factor in the Initiation and Progression of Insulin Resistance, the Metabolic Syndrome and Ischemic Heart Disease in South Asians. Lipids Health Dis. 2010, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Kruger, M.C.; Coetzer, H.; De Winter, R.; Gericke, G.; Van Papendorp, D.H. Calcium, Gamma-Linolenic Acid and Eicosapentaenoic Acid Supplementation in Senile Osteoporosis. Aging Clin. Exp. Res. 1998, 10, 385–394. [Google Scholar] [CrossRef]
- Itoh, S.; Taketomi, A.; Harimoto, N.; Tsujita, E.; Rikimaru, T.; Shirabe, K.; Shimada, M.; Maehara, Y. Antineoplastic Effects of Gamma Linolenic Acid on Hepatocellular Carcinoma Cell Lines. J. Clin. Biochem. Nutr. 2010, 47, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Scheim, D.E. Cytotoxicity of Unsaturated Fatty Acids in Fresh Human Tumor Explants: Concentration Thresholds and Implications for Clinical Efficacy. Lipids Health Dis. 2009, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Horrobin, D.F. Nutritional and Medical Importance of Gamma-Linolenic Acid. Prog. Lipid Res. 1992, 31, 163–194. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Chapkin, R.S. Importance of Dietary γ-Linolenic Acid in Human Health and Nutrition. J. Nutr. 1998, 128, 1411–1414. [Google Scholar] [CrossRef] [Green Version]
- Cameron, M.; Gagnier, J.J.; Chrubasik, S. Herbal Therapy for Treating Rheumatoid Arthritis. Cochrane Database Syst. Rev. 2011, 16, CD002948. [Google Scholar] [CrossRef]
- Wafa’a, A.; Schwartz, M.D.; Alrashdi, S.; Algren, A.D.; Morgan, B.W. Status Epilepticus Associated with Borage Oil Ingestion. J. Med. Toxicol. 2011, 7, 154–157. [Google Scholar]
- Tasset-Cuevas, I.; Fernández-Bedmar, Z.; Lozano-Baena, M.D.; Campos-Sánchez, J.; de Haro-Bailón, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Protective Effect of Borage Seed Oil and Gamma Linolenic Acid on DNA: In Vivo and In Vitro Studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.A.; Sun, M.; Jeong, J. Borage Oil Treated with Immobilized Lipase Inhibits Melanogenesis. Lipids 2020, 55, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical Fluid Extraction of Seed Oils—A Short Review of Current Trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Guzmán-Albores, J.M.; Bojórquez-Velázquez, E.; De León-Rodríguez, A.; de Jesús Calva-Cruz, O.; de la Rosa, A.P.B.; Ruíz-Valdiviezo, V.M. Comparison of Moringa Oleifera Oils Extracted with Supercritical Fluids and Hexane and Characterization of Seed Storage Proteins in Defatted Flour. Food Biosci. 2021, 40, 100830. [Google Scholar] [CrossRef]
- Ishak, I.; Hussain, N.; Coorey, R.; Abd Ghani, M. Optimization and Characterization of Chia Seed (Salvia hispanica L.) Oil Extraction Using Supercritical Carbon Dioxide. J. CO2 Util. 2021, 45, 101430. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Novel Seeds Pretreatment Techniques: Effect on Oil Quality and Antioxidant Properties: A Review. J. Food Sci. Technol. 2021, 58, 4451–4464. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Gandhi, N.; Tyagi, S.K.; Kaur, A.; Mahajan, B.V.C. Extraction and Characterization of Guava Seed Oil: A Novel Industrial Byproduct. LWT 2020, 132, 109882. [Google Scholar] [CrossRef]
- Gomez, A.M.; de la Ossa, E.M. Quality of Borage Seed Oil Extracted by Liquid and Supercritical Carbon Dioxide. Chem. Eng. J. 2002, 88, 103–109. [Google Scholar] [CrossRef]
- Soto, C.; Conde, E.; Moure, A.; Zúñiga, M.E.; Domínguez, H. Supercritical Extraction of Borage Seed Oil Coupled to Conventional Solvent Extraction of Antioxidants. Eur. J. Lipid Sci. Technol. 2008, 110, 1035–1044. [Google Scholar] [CrossRef]
- Temelli, F.; Güçlü-Üstündağ, Ö. Supercritical Technologies for Further Processing of Edible Oils. In Bailey’s Industrial Oil and Fat Products; Wiley Online Library: Hoboken, NJ, USA, 2005; Volume 5, pp. 397–432. [Google Scholar]
- Brignole, E.; Pereda, S. High-Pressure Fractionation and Extraction of Natural Oils. In Supercritical Fluid Science and Technology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 3, pp. 239–261. [Google Scholar]
- Da Porto, C.; Decorti, D.; Natolino, A. Separation of Aroma Compounds from Industrial Hemp Inflorescences (Cannabis sativa L.) by Supercritical CO2 Extraction and on-Line Fractionation. Ind. Crops Prod. 2014, 58, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Baldino, L.; Adami, R.; Reverchon, E. Concentration of Ruta Graveolens Active Compounds Using SC-CO2 Extraction Coupled with Fractional Separation. J. Supercrit. Fluids 2018, 131, 82–86. [Google Scholar] [CrossRef]
- El Marsni, Z.; Casas, L.; Mantell, C.; Rodríguez, M.; Torres, A.; Macias, F.A.; de la Ossa, E.J.M.; Molinillo, J.M.G.; Varela, R.M. Potential Allelopathic of the Fractions Obtained from Sunflower Leaves Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2011, 60, 28–37. [Google Scholar] [CrossRef]
- El Marsni, Z.; Casas, L.; Mantell, C.; Rodríguez, M.; Torres, A.; Macias, F.A.; de la Ossa, E.J.M. Allelopathic Properties of the Fractions Obtained from Sunflower Leaves Using Supercritical Carbon Dioxide: The Effect of Co-Solvent Addition. J. Supercrit. Fluids 2013, 82, 221–229. [Google Scholar] [CrossRef]
- Fuentes-Gandara, F.; Torres, A.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Varela, R.; de la Ossa-Fernández, E.J.M.; Macias, F.A. Selective Fractionation and Isolation of Allelopathic Compounds from Helianthus annuus L. Leaves by Means of High-Pressure Techniques. J. Supercrit. Fluids 2019, 143, 32–41. [Google Scholar] [CrossRef]
- Fernandes, J.B.; Lisboa, P.F.; Barbosa Mota, J.P.; Simões, P.C. Modelling and Simulation of a Complete Supercritical Fluid Extraction Plant with Countercurrent Fractionation Column. Sep. Sci. Technol. 2011, 46, 2088–2098. [Google Scholar] [CrossRef]
- Bejarano, A.; Simões, P.C.; del Valle, J.M. Fractionation Technologies for Liquid Mixtures Using Dense Carbon Dioxide. J. Supercrit. Fluids 2016, 107, 321–348. [Google Scholar] [CrossRef]
- Torres, C.F.; Fornari, T.; Torrelo, G.; Señoráns, F.J.; Reglero, G. Production of Phytosterol Esters from Soybean Oil Deodorizer Distillates. Eur. J. Lipid Sci. Technol. 2009, 111, 459–463. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Temelli, F. Column Fractionation of Canola Oil Deodorizer Distillate Using Supercritical Carbon Dioxide. J. Am. Oil Chem. Soc. 2007, 84, 953–961. [Google Scholar] [CrossRef]
- Brunner, G.; Machado, N.T. Process Design Methodology for Fractionation of Fatty Acids from Palm Fatty Acid Distillates in Countercurrent Packed Columns with Supercritical CO2. J. Supercrit. Fluids 2012, 66, 96–110. [Google Scholar] [CrossRef]
- Al-Darmaki, N.; Lu, T.; Al-Duri, B.; Harris, J.B.; Favre, T.L.F.; Bhaggan, K.; Santos, R.C.D. Isothermal and Temperature Gradient Supercritical Fluid Extraction and Fractionation of Squalene from Palm Fatty Acid Distillate Using Compressed Carbon Dioxide. J. Supercrit. Fluids 2012, 61, 108–114. [Google Scholar] [CrossRef]
- Catchpole, O.J.; Grey, J.B.; Noermark, K.A. Fractionation of Fish Oils Using Supercritical CO2 and CO2+ Ethanol Mixtures. J. Supercrit. Fluids 2000, 19, 25–37. [Google Scholar] [CrossRef]
- Uemori, C.; Kawamoto, Y.; Matsubara, T.; Sasaki, Y.; Tanaka, M.; Hoshino, M.; Sakamoto, J.; Quitain, A.T.; Sasaki, M.; Goto, M. Development of Separation Technology for Valuable Oil Mixture of Citrus Juice Waste. ARPN J. Eng. Appl. Sci. 2013, 13, 9237–9243. [Google Scholar]
- Del Rio, M.; Fernández-Martínez, J.; De Haro, A. Wild and Cultivated Borago officinalis L.: Sources of Gamma-Linolenic Acid. Grasas y Aceites 1993, 44, 125–126. [Google Scholar]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC); Association of Official Analytical Chemists: Arlington, TX, USA, 1990. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant Activity Index (AAI) by the 2, 2-Diphenyl-1-Picrylhydrazyl Method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Taribak, C.; Casas, L.; Mantell, C.; Elfadli, Z.; Metni, R.E.; Martínez de la Ossa, E.J. Quality of Cosmetic Argan Oil Extracted by Supercritical Fluid Extraction from Argania spinosa L. J. Chem. 2013, 2013, 408194. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Martín, E.; Otero, C. Different Enzyme Requirements for the Synthesis of Biodiesel: Novozym® 435 and Lipozyme® TL IM. Bioresour. Technol. 2008, 99, 277–286. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Loft, S. Effect of Chemopreventive Compounds from Brassica Vegetables on NAD (P) H: Quinone Reductase and Induction of DNA Strand Breaks in Murine Hepa1c1c7 Cells. Food Chem. Toxicol. 2003, 41, 455–462. [Google Scholar] [CrossRef]
- Pham, H.; Vang, K.; Ziboh, V.A. Dietary γ-Linolenate Attenuates Tumor Growth in a Rodent Model of Prostatic Adenocarcinoma via Suppression of Elevated Generation of PGE2 and 5S-HETE. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Anter, J.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Demyda-Peyras, S.; Moreno-Millán, M.; Alonso-Moraga, Á.; Muñoz-Serrano, A.; de Castro, M.D.L. A Pilot Study on the DNA-Protective, Cytotoxic, and Apoptosis-Inducing Properties of Olive-Leaf Extracts. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 723, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Villatoro-Pulido, M.; Font, R.; Saha, S.; Obregón-Cano, S.; Anter, J.; Muñoz-Serrano, A.; De Haro-Bailón, A.; Alonso-Moraga, A.; Del Río-Celestino, M. In Vivo Biological Activity of Rocket Extracts (Eruca vesicaria Subsp. Sativa (Miller) Thell) and Sulforaphane. Food Chem. Toxicol. 2012, 50, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Anazetti, M.C.; Melo, P.S.; Durán, N.; Haun, M. Comparative Cytotoxicity of Dimethylamide-Crotonin in the Promyelocytic Leukemia Cell Line (HL60) and Human Peripheral Blood Mononuclear Cells. Toxicology 2003, 188, 261–274. [Google Scholar] [CrossRef]
- Akao, Y.; Maruyama, H.; Matsumoto, K.; Ohguchi, K.; Nishizawa, K.; Sakamoto, T.; Araki, Y.; Mishima, S.; Nozawa, Y. Cell Growth Inhibitory Effect of Cinnamic Acid Derivatives from Propolis on Human Tumor Cell Lines. Biol. Pharm. Bull. 2003, 26, 1057–1059. [Google Scholar] [CrossRef] [Green Version]
- Melo, P.S.; Justo, G.Z.; de Azevedo, M.B.M.; Durán, N.; Haun, M. Violacein and Its β-Cyclodextrin Complexes Induce Apoptosis and Differentiation in HL60 Cells. Toxicology 2003, 186, 217–225. [Google Scholar] [CrossRef]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Selvaggini, R.; Montedoro, G.F.; Di Saverio, C.; Morozzi, G. Virgin Olive Oil Phenols Inhibit Proliferation of Human Promyelocytic Leukemia Cells (HL60) by Inducing Apoptosis and Differentiation. J. Nutr. 2006, 136, 614–619. [Google Scholar] [CrossRef] [Green Version]
- Yedjou, C.G.; Moore, P.; Tchounwou, P.B. Dose-and Time-Dependent Response of Human Leukemia (HL-60) Cells to Arsenic Trioxide Treatment. Int. J. Environ. Res. Public Health 2006, 3, 136–140. [Google Scholar] [CrossRef]
- Villatoro-pulido, M.; Font, R.; Obregón-cano, S.; Moreno-rojas, R. Cytotoxic and Genotoxic Effects of Metal (Oid) s Bioactivated in Rocket Leaves (Eruca vesicaria Subsp. Sativa Miller). Chemosphere 2013, 93, 2554–2561. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The Antioxidant Activity and Oxidative Stability of Cold-pressed Oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y.; Chi, X. Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage. Molecules 2020, 25, 1157. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Shahidi, F. Photooxidative Stability of Stripped and Non-Stripped Borage and Evening Primrose Oils and Their Emulsions in Water. Food Chem. 2002, 79, 47–53. [Google Scholar] [CrossRef]
- Namal Senanayake, S.P.J.; Shahidi, F. Oxidative Stability of Structured Lipids Produced from Borage (Borago officinalis L.) and Evening Primrose (Oenothera biennis L.) Oils with Docosahexaenoic Acid. J. Am. Oil Chem. Soc. 2002, 79, 1003–1013. [Google Scholar] [CrossRef]
- Soto, C.; Concha, J.; Zuniga, M.E. Antioxidant Content of Oil and Defatted Meal Obtained from Borage Seeds by an Enzymatic-Aided Cold Pressing Process. Process. Biochem. 2008, 43, 696–699. [Google Scholar] [CrossRef]
- Tamkutė, L.; Pukalskas, A.; Syrpas, M.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Fractionation of Cranberry Pomace Lipids by Supercritical Carbon Dioxide Extraction and On-Line Separation of Extracts at Low Temperatures. J. Supercrit. Fluids 2020, 163, 104884. [Google Scholar] [CrossRef]
- Kavousi, A.; Torabi, F.; Chan, C.W.; Shirif, E. Experimental Measurement and Parametric Study of CO2 Solubility and Molecular Diffusivity in Heavy Crude Oil Systems. Fluid Phase Equilib. 2014, 371, 57–66. [Google Scholar] [CrossRef]
- Catchpole, O.J.; Tallon, S.J.; Eltringham, W.E.; Grey, J.B.; Fenton, K.A.; Vagi, E.M.; Vyssotski, M.V.; MacKenzie, A.N.; Ryan, J.; Zhu, Y. The Extraction and Fractionation of Specialty Lipids Using near Critical Fluids. J. Supercrit. Fluids 2009, 47, 591–597. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated Methodology to Determine Antioxidant Capacity in Plant Foods, Oils and Beverages: Extraction, Measurement and Expression of Results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Oliveira, R.; Fátima Rodrigues, M.; Gabriela Bernardo-Gil, M. Characterization and Supercritical Carbon Dioxide Extraction of Walnut Oil. J. Am. Oil Chem. Soc. 2002, 79, 225–230. [Google Scholar] [CrossRef]
- Fernández-Bedmar, Z.; Anter, J.; de La Cruz-Ares, S.; Muñoz-Serrano, A.; Alonso-Moraga, Á.; Pérez-Guisado, J. Role of Citrus Juices and Distinctive Components in the Modulation of Degenerative Processes: Genotoxicity, Antigenotoxicity, Cytotoxicity, and Longevity in Drosophila. J. Toxicol. Environ. Health Part A 2011, 74, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Fernández, M.; Merinas-Amo, T.; Moreno-Millán, M.; Alonso-Moraga, Á.; Demyda-Peyrás, S. In Vivo and in Vitro Genotoxic and Epigenetic Effects of Two Types of Cola Beverages and Caffeine: A Multiassay Approach. Biomed Res. Int. 2016, 2016, 7574843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, P.C.; Carmelo, P.J.; Pereira, P.J.; Lopes, J.A.; da Ponte, M.N.; Brunner, G. Quality Assessment of Refined Olive Oils by Gas Extraction. J. Supercrit. Fluids 1998, 13, 337–341. [Google Scholar] [CrossRef]
- Vázquez, L.; Hurtado-Benavides, A.M.; Reglero, G.; Fornari, T.; Ibáñez, E.; Señoráns, F.J. Deacidification of Olive Oil by Countercurrent Supercritical Carbon Dioxide Extraction: Experimental and Thermodynamic Modeling. J. Food Eng. 2009, 90, 463–470. [Google Scholar] [CrossRef]

| Test | Separator 1 (S1) | Separator 2 (S2) |
|---|---|---|
| 1 | 200 bar/45 °C | atmospheric conditions |
| 2 | 100 bar/40 °C | atmospheric conditions |
| 3 | 90 bar/40 °C | atmospheric conditions |
| 4 | 70 bar/40 °C | atmospheric conditions |
| Fractionation Conditions | CO2 Density (kg/m3) in S1 | Separation Percentage (%) | Acidity Index (%) | EC50 Values (mg/mg DPPH) | |||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | ||
| 200 bar/45 °C | 865.66 | 34 ± 4 | 66 ± 3 | 2.7 ± 0.6 | 3.5 ± 1.2 | 150 ± 7 | 178 ± 7 |
| 100 bar/40 °C | 622.64 | 55 ± 3 | 45 ± 2 | 3.1 ± 0.9 | 7.0 ± 1.2 | 132 ± 8 | 182 ± 4 |
| 90 bar/40 °C | 484.09 | 82 ± 6 | 18 ± 4 | 3.0 ± 0.8 | 10.5 ± 1.9 | 138 ± 6 | 162 ± 9 |
| 70 bar/40 °C | 198.32 | 99± 1 | 1± 1 | 5.4 ± 0.6 | n.d. | 118 ± 7 | n.d. |
| Fractionating Conditions | Palmitic | Estearic | Oleic | Linoleic | γ-Linolenic | Eicosenoic | Erucic | |
|---|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 (n-6) | C20:1 | C22:1 | ||
| 200 bar/45 °C | S1 | 11.30 ± 1.50 | 4.07 ± 0.64 | 16.51 ± 1.91 | 37.21 ± 2.51 | 24.01 ± 2.25 | 4.11 ± 1.33 | 2.80 ± 1.91 |
| S2 | 11.76 ± 1.03 | 3.86 ± 0.64 | 16.78 ± 1.27 | 37.22 ± 1.99 | 24.07 ± 2.94 | 3.79 ± 1.09 | 2.53 ± 1.89 | |
| 100 bar/40 °C | S1 | 11.50 ± 1.64 | 3.89 ± 0.99 | 16.53 ± 1.27 | 37.29 ± 2.34 | 24.27 ± 3.03 | 3.94 ± 1.65 | 2.58 ± 1.67 |
| S2 | 12.89 ± 1.82 | 4.21 ± 0.81 | 17.29 ± 1.49 | 36.53 ± 2.12 | 23.11 ± 2.78 | 3.60 ± 1.69 | 2.36 ± 1.50 | |
| 90 bar/40 °C | S1 | 11.66 ± 1.95 | 3.84 ± 0.94 | 16.54 ± 1.31 | 37.31 ± 2.63 | 24.18 ± 2.69 | 3.81 ± 2.36 | 2.69 ± 1.56 |
| S2 | 13.35 ± 1.91 | 4.40 ± 0.64 | 17.68 ± 1.23 | 35.78 ± 2.54 | 22.77 ± 3.03 | 3.53 ± 02.29 | 2.51 ± 1.49 | |
| 70 bar/40 °C | S1 | 11.84 ± 1.92 | 3.93 ± 1.03 | 16.54 ± 1.44 | 37.13 ± 2.09 | 24.16 ± 2.38 | 3.80 ± 02.67 | 2.62 ± 1.82 |
| Extraction with hexane | 10.15 ± 1.88 | 4.12 ± 0.64 | 17.18 ± 1.23 | 38.01 ± 2.45 | 24.11 ± 2.94 | 4.18 ± 2.40 | 2.63 ± 1.49 | |
| ANOVA/p-value | 0.0678 | 0.3021 | 0.289 | 0.129 | 0.5673 | 0.3478 | 0.5871 | |
| Pressure | Extracts 35 °C | Raffinate 35 °C | Extracts 55 °C | Raffinate 55 °C | Extracts 75 °C | Raffinate 75 °C |
|---|---|---|---|---|---|---|
| 100 bar | 0.005 | 4.950 | 0 | 5.297 | 0 | 4.877 |
| 200 bar | 0.071 | 5.536 | 0.037 | 5.556 | 0.004 | 5.643 |
| 300 bar | 0.550 | 5.750 | 0.133 | 5.000 | 0.100 | 5.769 |
| 400 bar | 5.250 | 0 | 0.360 | 4.950 | 0.278 | 4.444 |
| Pressure | Raffinate 35 °C | Raffinate 55 °C | Raffinate 75 °C |
|---|---|---|---|
| 100 bar | 278 ± 7 | 240 ± 5 | 270 ± 8 |
| 200 bar | 237 ± 5 | 259 ± 7 | 251 ± 5 |
| 300 bar | 248 ± 5 | 283 ± 4 | 247 ± 4 |
| 400 bar | - | 284 ± 7 | 253 ± 6 |
| Fractionation Conditions | Palmitic | Estearic | Oleic | Linoleic | γ-Linolenic | Eicosenoic | Erucic |
|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 (n-6) | C20:1 | C22:1 | |
| Crude borage oil | 10.69 ± 1.97 | 4.61 ± 0.97 | 18.63 ± 2.91 | 37.45 ± 2.76 | 21.22 ± 3.01 | 4.34 ± 1.39 | 3.08 ± 1.17 |
| Raffinate | 10.60 ± 2.25 | 4.57 ± 0.88 | 18.66 ± 2.76 | 37.52 ± 3.05 | 21.34 ± 2.98 | 4.40 ± 1.48 | 2.94 ± 1.18 |
| Extract | 13.18 * ± 1.09 | 4.36 ± 0.91 | 20.02 ± 1.03 | 37.27 ± 2.93 | 19.87 ± 2.75 | 3.34 ± 1.26 | 1.96 ± 1.06 |
| ANOVA/p value | 0.036 | 0.5482 | 0.2138 | 0.9923 | 0.6237 | 0.1523 | 0.5488 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Cardoso, L.; Mantell, C.; Obregón, S.; Cejudo-Bastante, C.; Alonso-Moraga, Á.; de la Ossa, E.J.M.; de Haro-Bailón, A. Health-Promoting Properties of Borage Seed Oil Fractionated by Supercritical Carbon Dioxide Extraction. Foods 2021, 10, 2471. https://doi.org/10.3390/foods10102471
Casas-Cardoso L, Mantell C, Obregón S, Cejudo-Bastante C, Alonso-Moraga Á, de la Ossa EJM, de Haro-Bailón A. Health-Promoting Properties of Borage Seed Oil Fractionated by Supercritical Carbon Dioxide Extraction. Foods. 2021; 10(10):2471. https://doi.org/10.3390/foods10102471
Chicago/Turabian StyleCasas-Cardoso, Lourdes, Casimiro Mantell, Sara Obregón, Cristina Cejudo-Bastante, Ángeles Alonso-Moraga, Enrique J. Martínez de la Ossa, and Antonio de Haro-Bailón. 2021. "Health-Promoting Properties of Borage Seed Oil Fractionated by Supercritical Carbon Dioxide Extraction" Foods 10, no. 10: 2471. https://doi.org/10.3390/foods10102471
APA StyleCasas-Cardoso, L., Mantell, C., Obregón, S., Cejudo-Bastante, C., Alonso-Moraga, Á., de la Ossa, E. J. M., & de Haro-Bailón, A. (2021). Health-Promoting Properties of Borage Seed Oil Fractionated by Supercritical Carbon Dioxide Extraction. Foods, 10(10), 2471. https://doi.org/10.3390/foods10102471










