Valorization of Greenhouse Horticulture Waste from a Biorefinery Perspective
Abstract
1. Introduction
2. Materials and Methods
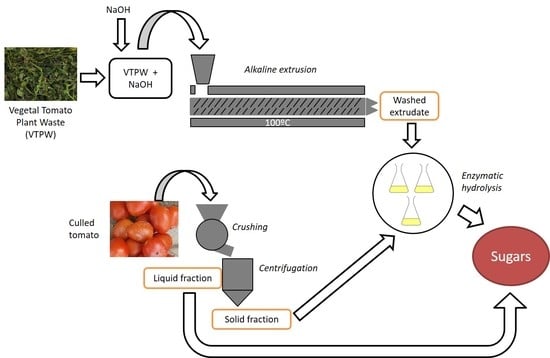
2.1. Raw Materials
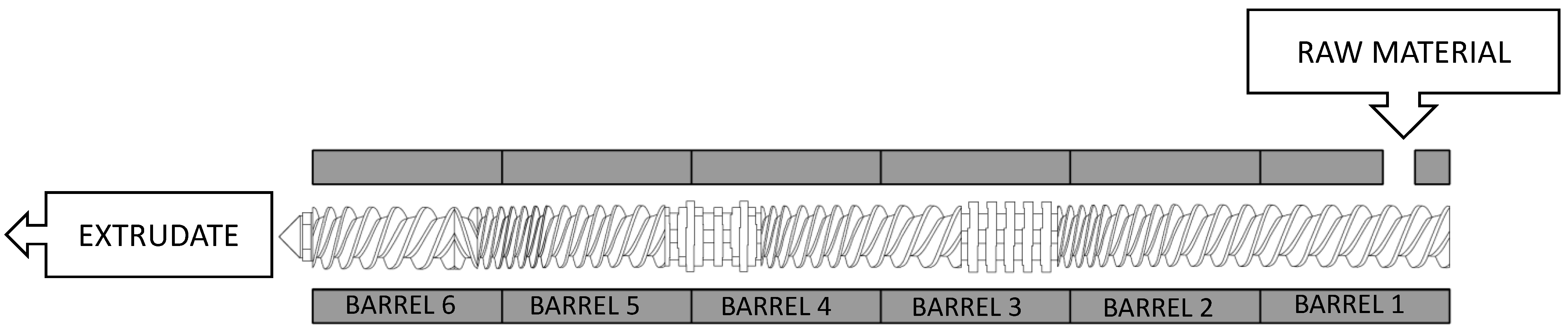
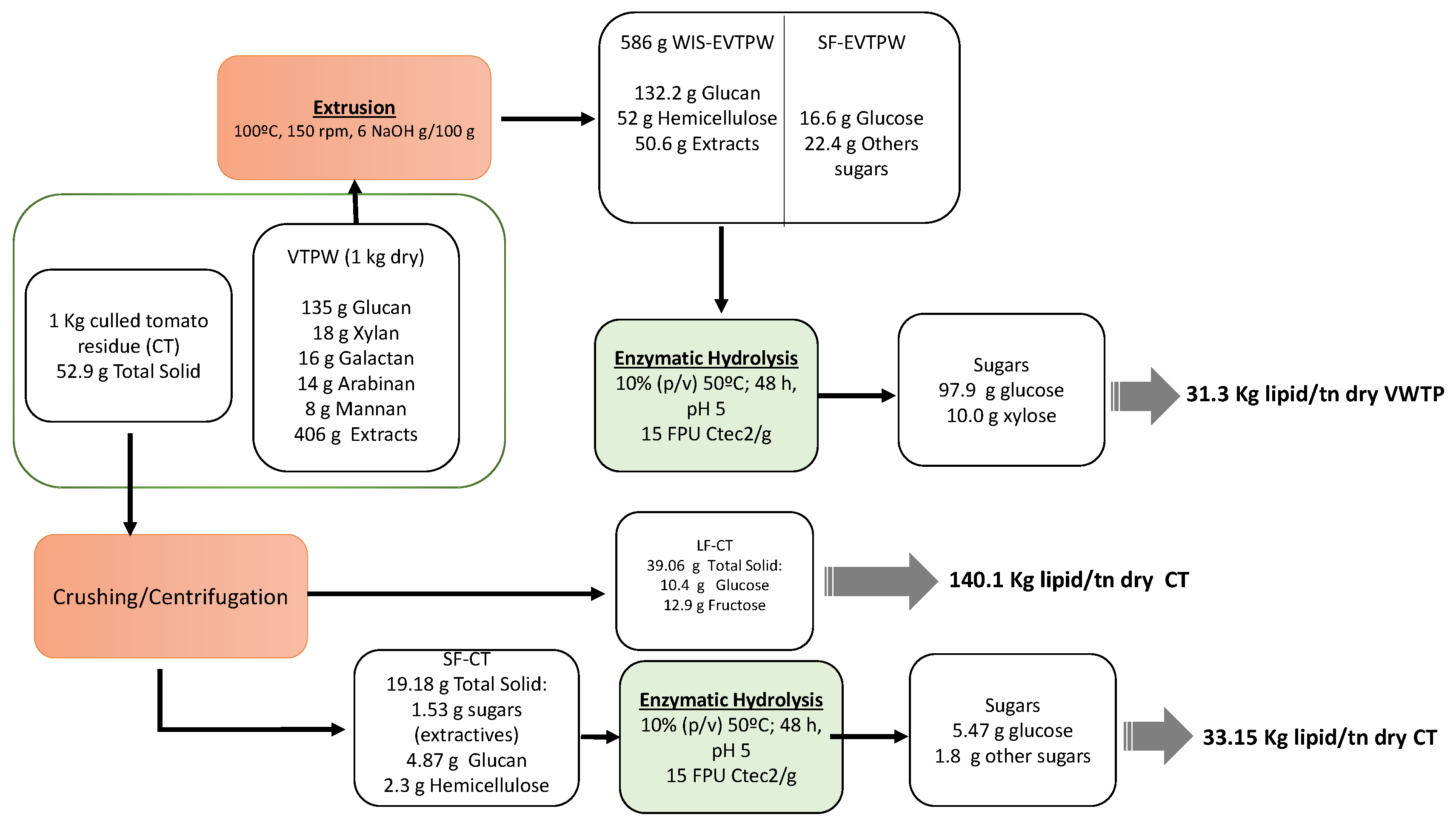
2.2. Extrusion Pretreatment
2.3. Extrudate Characterization
2.4. Culled Tomatoes Fractionation
2.5. Enzymatic Hydrolysis Test
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Vegetal Tomato Plant Waste
3.2. Extrusion Pretreatment of Vegetal Tomato Plant Waste
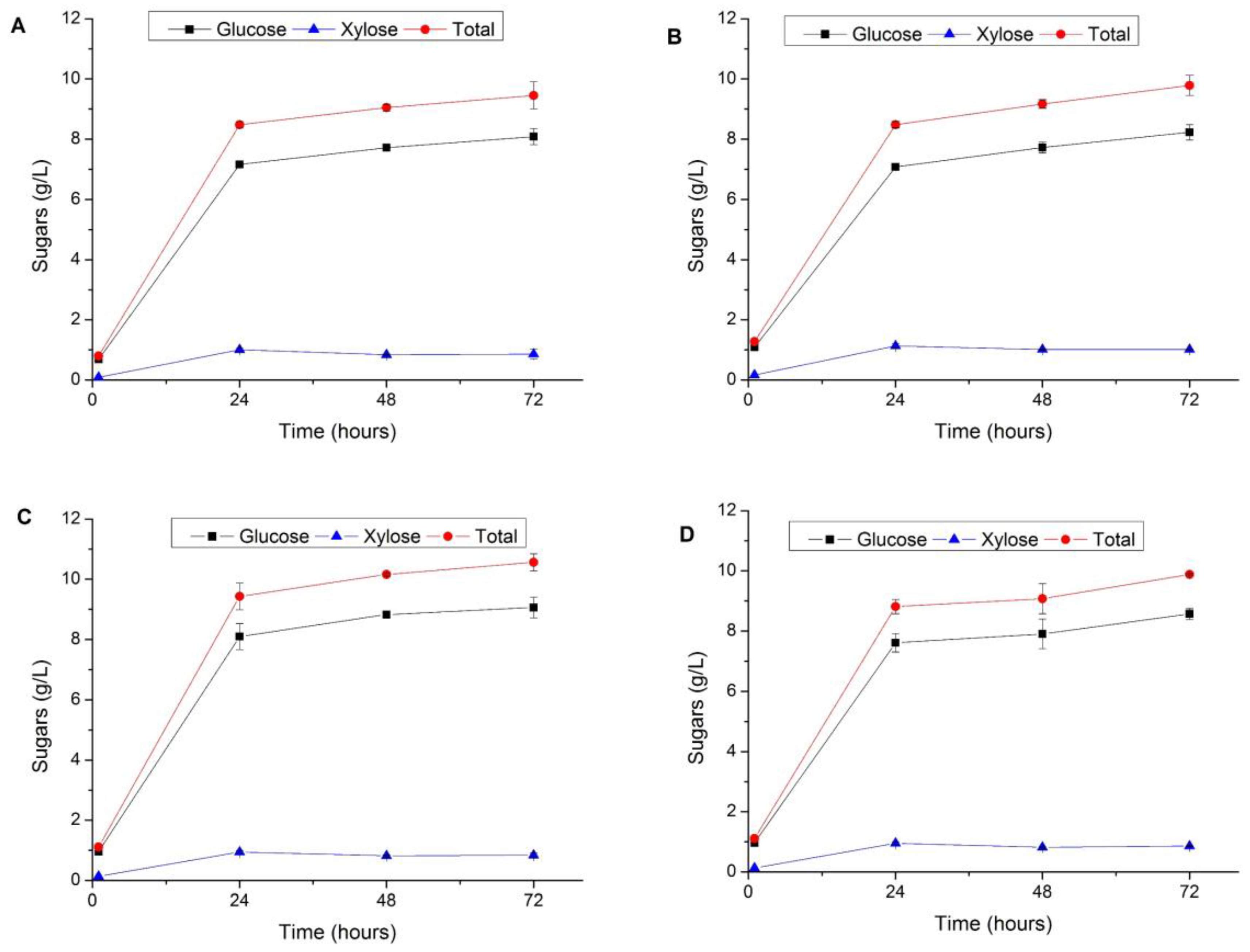
3.3. Enzymatic Hydrolysis of Pretreated Vegetal Tomato Plant Waste
3.4. Chemical Composition of Culled Tomato
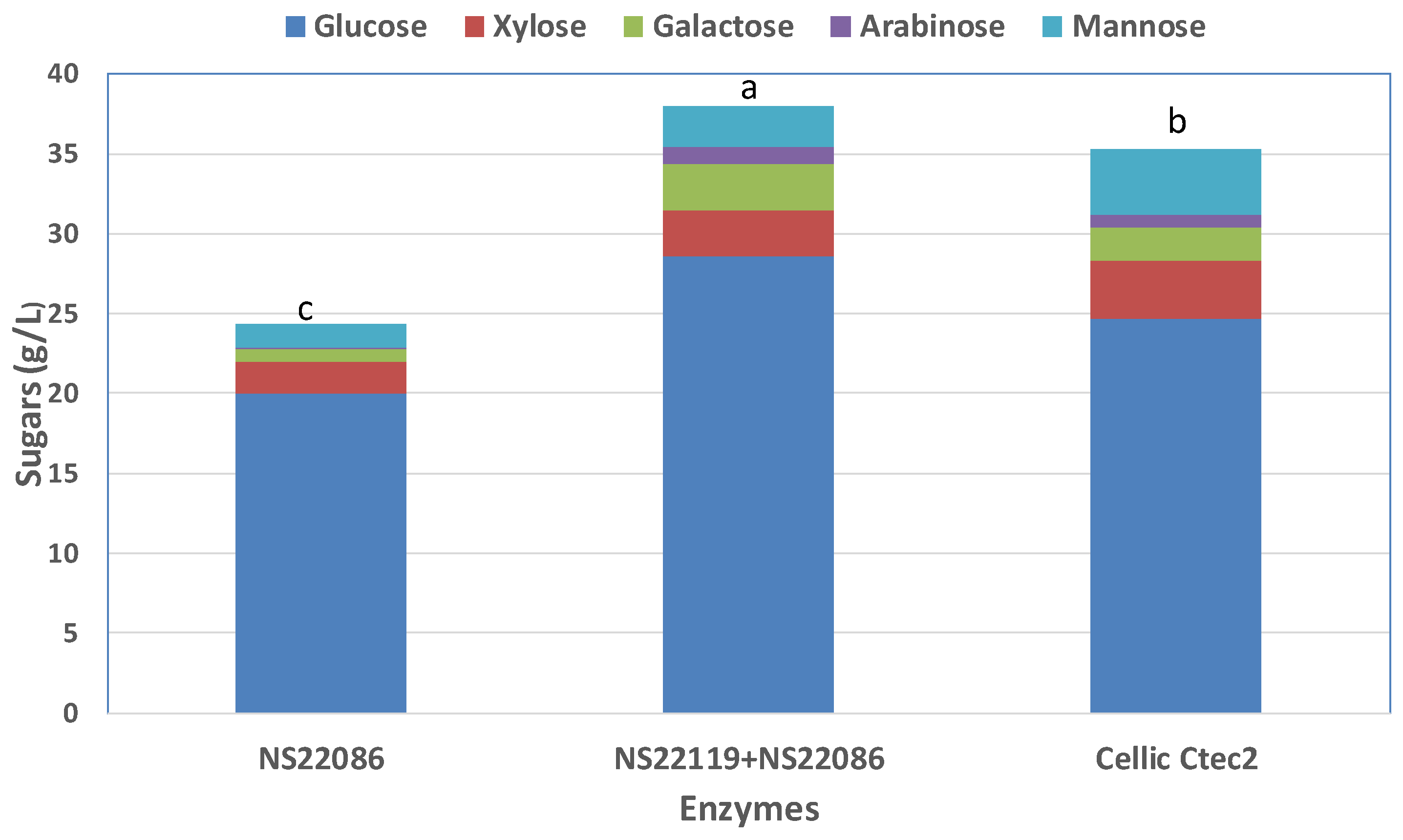
3.5. Fractionation of Culled Tomato and Enzymatic Hydrolysis
3.6. Sugars Production from the Organic Residues Derivied from Tomato Greenhouse Crops
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ballesteros, M.; Negro, M.J. Biorefineries for the valorization of food processing waste. In The Interaction of Food Industry and the Environment; Galanakis, C., Ed.; Academic Press: London, UK, 2020; pp. 155–190. [Google Scholar]
- Szabo, K.; Dulf, F.V.; Teleky, B.-E.; Eleni, P.; Boukouvalas, C.; Krokida, M.; Kapsalis, N.; Rusu, A.V.; Socol, C.T.; Vodnar, D.C. Evaluation of the bioactive compounds found in tomato seed oil and tomato peels influenced by industrial heat treatments. Foods 2021, 10, 110. [Google Scholar] [CrossRef]
- Georganas, A.; Giamouri, E.; Pappas, A.C.; Papadomichelakis, G.; Galliou, F.; Manios, T.; Tsiplakou, E.; Fegeros, K.; Zervas, G. Bioactive compounds in food waste: A review on the transformation of food waste to animal feed. Foods 2020, 9, 291. [Google Scholar] [CrossRef]
- Rossi, R. The EU Fruit and Vegetable Sector. Main Features, Challenges and Prospects. EPRS. European Parliamentary Research Service, 2019. PE 635.563. Available online: https://www.europarl.europa.eu/thinktank/en/document.html?reference=EPRS_BRI(2019)635563 (accessed on 20 November 2020).
- Eurostat. Available online: http://appsso.eurostat.ec.europa.eu/ (accessed on 11 January 2021).
- Faostat. Available online: http://www.fao.org/faostat/es (accessed on 11 January 2021).
- Lødval, T.; Droogenbroecck, B.V.; Kaniszewski, S.; Agati, G.; Verheul, M.; Skipnes, D. Valorization of tomato surplus and waste fractions: A case study using Norgay, Belgium, Poland, and Turkey as examples. Foods 2019, 8, 229. [Google Scholar] [CrossRef]
- Manzano-Agugliaro, F. Gasificación de residuos de invernadero para la obtención de energía eléctrica en el sur de España: Ubicación mediante SIG. Interciencia 2007, 32, 131–136. [Google Scholar]
- Duque-Azevedo, M.; Belmonte-Ureña, L.J.; Plaza-Úbeda, J.A.; Camacho-Ferre, F. The management of agricultural waste biomass in the framework of circular economy and bioeconomy: An opportunity for green house agriculture in Southeast Spain. Agronomy 2020, 10, 489. [Google Scholar] [CrossRef]
- Bedoić, R.; Ćosić, B.; Duić, N. Technical potential and geographic distribution of agriculture residues, co-products and by-products in the European Union. Sci. Total Environ. 2019, 686, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gómez, M.J.; Romero, E.; Nogales, R. Feasibility of vermicomposting for vegetable greenhouse waste recycling. Bioresour. Technol. 2010, 101, 9654–9660. [Google Scholar] [CrossRef]
- Pane, C.; Celano, G.; Piccolo, A.; Villecco, D.; Spaccini, R.; Palese, A.M.; Zaccardelli, M. Effects of on-farm composted tomato residues on soil biological activity and yields in a tomato cropping system. Chem. Biol. Technol. Agric. 2015, 2, 4. [Google Scholar] [CrossRef]
- Reinoso Moreno, J.V.; Pinna-Hernández, G.; Fernández Fernández, M.D.; Sánchez Molina, J.A.; Rodríguez Díaz, F.; López Hernández, J.C.; Acién-Fernández, F.G. Optimal processing of greenhouse crop residues to use as energy and CO2 sources. Ind. Crop. Prod. 2019, 137, 662–671. [Google Scholar] [CrossRef]
- Monterumici, C.M.; Rosso, D.; Montoneri, E.; Ginepro, M.; Baglieri, A.; Novotny, E.H.; Kwapinski, W.; Negre, M. Processed vs. non-processed biowastes for agriculture: Effects of post-harvest tomato plants and biochar on radish growth, chlorophyll content and protein production. Int. J. Mol. Sci. 2015, 16, 8826–8843. [Google Scholar] [CrossRef] [PubMed]
- Tsakona, S.; Papadaki, A.; Kopsahelis, N.; Kachrimanidou, V.; Papanikolaou, S.; Koutinas, A. Development of a circular oriented bioprocess for microbial oil production using diversified mixed confectionery side-streams. Foods 2019, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for lignocellulosic biomass deconstruction within a biorefinery perspective. In Biofuels: Alternative Feedstock and Conversion Process for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khnal, S.K., Ricke, S., Eds.; Academic Press: Boca Raton, FL, USA, 2019; pp. 379–399. [Google Scholar]
- Duque, A.; Manzanares, P.; Ballesteros, M. Extrusion as a pretreatment for lignocellulosic biomass: Fundamentals and applications. Ren. Energy 2017, 114, 1427–1441. [Google Scholar] [CrossRef]
- Mozhiarasi, V.; Weichgrebe, D.; Srinivasan, S.V. Enhancement of methane production from vegetable, fruit and flower market wastes using extrusion as pretreatment and kinetic modeling. Water Air Soil Pollut. 2020, 231, 126. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Negro, M.J.; Oliva, J.M.; Sáez, F.; Ballesteros, M. Study of process configuration and catalyst concentration in integrated alkaline extrusion of barley straw for bioethanol production. Fuel 2014, 134, 448–454. [Google Scholar] [CrossRef]
- Chen, W.H.; Xu, Y.Y.; Hwang, W.S.; Wang, J.B. Pretreatment of rice straw using an extrusion/extraction process at bench-scale for producing cellulosic ethanol. Bioresour. Technol. 2011, 102, 10451–10458. [Google Scholar] [CrossRef]
- Han, S.; Park, C.; Endo, T.; Febrianto, F.; Kim, N.H.; Lee, S.H. Extrusion process to enhance the pretreatment effect of ionic liquid for improving enzymatic hydrolysis of lignocellulosic biomass. Wood Sci. Technol. 2020, 54, 599–613. [Google Scholar] [CrossRef]
- Ai, B.; Li, W.; Woomer, J.; Li, M.; Pu, Y.; Sheng, Z.; Zheng, L.; Adedeji, A.; Ragauskas, A.J.; Shi, J. Natural deep eutectic solvent mediated extrusion for continuous high-solid pretreatment of lignocellulosic biomass. Green Chem. 2020, 22, 6372–6383. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Negro, J.M.; Oliva, J.M.; González, A.; Ballesteros, M. Sugar production from barley straw biomass pretreated by combined alkali and enzymatic extrusion. Bioresour. Technol. 2014, 158, 162–268. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.; Doménech, P.; Álvarez, C.; Ballesteros, M.; Manzanares, P. Study of the bioprocess conditions to produce bioetanol from barley straw pretreated by combined soda and enzyme-catalyzed extrusion. Ren. Energy 2020, 158, 263–270. [Google Scholar] [CrossRef]
- Moro, M.K.; Teixeira, R.S.S.; da Silva, A.S.; Duarte, D.; Fujimoto, M.D.; Melo, P.A.; Secchi, A.R.; Bon, E.P.S. Continuous pretreatment of sugarcane biomass using a twin-screw extruder. Ind. Crop. Prod. 2017, 97, 509–517. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, I.; Ballesteros, M.J.; Negro, J.M.; Oliva, F.; Sáez, F.; Ballesteros, M. Optimization of integrated alkaline-extrusion pretreatment of barley straw for sugar production by enzymatic hydrolysis. Process Biochem. 2013, 48, 775–781. [Google Scholar] [CrossRef]
- Wang, X.; He, X.; Yan, L.; Wang, J.; Hu, X.; Sun, Q.; Zhang, H. Enhancing enzymatic hydrolysis of corn stover by twin-screw extrusion pretreatment. Ind. Crop. Prod. 2020, 143, 111960. [Google Scholar] [CrossRef]
- Kang, K.E.; Han, M.; Moon, S.K.; Kang, H.W.; Kim, Y.; Cha, Y.L.; Choi, G.W. Optimization of alkali-extrusion pretreatment with twin-screw for bioethanol production from Miscanthus. Fuel 2013, 109, 520–526. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; González, A.; Ballesteros, M. Study of the application of alkaline extrusion to the pretreatment of eucalyptus biomass as first step in a bioethanol production process. Energies 2018, 11, 2961. [Google Scholar] [CrossRef]
- Negro, M.J.; Duque, A.; Manzanares, P.; Sáez, F.; Oliva, J.M.; Ballesteros, I.; Ballesteros, M. Alkaline twin-screw extrusion fractionation of olive-tree pruning biomass. Ind. Crops Prod. 2015, 74, 336–341. [Google Scholar] [CrossRef]
- Sluiter, A.; Hyman, D.; Payne, C.; Wolfe, J. Determination of Insoluble Solids in Pretreated Biomass Material Laboratory Analytical Procedure; NREL/TP-510-42627; National Renewable Energy Laboratory: Golden, Colorado, 2008.
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Ahmed, S.; Bin, R.F.; Ahmed, S.; Mohammad Shohael, A. Insights into the bioactive compounds, antioxidant potential and TLC profiling of different extracts of tomato plants. Jahangirnagar Univ. J. Biol. Sci. 2018, 7, 65–77. [Google Scholar] [CrossRef]
- Nisticò, R.; Evon, P.; Labonne, L.; Vaca-Medina, G.; Montoneri, E.; Vaca-Garcia, C.; Negre, M. Post-harvest tomato plants and urban food wastes for manufacturing plastic films. J. Clean. Prod. 2017, 167, 68–74. [Google Scholar] [CrossRef]
- Ventura, M.R.; Pieltin, M.C.; Castañon, J.I.R. Evaluation of tomato crop by-products as feed for goats. Anim. Feed. Sci. Technol. 2009, 154, 271–277. [Google Scholar] [CrossRef]
- Gary, C.; Bertin, N.; Frossard, J.S.; Lebot, J. High mineral contents explain the low construction cost of leaves, stems and fruits of tomato plants. J. Exp. Botany 1998, 49, 49–57. [Google Scholar] [CrossRef]
- Doménech, P.; Duque, A.; Higueras, I.; Iglesias, R.; Manzanares, P. Biorefinery of the olive tree—production of sugars from enzymatic hydrolysis of olive stone pretreated by alkaline extrusion. Energies 2020, 13, 4517. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Duque, A.; Sáez, F.; Manzanares, P.; García-Cruz, C.H.; Ballesteros, M. Sugar production from wheat Straw by alkaline extrusion and enzymatic hydrolysis. Ren. Energy 2016, 86, 1060–1068. [Google Scholar] [CrossRef]
- Karamerou, E.E.; Webb, C. Cultivation modes for microbial oil production using oleaginous yeasts—A review. Biochem. Eng. J. 2019, 151, 107322. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeast. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2021, 113, 1031–1051. [Google Scholar] [CrossRef]
| Component | % (w/w) |
|---|---|
| Extracts | 40.6 ± 0.4 |
| Aqueous extract | 35.0 ± 0.3 |
| Organic solvent- extract | 5.6 ± 0.3 |
| Glucan | 13.5 ± 0.1 |
| Hemicelluloses | 5.6 ± 0.1 |
| Xylan | 1.8 ± 0.1 |
| Galactan | 1.6 ± 0.1 |
| Arabinan | 1.4 ± 0.0 |
| Mannan | 0.8 ± 0.0 |
| Acetyl groups | 1.1 ± 0.0 |
| Acid-insoluble lignin | 9.4 ± 1.1 |
| Acid-soluble lignin | 3.6 ± 0.3 |
| Whole Ash | 17.5 ± 0.7 |
| Parameter | One-Run | Three-Run | ||
|---|---|---|---|---|
| 6% NaOH (w/w) | 12% NaOH (w/w) | 6% NaOH (w/w) | 12% NaOH (w/w) | |
| g WIS/100 g extrudate | 56.6 a,b | 49.5 b,c | 56.9 a | 46.8 c |
| Mass yields (%) | 58.7 a | 54.4 b | 58.3 a | 51.1 b |
| Component | Extrusion Conditions | |||
|---|---|---|---|---|
| One-Run | Three-Run | |||
| 6% NaOH (w/w) | 12% NaOH (w/w) | 6% NaOH (w/w) | 12% NaOH (w/w) | |
| Extracts | 8.6 ± 0.5 | 12.8 ± 0.7 | 6.8 ± 0.4 | 7.1 ± 0.5 |
| Glucan | 22.5 ± 0.8 a | 20.3 ± 0.9 ab | 21.6 ± 0.2 ab | 19.2 ± 0.7 b |
| Xylan | 3.6 ± 0.2 b | 2.8 ± 0.1 a | 3.9 ± 0.3 b | 2.5 ± 0.1 a |
| Galactan | 3.4 ± 0.2 b | 3.3 ± 0.1 b | 3.2 ± 0.1 b | 2.5 ± 0.1 a |
| Arabinan | 1.8 ± 0.1 ab | 1.9 ± 0.1 a | 1.7 ± 0.0 b | 1.3 ± 0.1 c |
| Mannan | 1.1 ± 0.1 b | 0.9 ± 0.0 b | 1.1 ± 0.0 a | 0.7 ± 0.0 c |
| Acetyl-groups | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 |
| Lignin | 23.0 ± 0.4 b | 26.0 ± 0.7 ab | 23.1 ± 0.6 ab | 23.1 ± 0.3 a |
| Ash | 14.8 ± 0.1 b | 16.9 ± 0.1 a | 17.1 ± 0.1 a | 17.0 ± 0.2 a |
| Component | Extrusion Conditions | |||
|---|---|---|---|---|
| One-Run | Three-Run | |||
| 6% NaOH (w/w) | 12% NaOH (w/w) | 6% NaOH (w/w) | 12% NaOH (w/w) | |
| Sucrose | 0.5 | 0.4 | 0.6 | 0.4 |
| Glucose | 1.7 | 1.8 | 2.4 | 2.7 |
| Xylose | 0.1 | 0.2 | 0.1 | 0.1 |
| Galactose | 0.6 | 1.1 | 0.8 | 1.1 |
| Arabinose | 0.3 | 0.3 | 0.2 | 0.2 |
| Mannose | 0.1 | 0.2 | 0.2 | 0.2 |
| Fructose | 0.7 | 0.1 | 0.7 | 0.3 |
| Total sugars | 3.9 | 4.0 | 4.9 | 5.0 |
| Extrusion Conditions | EHg (%) | Glucose Yield (g/100 g WIS) | Overall Glucose Yield (g/100 g VTPW) | EHx (%) | Xylose Yield (g/100 g dry WIS) | Overall Xylose Yield (g/100 g VTPW) | |
|---|---|---|---|---|---|---|---|
| 6% (w/w) NaOH | One-run | 67.3 b | 16.7 a | 9.8 a | 41.4 b | 1.7 a | 1.0 a |
| Three-run | 71.8 ab | 16.0 a | 8.7 a | 57.5 a | 1.8 a | 1.0 a | |
| 12% (w/w) NaOH | One-run | 71.6 ab | 17.0 a | 9.9 a | 47.1 ab | 1.5 a | 0.9 a |
| Three-run | 79.4 a | 16.8 a | 8.6 a | 56.7 a | 1.6 a | 0.8 a | |
| Component | % (w/w) DW |
|---|---|
| Total extract | 69.1 ± 5.2 |
| Aqueous extract | 65.9 ± 4.5 |
| Organic extract | 3.3 ± 1.0 |
| Structural carbohydrates | 12.5 ± 0.1 |
| Glucan | 8.0 ± 0.1 |
| Xylan | 1.3 ± 0.1 |
| Galactan | 1.3 ± 0.0 |
| Arabinan | 0.6 ± 0.0 |
| Mannan | 1.3 ± 0.1 |
| Acid Insoluble solids | 9.3 ± 0.8 |
| Ash | 6.9 ± 0.1 |
| Nitrogen | 1.5 ± 0.1 |
| Sugars | % (w/w) DW | |
|---|---|---|
| Monomers | Oligomers | |
| Glucose | 17.6 ± 3.0 | 0.1 ± 0.0 |
| Xylose | 0.3 ± 0.0 | 0.1± 0.0 |
| Galactose | 0.3 ± 0.1 | 0.9 ± 0.1 |
| Arabinose | 0.1 ± 0.1 | 0.6 ± 0.0 |
| Mannose | 0.5 ± 0.1 | n.f. |
| Fructose | 23.7 ± 2.0 | n.f. |
| Sucrose | 0.3 ± 0.0 | |
| Component | % (w/w) DW |
|---|---|
| Total extract | 31.5 ± 0.4 |
| Aqueous extract | 20.7 ± 0.3 |
| Sugars | 8.3 ± 0.2 |
| Inorganic compounds | 3.3 ± 0.1 |
| Organic extract | 10.8 ± 0.2 |
| Structural carbohydrates | 37.1 ± 0.3 |
| Glucan | 25.4 ± 0.4 |
| Xylan | 3.3 ± 0.2 |
| Galactan | 2.9 ± 0.1 |
| Arabinan | 1.3 ± 0.1 |
| Mannan | 4.30 ± 0.1 |
| Ash | 4.6 ± 0.2 |
| Nitrogen | 2.5 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, A.D.; Duque, A.; González, A.; Ballesteros, I.; Negro, M.J. Valorization of Greenhouse Horticulture Waste from a Biorefinery Perspective. Foods 2021, 10, 814. https://doi.org/10.3390/foods10040814
Moreno AD, Duque A, González A, Ballesteros I, Negro MJ. Valorization of Greenhouse Horticulture Waste from a Biorefinery Perspective. Foods. 2021; 10(4):814. https://doi.org/10.3390/foods10040814
Chicago/Turabian StyleMoreno, Antonio D., Aleta Duque, Alberto González, Ignacio Ballesteros, and María José Negro. 2021. "Valorization of Greenhouse Horticulture Waste from a Biorefinery Perspective" Foods 10, no. 4: 814. https://doi.org/10.3390/foods10040814
APA StyleMoreno, A. D., Duque, A., González, A., Ballesteros, I., & Negro, M. J. (2021). Valorization of Greenhouse Horticulture Waste from a Biorefinery Perspective. Foods, 10(4), 814. https://doi.org/10.3390/foods10040814