Effect of Drying Methods on Volatile Compounds of Burdock (Arctium lappa L.) Root Tea as Revealed by Gas Chromatography Mass Spectrometry-Based Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Hot-Air Drying (HD) Procedure
2.2.2. Sun Drying (SD) Procedure
2.2.3. Vacuum Freeze-Drying (VFD) Procedure
2.2.4. Vacuum Drying (VD) Procedure
2.2.5. Natural Drying (ND) Procedure
2.3. Color Measurement
2.4. Water Activity (Aw) Determination
2.5. Preparation of BR Powder
2.6. Preparation of QC Samples
2.7. HS-GC-MS Conditions
2.8. Data Processing
2.9. Statistical Analysis
3. Results and Discussion
3.1. Appearance of Dried Burdock
3.2. Optimization of HS-GC-MS Parameters
3.3. Volatile Compounds Characterization by HS-GC-MS
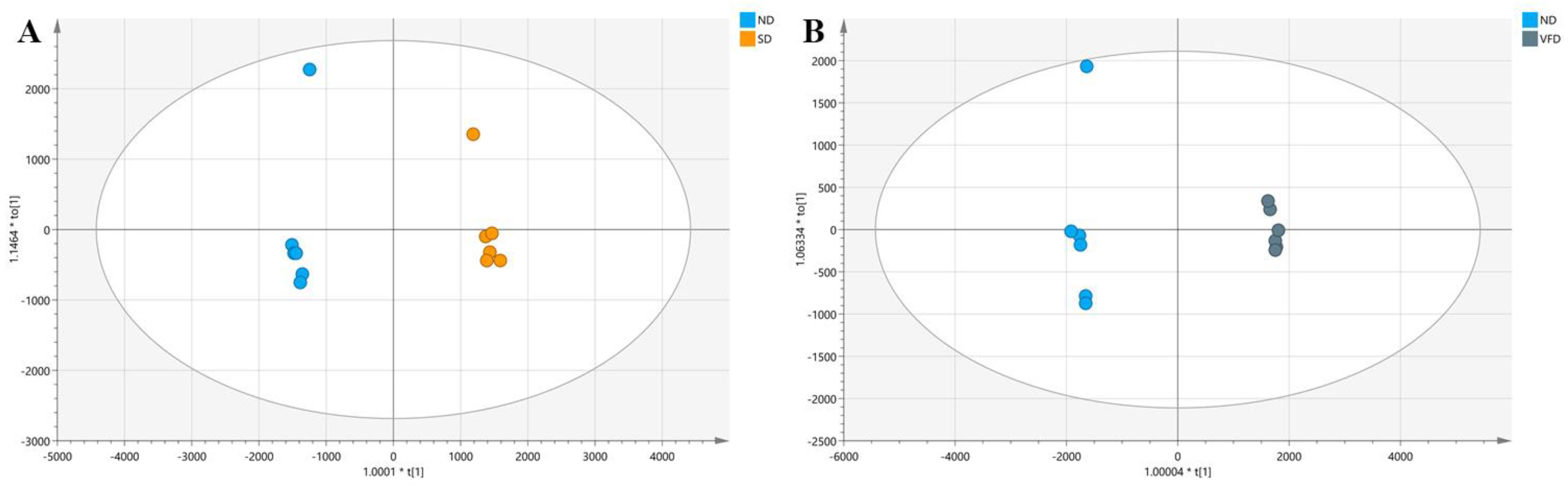
3.4. Comparisons of SD, VFD, HD, VD, and ND on the Volatile Compounds of BR by OPLS-DA
3.4.1. Comparison between SD and ND
3.4.2. Comparison between VFD and ND
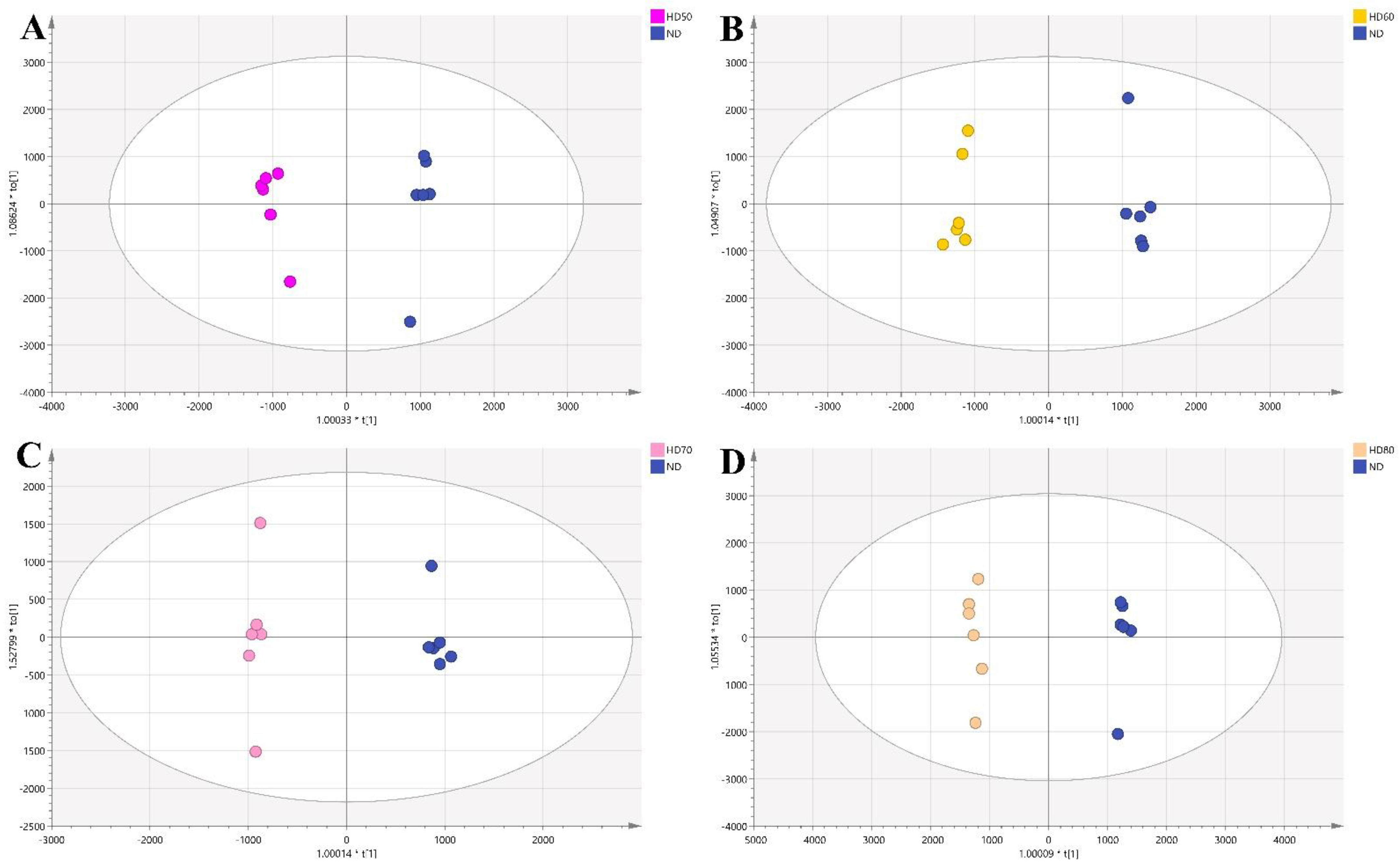
3.4.3. Comparison between HD and ND
3.4.4. Comparison between VD and ND
3.5. HCA of Volatile Compounds in BR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Wang, J.; Zhang, Z.; Xu, J.; Xie, Z.; Slavin, M.; Gao, X. In vitro and in vivo antioxidant activity of a fructan from the roots of Arctium lappa L. Int. J. Biol. Macromol. 2014, 65, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Maghsoumi-Norouzabad, L.; Shishehbor, F.; Abed, R.; Zare Javid, A.; Eftekhar-Sadat, B.; Alipour, B. Effect of Arctium lappa linne (Burdock) root tea consumption on lipid profile and blood pressure in patients with knee osteoarthritis. J. Herbal Med. 2019, 17–18, 100266. [Google Scholar] [CrossRef]
- Predes, F.S.; Ruiz, A.L.; Carvalho, J.E.; Foglio, M.A.; Dolder, H. Antioxidative and in vitro antiproliferative activity of Arctium lappa root extracts. BMC Complement. Altern. Med. 2011, 11, 25. [Google Scholar] [CrossRef]
- Tousch, D.; Bidel, L.P.; Cazals, G.; Ferrare, K.; Leroy, J.; Faucanie, M.; Chevassus, H.; Tournier, M.; Lajoix, A.D.; Azay-Milhau, J. Chemical analysis and antihyperglycemic activity of an original extract from burdock root (Arctium lappa). J. Agric. Food Chem. 2014, 62, 7738–7745. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, P.; Wang, C.; Jiang, Q.; Zhang, L.; Cao, Y.; Zhong, W.; Wang, C. Protective effects of Arctium lappa L. root extracts (AREs) on high fat diet induced quail atherosclerosis. BMC Complement. Altern. Med. 2016, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.; Leung, G.P.; Yu, P.H.; Chan, S.W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Haenly, J.M. Identification of Hydroxycinnamoylquinic Acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. J. Agric. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Kan, J.; Zhang, X.; Wu, X.; Sun, R.; Tang, S.; Liu, J.; Qian, C.; Jin, C. Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice. Carbohydr. Polym. 2019, 213, 89–99. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Liu, P.; Li, M.; Dong, H.; Qiao, X. Semi-preparative separation of 10 caffeoylquinic acid derivatives using high speed counter-current chromatogaphy combined with semi-preparative HPLC from the roots of burdock (Arctium lappa L.). Molecules 2018, 23, 429. [Google Scholar] [CrossRef]
- Jiang, X.-W.; Bai, J.-P.; Zhang, Q.; Hu, X.-L.; Tian, X.; Zhu, J.; Liu, J.; Meng, W.-H.; Zhao, Q.-C. Caffeoylquinic acid derivatives from the roots of Arctium lappa L. (burdock) and their structure–activity relationships (SARs) of free radical scavenging activities. Phytochem. Lett. 2016, 15, 159–163. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kuhnert, N. Identification and characterization of five new classes of chlorogenic acids in burdock (Arctium lappa L.) roots by liquid chromatography/tandem mass spectrometry. Food Funct. 2011, 2, 63–71. [Google Scholar] [CrossRef]
- Du, L.L.; Fu, Q.Y.; Xiang, L.P.; Zheng, X.Q.; Lu, J.L.; Ye, J.H.; Li, Q.S.; Polito, C.A.; Liang, Y.R. Tea polysaccharides and their bioactivities. Molecules 2016, 21, 1449. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.M.; Ida, E.I.; Falcão, H.G.; de Rezende, T.A.M.; de Santana Silva, J.; Fernandes Alves, C.C.; da Silva Pereira, M.A.; Egea, M.B. Evaluating technological quality of okara flours obtained by different drying processes. LWT-Food Sci. Technol. 2020, 123, 109062. [Google Scholar] [CrossRef]
- Ye, S.; Wang, Z.; Shen, J.; Shao, Q.; Fang, H.; Zheng, B.; Younis, A. Sensory qualities, aroma components, and bioactive compounds of Anoectochilus roxburghii (Wall.) Lindl. as affected by different drying methods. Ind. Crops Prod. 2019, 134, 80–88. [Google Scholar] [CrossRef]
- Sárosi, S.; Sipos, L.; Kókai, Z.; Pluhár, Z.; Szilvássy, B.; Novák, I. Effect of different drying techniques on the aroma profile of Thymus vulgaris analyzed by GC–MS and sensory profile methods. Ind. Crops Prod. 2013, 46, 210–216. [Google Scholar] [CrossRef]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef]
- Kopyt’ko, Y.F.; Kir’yanov, A.A.; Stikhin, Y.V.; Stikhin, V.A.; Sokol’skaya, T.A. Composition of volatile substances and fatty acids isolated from the juice of woolly burdock leaves. Pharm. Chem. J. 2003, 37, 325–326. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass spectrometry-based metabolomics of volatiles as a new tool for understanding aroma and flavour chemistry in processed food products. Metabolomics 2019, 15, 41. [Google Scholar] [CrossRef]
- Li, X.; Tsuta, M.; Hayakawa, F.; Nakano, Y.; Kazami, Y.; Ikehata, A. Estimating the sensory qualities of tomatoes using visible and near-infrared spectroscopy and interpretation based on gas chromatography-mass spectrometry metabolomics. Food Chem. 2021, 343, 128470. [Google Scholar] [CrossRef]
- Khodadadi, M.; Pourfarzam, M. A review of strategies for untargeted urinary metabolomic analysis using gas chromatography-mass spectrometry. Metabolomics 2020, 16, 66. [Google Scholar] [CrossRef]
- Lv, M.; Sun, J.; Min, W.; Fan, H.; Zunjian, Z.; Fengguo, X. Comparative analysis of volatile oils in the stems and roots of Ephedra sinica via GC-MS-based plant metabolomics. Chin. J. Nat. Med. 2016, 14, 133–140. [Google Scholar] [CrossRef]
- Jing, N.; Wang, M.; Gao, M.; Zhong, Z.; Ma, Y.; Wei, A. Color sensory characteristics, nutritional components and antioxidant capacity of Zanthoxylum bungeanum Maxim. as affected by different drying methods. Ind. Crops Prod. 2021, 160, 113167. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, X.; Guo, X.; Guo, Q.; Li, D. Metabolomics characterization of two Apocynaceae plants, Catharanthus roseus and Vinca minor, using GC-MS and LC-MS methods in combination. Molecules 2017, 22, 997. [Google Scholar] [CrossRef]
- Perez de Souza, L.; Alseekh, S.; Naake, T.; Fernie, A. Mass spectrometry-based untargeted plant metabolomics. Curr. Protoc. Plant Biol. 2019, 4, e20100. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Moro, T.M.A.; Celegatti, C.M.; Pereira, A.P.A.; Lopes, A.S.; Barbin, D.F.; Pastore, G.M.; Clerici, M.T.P.S. Use of burdock root flour as a prebiotic ingredient in cookies. LWT-Food Sci. Technol. 2018, 90, 540–546. [Google Scholar] [CrossRef]
- Rahman, M.S. Water activity and glass transition of foods. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Zhang, W.; Liang, X. Headspace gas chromatography-mass spectrometry for volatile components analysis in Ipomoea Cairica (L.) sweet leaves: Natural deep eutectic solvents as green extraction and dilution matrix. Foods 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Errabelli, R.; Feener, D.H.; Noble, K.; Attygalle, A.B. Identification of alkylpyrazines by gas chromatography mass spectrometry (GC-MS). J. Chromatogr. A 2019, 1589, 149–161. [Google Scholar] [CrossRef]
- Jena, S.; Ray, A.; Sahoo, A.; Panda, P.C.; Nayak, S. Deeper insight into the volatile profile of essential oil of two Curcuma species and their antioxidant and antimicrobial activities. Ind. Crops Prod. 2020, 155, 112380. [Google Scholar] [CrossRef]
- Mo, X.; Peng, X.; Liang, X.; Fang, S.; Xie, H.; Chen, J.; Meng, Y. Development of antifungal gelatin-based nanocomposite films functionalized with natamycin-loaded zein/casein nanoparticles. Food Hydrocoll. 2021, 113, 106506. [Google Scholar] [CrossRef]
- Lou, Z.; Liu, Y.; Hong, Y.; Song, X.; Wang, H.; Ai, L. Anti-biofilm activities and chemical composition of essential oil from burdock leaf. Food Sci. Technol. Res. 2013, 19, 915–921. [Google Scholar] [CrossRef][Green Version]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K.; Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197 Pt B, 1292–1300. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, G.; Huang, F.; Banda, M.; Reed, E. Antineoplastic effect of beta-elemene on prostate cancer cells and other types of solid tumour cells. J. Pharm. Pharmacol. 2010, 62, 1018–1027. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Prasad, S.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a novel compound (beta-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: Comparison with curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, R.E.; Diaz, D.; Duarte, G.; Lappe-Oliveras, P.; Sanchez, S.; Macias-Rubalcava, M.L. Antifungal volatile organic compounds from the endophyte Nodulisporium sp. strain GS4d2II1a: A qualitative change in the intraspecific and interspecific interactions with Pythium aphanidermatum. Microbial. Ecol. 2016, 71, 347–364. [Google Scholar] [CrossRef]
- Clerck, C.; Maso, S.D.; Parisi, O.; Dresen, F.; Zhiri, A.; Jijakli, M.H. Screening of antifungal and antibacterial activity of 90 commercial essential oils against 10 pathogens of agronomical importance. Foods 2020, 9, 1418. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Mohammadi, X.; Deng, Y.; Matinfar, G.; Singh, A.; Mandal, R.; Pratap-Singh, A. Impact of three different dehydration methods on nutritional values and sensory quality of dried broccoli, oranges, and carrots. Foods 2020, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, X.; Zhang, Q.; Giovanni, V.; Meng, X. Key composition optimization of meat processed protein source by vacuum freeze-drying technology. Saudi J. Biol. Sci. 2018, 25, 724–732. [Google Scholar] [CrossRef]
- Petricevic, S.; Marusic Radovcic, N.; Lukic, K.; Listes, E.; Medic, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Carballo, J.; Franco, D. Effect of the inclusion of chestnut in the finishing diet on volatile compounds of dry-cured ham from celta pig breed. J. Integr. Agric. 2013, 12, 2002–2012. [Google Scholar] [CrossRef]
- Gonçalves, G.M.S.; Barros, P.P.; de Silva, G.H.; Fedes, G.R. The essential oil of Curcuma longa rhizomes as an antimicrobial and its composition by gas chromatography/mass spectrometry. Rev. Ciênc. Méd. 2019, 28, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, M.; Liu, F.; Feng, X.; Ibrahim, S.A.; Cheng, L.; Huang, W. Effects of freeze drying and hot-air drying on the physicochemical properties and bioactivities of polysaccharides from Lentinula edodes. Int. J. Biol. Macromol. 2020, 145, 476–483. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Pei, Z.; Wei, P.; Xiang, D.; Cao, X.; Shen, X.; Li, C. Volatile flavour components and the mechanisms underlying their production in golden pompano (Trachinotus blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC-MS. Food Res. Int. 2019, 123, 217–225. [Google Scholar] [CrossRef]
- Demarchi, S.M.; Torrez Irigoyen, R.M.; Giner, S.A. Vacuum drying of rosehip leathers: Modelling of coupled moisture content and temperature curves as a function of time with simultaneous time-varying ascorbic acid retention. J. Food Eng. 2018, 233, 9–16. [Google Scholar] [CrossRef]




| Treatment 1 | a* | b* | L* | ΔE |
|---|---|---|---|---|
| HD50 | 3.45 ± 0.23 | 23.41 ± 0.42 | 70.34 ± 0.73 | 4.68 |
| HD60 | 2.01 ± 1.02 | 24.87 ± 1.24 | 71.87 ± 0.93 | 6.89 |
| HD70 | 2.70 ± 0.47 | 23.12 ± 0.57 | 64.37 ± 1.04 | 4.97 |
| HD80 | 2.73 ± 0.22 | 26.22 ± 1.07 | 72.86 ± 0.59 | 7.55 |
| VD50 | 4.05 ± 0.37 | 17.39 ± 0.95 | 62.34 ± 0.90 | 8.27 |
| VD60 | 2.78 ± 0.32 | 17.75 ± 0.58 | 53.70 ± 1.33 | 15.16 |
| VD70 | 3.63 ± 0.14 | 14.16 ± 1.59 | 64.02 ± 1.40 | 10.40 |
| VD80 | 4.06 ± 1.54 | 20.77 ± 2.71 | 64.32 ± 1.56 | 4.82 |
| ND | 2.40 ± 1.44 | 20.05 ± 4.54 | 66.06 ± 0.43 | 5.74 |
| SD | 1.96 ± 0.24 | 26.35 ± 0.38 | 76.56 ± 1.02 | 10.99 |
| VFD | 2.75 ± 0.73 | 16.76 ± 2.83 | 66.86 ± 0.51 | 7.93 |
| FP | 6.83 ± 0.61 | 23.56 ± 0.48 | 67.11 ± 0.87 | / |
| No. | Compounds | CAS | RT/min | Formula | Mw | Score | RIt | RIc |
|---|---|---|---|---|---|---|---|---|
| 1 | Chloromethane | 74-87-3 | 1.911 | CH3Cl | 50.49 | 992 | 329 | 484.2 |
| 2 | Methanethiol | 74-93-1 | 2.448 | CH4S | 48.11 | 930 | 401 | 497.0 |
| 3 | Formic acid | 64-18-6 | 3.116 | C2H6O | 46.07 | 803 | 526 | 522.7 |
| 4 | Propanal | 123-38-6 | 3.439 | C3H6O | 58.08 | 986 | 461 | 532.5 |
| 5 | Dimethyl sulfide | 75-18-3 | 3.653 | C2H6S | 62.13 | 937 | 520 | 539.2 |
| 6 | Isopropyl Alcohol | 67-63-0 | 3.846 | C3H8O | 60.10 | 974 | 486 | 545.7 |
| 7 | Methylene chloride | 75-09-2 | 4.194 | CH2Cl2 | 84.93 | 862 | 528 | 556.4 |
| 8 | 2-Butanone | 78-93-3 | 4.998 | C4H8O | 72.11 | 814 | 598 | 581.7 |
| 9 | 3-Methylfuran | 930-27-8 | 5.97 | C5H6O | 82.10 | 940 | 614 | 612.2 |
| 10 | Butanal | 123-72-8 | 6.56 | C4H8O | 72.11 | 855 | 593 | 631.0 |
| 11 | 2,3-Dimethoxy-1,4-dioxane | 23918-30-1 | 6.747 | C6H12O4 | 148.16 | 731 | / | 636.7 |
| 12 | 3-Methylbutanal | 590-86-3 | 8.36 | C5H10O | 86.13 | 934 | 652 | 687.6 |
| 13 | 2-Methylbutanal | 96-17-3 | 8.622 | C5H10O | 86.13 | 939 | 662 | 695.8 |
| 14 | Acetic acid | 64-19-7 | 9.164 | C2H4O | 60.05 | 984 | 610 | 712.8 |
| 15 | 2-Ethylfuran | 3208-16-0 | 9.273 | C6H8O | 96.13 | 949 | 703 | 716.4 |
| 16 | 1-Penten-3-one | 1629-58-9 | 9.52 | C5H8O | 84.12 | 841 | 681 | 724.2 |
| 17 | Pentanal | 110-62-3 | 9.775 | C5H10O | 86.13 | 871 | 699 | 732.2 |
| 18 | 2,3-Pentanedione | 600-14-6 | 9.974 | C5H8O2 | 100.12 | 904 | 698 | 734.5 |
| 19 | 1-Chloropentane | 543-59-9 | 11.043 | C5H11Cl | 106.59 | 891 | 742 | 772.2 |
| 20 | Toluene | 108-88-3 | 11.539 | C7H8 | 92.14 | 831 | 763 | 787.8 |
| 21 | Hexanal | 66-25-1 | 12.893 | C6H12O | 100.16 | 952 | 800 | 830.6 |
| 22 | Methylpyrazine | 109-08-0 | 13.608 | C5H6N2 | 94.11 | 827 | 831 | 861.1 |
| 23 | Furfural | 98-01-1 | 14.477 | C5H4O2 | 96.08 | 959 | 833 | 892.6 |
| 24 | 2-n-Butyl furan | 4466-24-4 | 14.851 | C8H12O | 124.18 | 850 | 893 | 907.0 |
| 25 | 2-Heptanone | 110-43-0 | 15.411 | C7H14O | 114.19 | 781 | 891 | 930.1 |
| 26 | Heptanal | 111-71-7 | 15.598 | C7H14O | 114.19 | 860 | 901 | 937.7 |
| 27 | 2,5-Dimethylpyrazine | 123-32-0 | 15.818 | C6H8N2 | 108.14 | 885 | 917 | 946.8 |
| 28 | 1-(2-Furanyl)-ethanone | 1192-62-7 | 16.382 | C6H6O2 | 110.11 | 964 | 911 | 970.0 |
| 29 | 2-Pentylfuran | 3777-69-3 | 17.267 | C9H14O | 138.21 | 956 | 993 | 1007.1 |
| 30 | Benzaldehyde | 100-52-7 | 17.542 | C7H6O | 106.12 | 952 | 962 | 1019.6 |
| 31 | 5-Methyl-2-furaldehyde | 620-02-0 | 17.768 | C6H6O2 | 110.11 | 907 | 980 | 1029.9 |
| 32 | Phenol | 108-95-2 | 19.193 | C6H6O | 94.11 | 905 | 980 | 993.9 |
| 33 | Pyrazinamide | 98-96-4 | 19.403 | C5H5N3O | 123.11 | 844 | 1250 | 1104.7 |
| 34 | Benzeneacetaldehyde | 122-78-1 | 19.472 | C8H8O | 120.15 | 942 | 1051 | 1108.1 |
| 35 | 3,5-Octadien-2-one | 38284-27-4 | 19.83 | C8H12O | 124.18 | 705 | 1091 | 1125.6 |
| 36 | Nonanal | 124-19-6 | 20.176 | C9H18O | 142.24 | 767 | 1104 | 1142.7 |
| 37 | 1-(1H-pyrrol-2-yl)-ethanone | 1072-83-9 | 20.356 | C6H7NO | 109.13 | 852 | 1072 | 1151.8 |
| 38 | 2-Methoxy-3-(2-methylpropyl)-pyrazine | 24683-00-9 | 21.41 | C9H14N2O | 166.22 | 871 | 1183 | 1205.2 |
| 39 | Decanal | 112-31-2 | 22.1 | C10H20O | 156.27 | 772 | 1206 | 1249.0 |
| 40 | 3-Phenylfuran | 13679-41-9 | 22.637 | C10H8O | 144.17 | 845 | 1226 | 1283.0 |
| 41 | β-Elemene | 515-13-9 | 24.375 | C15H24 | 204.35 | 910 | 1391 | 1431.7 |
| 42 | 1-Pentadecene | 13360-61-7 | 24.975 | C15H30 | 210.40 | 915 | 1492 | 1491.1 |
| 43 | γ-Curcumene | 451-55-8 | 25.172 | C15H24 | 204.35 | 814 | 1480 | 1509.6 |
| 44 | 1-Methyl-4-(6-methylhept-5-en-2-yl) benzene | 644-30-4 | 25.251 | C15H22 | 202.34 | 936 | 1483 | 1516.7 |
| 45 | β-Selinene | 17066-67-0 | 25.613 | C15H24 | 204.35 | 870 | 1486 | 1548.9 |
| 46 | α-Selinene | 473-13-2 | 25.67 | C15H24 | 204.35 | 767 | 1494 | 1554.0 |
| 47 | Heptadeca-1,8,11-triene | 56134-03-3 | 27.194 | C17H30 | 234.42 | 865 | 1665 | 1675.7 |
| 48 | Heptadeca-1,8,11,14-tetraene | 10482-53-8 | 27.333 | C17H28 | 232.40 | 955 | 1664 | 1686.1 |
| 49 | β-Eudesmol | 473-15-4 | 28.444 | C15H26O | 222.37 | 819 | 1649 | 1754.9 |
| RT/min | Compound | HD50/ND | HD60/ND | HD70/ND | HD80/ND | SD/ND | VD50/ND | VD60/ND | VD70/ND | VD80/ND | VFD/ND |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.911 | Chloromethane | 2.52 | 2.54 | / | 3.50 | 2.73 | 3.72 | / | / | 0.76 | 2.33 |
| 2.318 | Methanethiol | / | / | / | 1.83 | / | / | / | / | / | / |
| 3.115 | Unknown | 2.76 | 6.23 | 3.33 | 4.82 | 2.65 | 2.76 | 5.30 | 6.02 | 5.35 | 6.47 |
| 3.116 | Formic acid | 2.83 | 6.40 | 3.40 | 4.94 | 2.79 | 2.83 | 5.46 | 6.22 | 5.51 | 6.65 |
| 3.439 | Propanal | 1.39 | 1.42 | / | 1.51 | 1.28 | 1.32 | / | / | / | / |
| 3.557 | Unknown | / | / | / | 0.70 | / | / | 0.62 | / | 0.62 | 1.65 |
| 3.625 | Unknown | / | / | / | 0.65 | 1.24 | / | 0.57 | / | 0.60 | 1.66 |
| 3.653 | Dimethyl sulfide | / | 0.34 | / | 0.65 | 1.32 | / | 0.52 | / | 0.61 | 1.75 |
| 3.846 | Isopropyl alcohol | / | 12.12 | 17.35 | / | 12.68 | / | / | / | / | / |
| 4.194 | Methylene chloride | / | / | / | / | / | / | 28.90 | 33.94 | / | / |
| 4.595 | Unknown | 2.55 | 2.66 | / | 3.45 | 4.18 | 3.14 | / | / | / | 2.48 |
| 4.998 | 2-Butanone | 1.30 | / | / | 1.52 | 1.76 | 1.53 | / | / | 1.53 | 1.85 |
| 6.219 | Unknown | 0.27 | 0.22 | 0.10 | 0.21 | 0.35 | 0.21 | 0.15 | 0.07 | 0.10 | 0.09 |
| 6.327 | Unknown | / | / | / | / | / | 1.67 | / | / | / | / |
| 8.360 | 3-Methylbutanal | / | 0.71 | / | 1.36 | 1.75 | / | / | / | / | 1.63 |
| 8.622 | 2-Methylbutanal | 1.32 | 0.75 | / | 1.33 | 1.56 | 1.60 | / | / | / | 1.78 |
| 9.164 | Acetic acid | / | 1.57 | / | 1.61 | 1.57 | 2.08 | 1.90 | / | / | / |
| 9.273 | 2-Ethylfuran | 0.42 | 0.45 | 0.20 | 0.26 | 0.40 | 0.35 | 0.28 | 0.13 | 0.16 | 0.10 |
| 9.775 | Pentanal | 1.41 | / | / | 1.57 | 1.41 | / | / | / | / | 0.50 |
| 9.974 | 2,3-Pentanedione | / | / | / | 21.39 | / | / | / | / | / | / |
| 9.996 | Unknown | / | 3.49 | 3.82 | / | / | / | / | 3.61 | / | / |
| 11.043 | 1-Chloropentane | 2.94 | 2.55 | / | 3.06 | / | 2.55 | / | / | / | / |
| 11.190 | Unknown | 2.36 | 3.12 | / | 3.90 | / | 4.79 | 4.22 | / | / | / |
| 11.311 | Unknown | / | 2.61 | / | / | / | / | / | / | / | / |
| 12.481 | Unknown | / | 0.26 | / | / | 2.79 | / | / | / | / | / |
| 12.893 | Hexanal | / | / | 0.22 | / | / | 0.55 | 0.47 | 0.31 | 0.45 | 0.22 |
| 13.608 | Methylpyrazine | 0.50 | / | / | / | 0.34 | 0.54 | / | / | / | / |
| 14.477 | Furfural | 3.66 | / | / | 2.85 | 3.77 | 3.85 | 1.55 | / | / | / |
| 14.666 | Unknown | / | 0.16 | 0.16 | 0.10 | / | 0.20 | / | 0.06 | 0.08 | / |
| 15.081 | Unknown | / | / | 0.61 | / | / | / | / | / | / | / |
| 15.411 | 2-Heptanone | 0.57 | / | 0.32 | / | / | 0.51 | 0.37 | 0.23 | 0.27 | 0.25 |
| 15.818 | 2,5-Dimethylpyrazine | 0.67 | / | 1.68 | / | 0.42 | 0.49 | / | 1.32 | / | / |
| 16.382 | 1-(2-Furanyl)-ethanone | 4.74 | / | / | 2.46 | 4.08 | 5.47 | / | / | / | 2.04 |
| 16.722 | Unknown | 0.28 | 0.18 | 0.30 | / | 3.80 | / | / | 0.88 | / | / |
| 17.267 | 2-Pentylfuran | / | / | 0.37 | / | / | / | 0.54 | 0.35 | 0.45 | 0.21 |
| 17.542 | Benzaldehyde | / | / | 0.18 | / | / | / | 0.67 | / | / | / |
| 17.768 | 5-Methyl-2-furancarboxaldehyde | 4.05 | / | / | 2.86 | 4.19 | 5.26 | / | / | / | / |
| 17.948 | Unknown | / | / | 1.84 | / | / | / | / | / | / | / |
| 19.074 | Unknown | / | / | / | 5.28 | / | / | / | / | / | / |
| 19.193 | Phenol | / | / | / | / | / | / | 62.08 | 10.54 | / | / |
| 19.403 | Pyrazinamide | / | / | 2.42 | / | / | / | / | / | 2.48 | / |
| 19.472 | Benzeneacetaldehyde | / | / | / | / | / | / | 0.72 | / | / | 1.46 |
| 19.509 | Unknown | / | / | 2.84 | / | / | / | / | / | / | / |
| 19.830 | 3,5-Octadien-2-one | / | / | / | 5.08 | / | / | / | / | 5.34 | / |
| 20.110 | Unknown | / | / | / | / | 3.72 | / | / | / | / | / |
| 20.176 | Nonanal | / | / | 0.27 | / | / | / | / | / | / | / |
| 20.356 | 1-(1H-pyrrol-2-yl)-ethanone | / | / | 1.93 | / | 2.21 | 2.61 | / | / | 2.10 | 3.75 |
| 21.194 | Unknown | 3.41 | / | / | / | / | / | / | / | / | / |
| 21.410 | 2-Methoxy-3-(2-methylpropyl)-pyrazine | 2.95 | / | / | / | 3.89 | 2.78 | / | / | / | 4.81 |
| 21.973 | Unknown | / | / | / | 12.22 | / | / | / | / | 10.39 | / |
| 21.977 | Unknown | / | / | 4.66 | 22.67 | / | 6.19 | 7.59 | 11.92 | 13.95 | 10.52 |
| 22.100 | Decanal | / | / | 0.25 | / | / | / | / | / | / | / |
| 22.761 | Unknown | / | / | / | / | / | / | 42.63 | / | / | / |
| 23.031 | Unknown | / | / | / | / | / | 1.84 | / | / | / | / |
| 24.375 | β-Elemene | / | / | / | 1.71 | 3.25 | 1.83 | / | / | / | / |
| 24.616 | Unknown | / | / | / | / | / | / | 0.24 | / | / | / |
| 24.881 | Unknown | / | / | / | / | / | / | / | / | / | 6.38 |
| 24.975 | 1-Pentadecene | / | 0.65 | 0.63 | / | / | / | / | 0.61 | 0.50 | 0.31 |
| 25.172 | γ-Curcumene | / | / | 1.77 | / | / | / | / | / | / | / |
| 25.251 | 1-Methyl-4-(6-methylhept-5-en-2-yl) benzene | 1.44 | / | / | / | 0.12 | / | / | / | 0.21 | 0.54 |
| 25.316 | Unknown | / | / | / | / | 3.94 | / | / | / | / | / |
| 25.361 | Unknown | / | / | / | / | / | 29.56 | / | / | 7.37 | / |
| 25.547 | Unknown | 2.40 | / | / | / | / | / | / | / | / | / |
| 25.613 | β-Selinene | 1.84 | / | / | / | 3.89 | 2.11 | / | / | / | / |
| 25.670 | α-Selinene | 1.87 | / | / | 1.96 | 3.78 | 1.90 | / | / | / | / |
| 26.828 | Unknown | / | 2.66 | / | / | 3.31 | 2.15 | / | / | / | 28.47 |
| 27.194 | Heptadeca-1,8,11-triene | / | / | / | / | / | / | / | / | 0.63 | 0.36 |
| 27.333 | Heptadeca-1,8,11,14-tetraene | 1.26 | / | / | / | / | / | / | / | / | 0.41 |
| 27.418 | Unknown | 2.10 | / | / | / | / | / | / | / | / | / |
| 27.521 | Unknown | / | / | 7.27 | / | / | / | / | / | / | / |
| 27.577 | Unknown | / | / | / | / | / | / | / | / | 4.41 | / |
| 28.444 | β-Eudesmol | / | / | / | 13.25 | / | 31.16 | / | / | / | / |
| 28.517 | Unknown | / | / | / | / | / | 11.25 | / | / | / | / |
| 28.903 | Unknown | / | / | 2.24 | / | / | / | 2.25 | / | 2.14 | 2.80 |
| 29.377 | Unknown | / | / | / | / | / | / | / | / | / | 8.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Guo, Z.; Fang, S.; Gu, J.; Liang, X. Effect of Drying Methods on Volatile Compounds of Burdock (Arctium lappa L.) Root Tea as Revealed by Gas Chromatography Mass Spectrometry-Based Metabolomics. Foods 2021, 10, 868. https://doi.org/10.3390/foods10040868
Xia J, Guo Z, Fang S, Gu J, Liang X. Effect of Drying Methods on Volatile Compounds of Burdock (Arctium lappa L.) Root Tea as Revealed by Gas Chromatography Mass Spectrometry-Based Metabolomics. Foods. 2021; 10(4):868. https://doi.org/10.3390/foods10040868
Chicago/Turabian StyleXia, Junjie, Zili Guo, Sheng Fang, Jinping Gu, and Xianrui Liang. 2021. "Effect of Drying Methods on Volatile Compounds of Burdock (Arctium lappa L.) Root Tea as Revealed by Gas Chromatography Mass Spectrometry-Based Metabolomics" Foods 10, no. 4: 868. https://doi.org/10.3390/foods10040868
APA StyleXia, J., Guo, Z., Fang, S., Gu, J., & Liang, X. (2021). Effect of Drying Methods on Volatile Compounds of Burdock (Arctium lappa L.) Root Tea as Revealed by Gas Chromatography Mass Spectrometry-Based Metabolomics. Foods, 10(4), 868. https://doi.org/10.3390/foods10040868