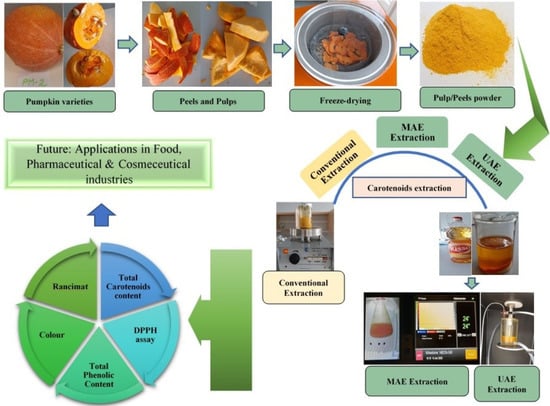
Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
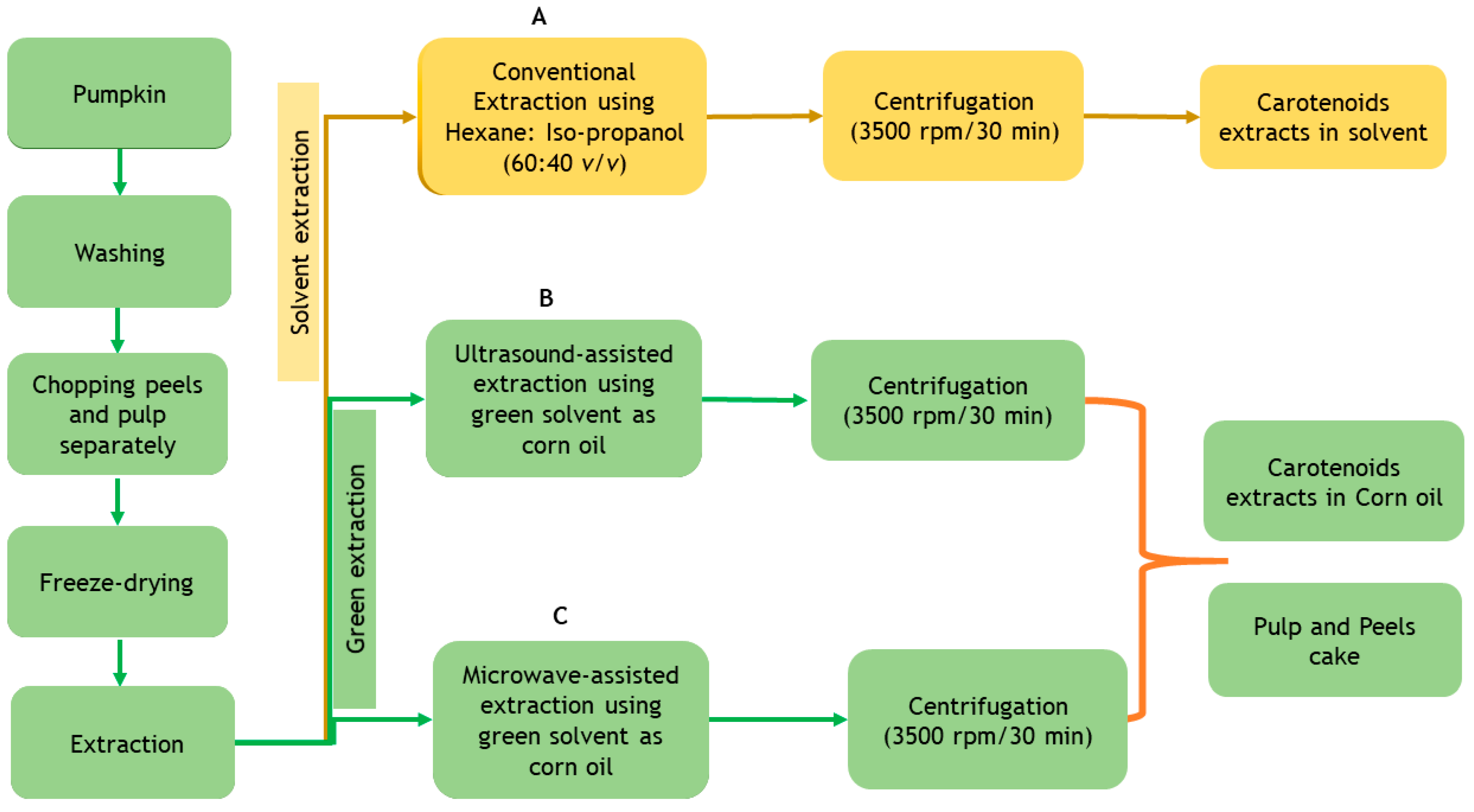
2.2. Sample Preparation
2.3. Extraction Methods for Carotenoids from Pumpkin Peels and Pulp
2.3.1. Conventional Solvent Extraction (CE)
2.3.2. Ultrasound-Assisted Extraction (UAE) Using Corn Oil (CO) as Green Solvent
2.3.3. Microwave-Assisted Extraction (MAE) Using Corn Oil (CO) as Green Solvent
2.4. Determination of Total Carotenoids Content (TCC)
2.5. Determination of Total Phenolic Content (TPC)
2.5.1. Preparation of Extract for TPC
2.5.2. Colourimetric Determination of Total Phenolic Content (TPC)
2.6. DPPH Analysis
2.6.1. Extraction Procedure for DPPH
2.6.2. Determination of DPPH
2.7. Colour Analysis
2.8. Oxidative Susceptibility by Rancimat Method
2.9. Statistical Analysis
3. Results and Discussion
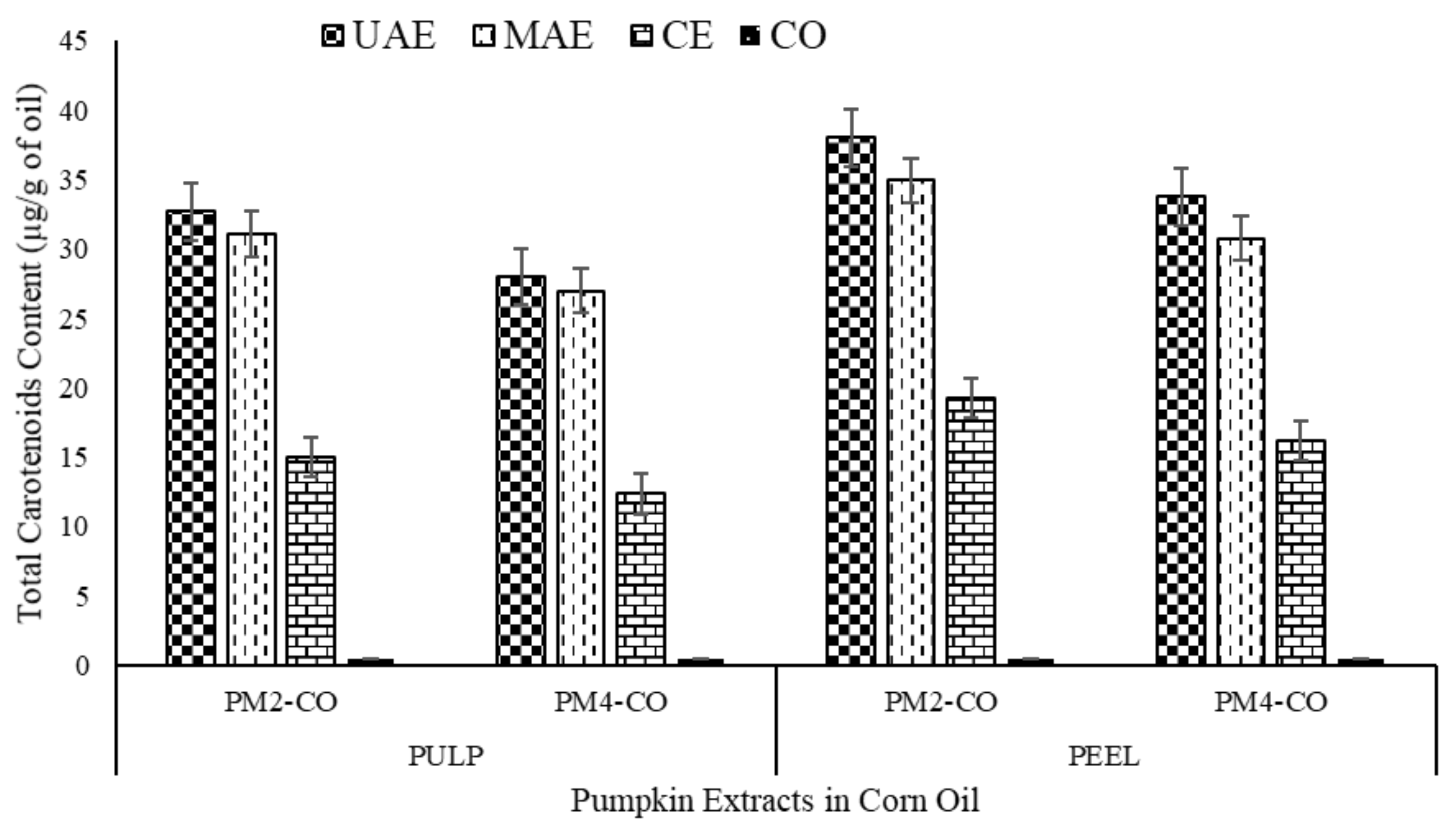
3.1. Total Carotenoids Content (TCC)
3.2. Antioxidant Study
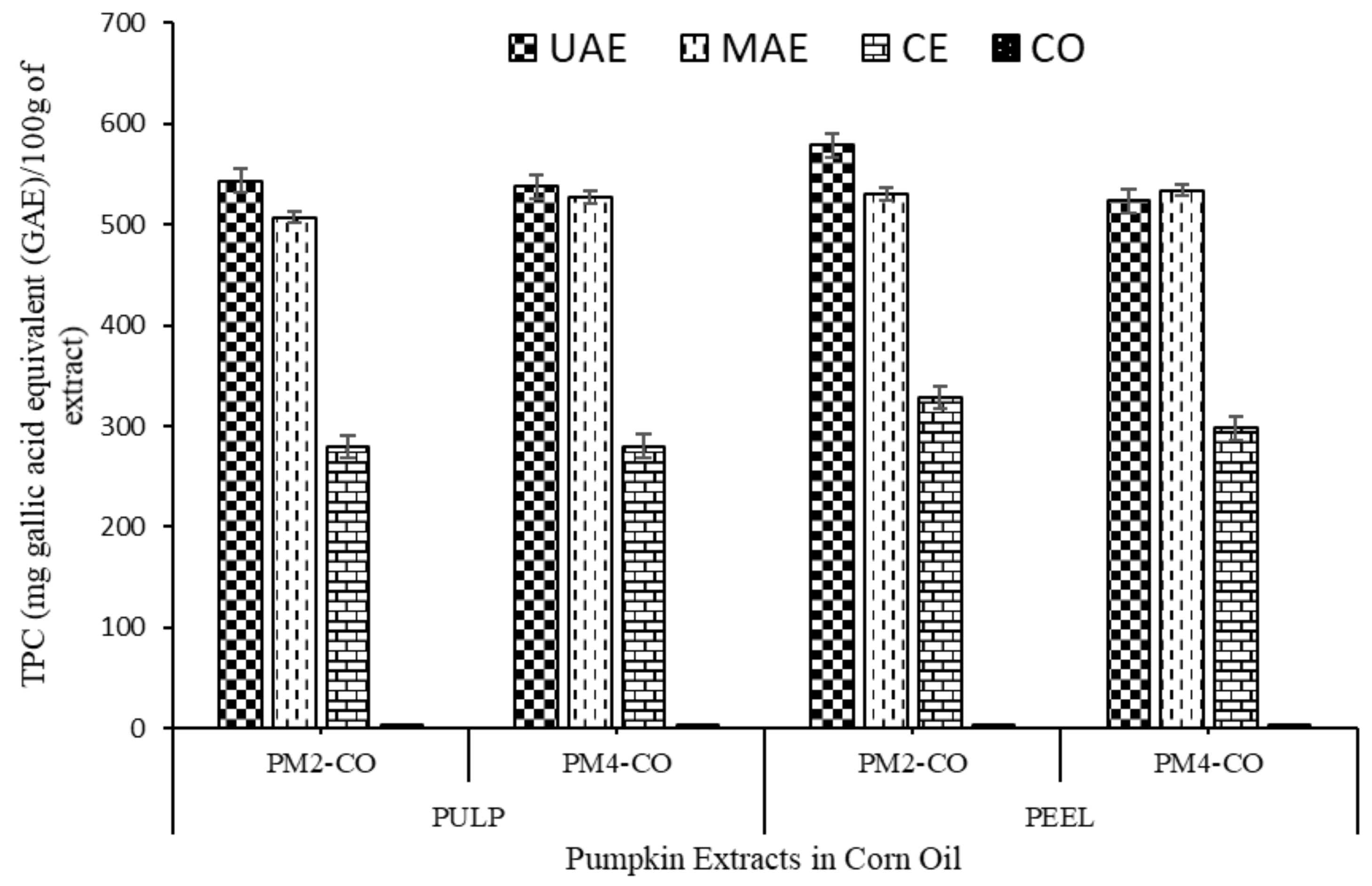
3.2.1. Total Phenolic Content (TPC)
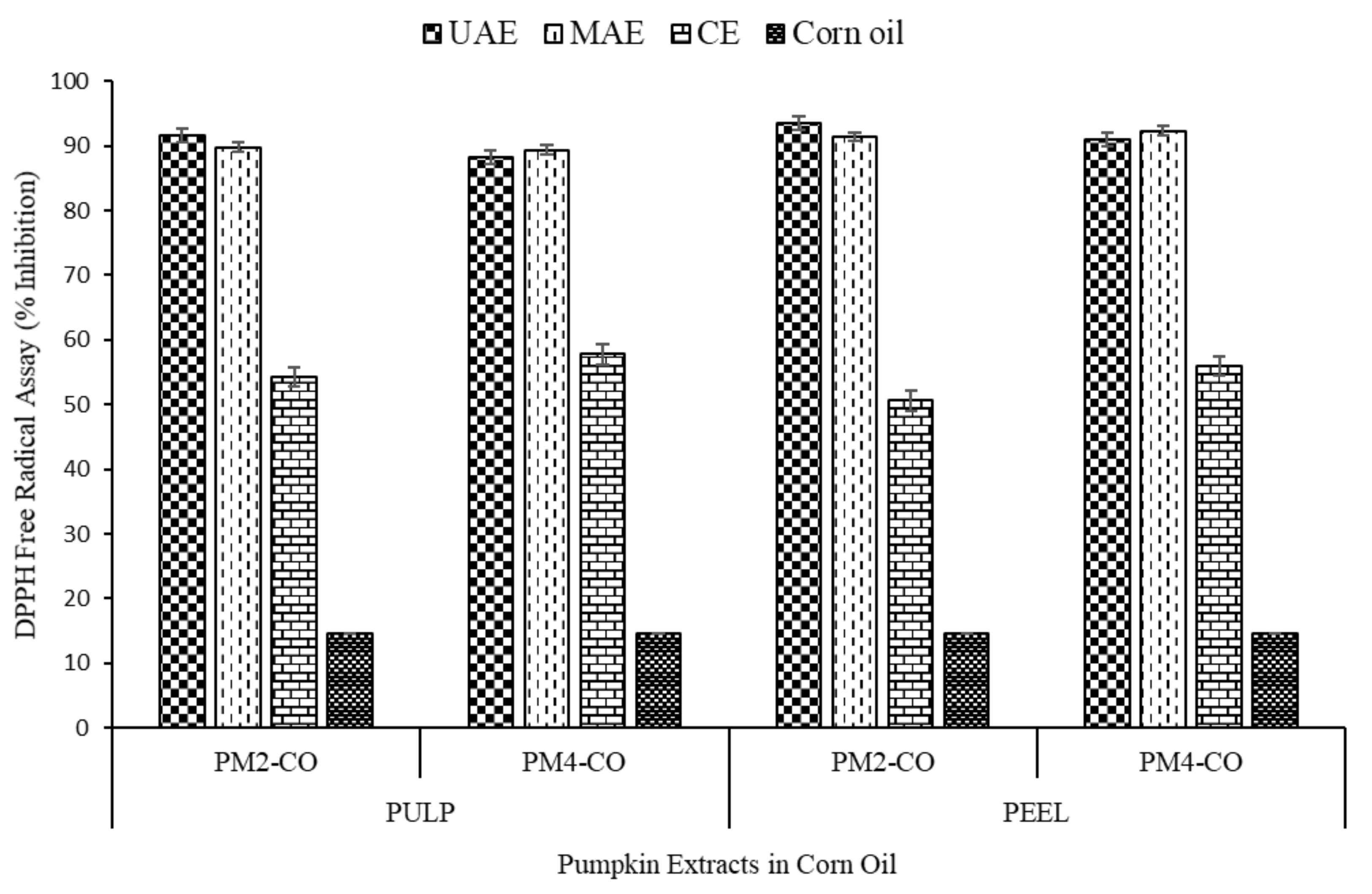
3.2.2. DPPH Antioxidant Activity
3.3. Colour
3.4. Determination of Susceptibility to Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Potera, C. Diet and nutrition: The artificial food dye blues. Environ. Health Persp. 2010, 118, A428–A431. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Joshi, V.K.; Kumar, A.; Kumar, V. Antimicrobial, antioxidant and phyto-chemicals from fruit and vegetable wastes: A review. Int. J. Food. Ferment. Technol. 2012, 2, 123–136. [Google Scholar]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources—a positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Sudheer, S.; Gupta, V.K.; Bhat, R. Engineered microbes for pigment production using waste biomass. Curr. Genom. 2020, 21, 80–95. [Google Scholar] [CrossRef]
- Lozada, M.I.O.; Maldonade, I.R.; Rodrigues, D.B.; Santos, D.S.; Sanchez, B.A.O.; de Souza, P.E.N.; Longo, J.P.; Amaro, G.B.; de Oliveira, L.D.L. Physicochemical characterization and nano-emulsification of three species of pumpkin seed oils with focus on their physical stability. Food Chem. 2021, 343, 128512. [Google Scholar] [CrossRef]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Antioxidant potential of phytochemicals in pumpkin varieties belonging to Cucurbita moschata and Cucurbita pepo species. CyTA J. Food 2020, 18, 472–484. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Corbu, A.R.; Rotaru, A.; Nour, V. Edible vegetable oils enriched with carotenoids extracted from by-products of sea buckthorn (Hippophae rhamnoides ssp. sinensis): The investigation of some characteristic properties, oxidative stability and the effect on thermal behaviour. J. Therm. Anal. Calorim. 2020, 142, 735–747. [Google Scholar] [CrossRef]
- Sabio, E.; Lozano, M.; Montero de Espinosa, V.; Mendes, R.L.; Pereira, A.P.; Palavra, A.F.; Coelho, J.A. Lycopene and β-carotene extraction from tomato processing waste using supercritical CO2. Ind. Eng. Chem. Res. 2003, 42, 6641–6646. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Ordoñez-Santos, L.E.; Esparza-Estrada, J.; Vanegas-Mahecha, P. Ultrasound-assisted extraction of total carotenoids from mandarin epicarp and application as natural colorant in bakery products. LWT 2020, 139, 110598. [Google Scholar] [CrossRef]
- Sun, M.; Temelli, F. Supercritical carbon dioxide extraction of carotenoids from carrot using canola oil as a continuous co-solvent. J. Supercrit. Fluids 2006, 37, 397–408. [Google Scholar] [CrossRef]
- Vasapollo, G.; Longo, L.; Rescio, L.; Ciurlia, L. Innovative supercritical CO2 extraction of lycopene from tomato in the presence of vegetable oil as co-solvent. J. Supercrit. Fluids 2004, 29, 87–96. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2017, 6, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.; Michos, C.; Badeka, A.; Kontakos, S.; Stratis, I.; Kontominas, M.G. Classification of Western Greek virgin olive oils according to geographical origin based on chromatographic, spectroscopic, conventional and chemometric analyses. Food Res. Int. 2013, 54, 1950–1958. [Google Scholar] [CrossRef]
- Fuentes, E.; Báez, M.E.; Bravo, M.; Cid, C.; Labra, F. Determination of total phenolic content in olive oil samples by UV–visible spectrometry and multivariate calibration. Food Anal. Methods 2012, 5, 1311–1319. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska-Czerniak, A.; Trokowski, K.; Karlovits, G.; Szłyk, E. Effect of refining processes on antioxidant capacity, total contents of phenolics and carotenoids in palm oils. Food Chem. 2011, 129, 1187–1192. [Google Scholar] [CrossRef]
- X-Rite Model: 964. Available online: https://www.xrite.com/categories/portable-spectrophotometers/964 (accessed on 30 March 2021).
- Zhang, X.; Cavender, G.A.; Lewandowski, K.R.; Cox, G.O.; Paton, C.M. Sensory Analysis of a Processed Food Intended for Vitamin A Supplementation. Foods 2020, 9, 232. [Google Scholar] [CrossRef]
- Segura, L.I.; Salvadori, V.O.; Goñi, S.M. Characterisation of liquid food colour from digital images. Int. J. Food Prop. 2017, 20, S467–S477. [Google Scholar] [CrossRef]
- Jackman, P.; Sun, D.-W.; ElMasry, G. Robust color calibration of an imaging system using a color space transform and advanced regression modeling. Meat Sci. 2012, 91, 402–407. [Google Scholar] [CrossRef]
- Girolami, A.; Napolitano, F.; Faraone, D.; Braghieri, A. Measurement of meat color using a computer vision system. Meat Sci. 2013, 93, 111–118. [Google Scholar] [CrossRef]
- Pauli, H. Proposed extension of the CIE recommendation on Uniform color spaces, color difference equations, and metric color terms. J. Opt. Soc. Am. 1976, 66, 866–867. [Google Scholar] [CrossRef]
- ISO. ISO 6886:2009; Animal and Vegetable Fats and Oils—Determination of Oxidation Stability (Accelerated Oxidation Test); International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Nour, V.; Corbu, A.R.; Rotaru, P.; Karageorgou, I.; Lalas, S. Effect of carotenoids, extracted from dry tomato waste, on the stability and characteristics of various vegetable oils. Grasas Aceites 2018, 69, e238. [Google Scholar] [CrossRef]
- De Carvalho, L.M.J.; Gomes, P.B.; Godoy, R.L.D.O.; Pacheco, S.; Monte, P.H.F.D.; de Carvalho, J.L.V.; Nutti, M.R.; Neves, A.C.L.; Vieira, A.C.R.A.; Ramos, S.R.R. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef]
- Song, J.; Yang, Q.; Huang, W.; Xiao, Y.; Li, D.; Liu, C. Optimization of trans lutein from pumpkin (Cucurbita moschata) peel by ultrasound-assisted extraction. Food Bioprod. Process. 2018, 107, 104–112. [Google Scholar] [CrossRef]
- Salami, A.; Asefi, N.; Kenari, R.E.; Gharekhani, M. Extraction of pumpkin peel extract using supercritical CO2 and subcritical water technology: Enhancing oxidative stability of canola oil. J. Food Sci. Technol. 2021, 58, 1101–1109. [Google Scholar] [CrossRef]
- Azizah, A.H.; Wee, K.C.; Azizah, O.; Azizah, M. Effect of boiling and stir frying on total phenolics, carotenoids and radical scavenging activity of pumpkin (Cucurbita moschata). Int. Food Res. J. 2009, 16, 45–51. [Google Scholar]
- Kandlakunta, B.; Rajendran, A.; Thingnganing, L. Carotene content of some common (cereals, pulses, vegetables, spices and condiments) and unconventional sources of plant origin. Food Chem. 2008, 106, 85–89. [Google Scholar] [CrossRef]
- Khajeh, M. Optimization of process variables for essential oil components from Satureja hortensis by supercritical fluid extraction using Box-Behnken experimental design. J. Supercrit. Fluids 2011, 55, 944–948. [Google Scholar] [CrossRef]
- Mala, K.S.; Kurian, A.E. Nutritional composition and antioxidant activity of pumpkin wastes. Int. J. Pharm. Chem. Biol. Sci. 2016, 6, 336–344. [Google Scholar]
- Altemimi, A.; Watson, D.G.; Choudhary, R.; Dasari, M.R.; Lightfoot, D.A. Ultrasound assisted extraction of phenolic compounds from peaches and pumpkins. PLoS ONE 2016, 11, e0148758. [Google Scholar] [CrossRef]
- Wanna, C. Free radical scavenging capacity and total phenolic contents in peel and fleshy crude extracts of selected vegetables. Pharmacog. J. 2019, 11, 1351–1358. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. Application of multivariate statistical techniques to assess the phenolic compounds and the in vitro antioxidant activity of commercial grape cultivars. J. Chemomet. 2018, e3073. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. Measurement, correlation, and thermodynamic properties for solubilities of bioactive compound (−)-epicatechin in different pure solvents at 298.15K to 338.15K. J. Mol. Liq. 2018, 264, 269–274. [Google Scholar] [CrossRef]
- Zhou, C.L.; Mi, L.; Hu, X.Y.; Zhu, B.H. Evaluation of three pumpkin species: Correlation with physicochemical, antioxidant properties and classification using SPME-GC–MS and E-nose methods. J. Food Sci. Technol. 2017, 54, 3118–3131. [Google Scholar] [CrossRef] [PubMed]
- Benakmoum, A.; Abbeddou, S.; Ammouche, A.; Kefalas, P.; Gerasopoulos, D. Valorisation of low quality edible oil with tomato peel waste. Food Chem. 2008, 110, 684–690. [Google Scholar] [CrossRef]
| Sample Names | Codes | UAE (Amplitude-20%, 30 Min) | MAE (130 W, 30 Min) | CE (Hex: IPA, 60:40 v/v, 60 Min) |
|---|---|---|---|---|
| Cucurbita maxima Var. Gold Nugget | PM2 | Pulp-CO | Pulp-CO | Pulp |
| Peel-CO | Peel-CO | Peel | ||
| Cucurbita maxima Var. Amoro F1 | PM4 | Pulp-CO | Pulp-CO | Pulp |
| Peel-CO | Peel-CO | Peel |
| Sample Names | TCC (µg/g of Oil Extracts) | Total Phenolic Content (TPC) (mg Gallic Acid Equivalent (GAE)/g of Extract) | DPPH Free Radical Assay (% Inhibition) | |||
|---|---|---|---|---|---|---|
| Pulp | Peel | Pulp | Peel | Pulp | Peel | |
| PM2-UAE-CO | 32.69 ± 2.01 c,A | 38.03 ± 4.21 d,B | 555.20 ± 10.69 e,A | 588.68 ± 7.26 f,B | 91.55 ± 1.80 c,A | 93.53 ± 0.30 c,A |
| PM2-MAE-CO | 31.067 ± 2.45 c,A | 34.94 ± 3.60 c,d,A | 527.20 ± 5.69 d,A | 554.54 ± 10.25 e,B | 89.82 ± 1.36 c,A | 91.35 ± 0.94 c,A |
| PM4-UAE-CO | 28.01 ± 6.07 c,A | 33.78 ± 1.76 c,d,A | 524.48 ± 9.89 d,A | 547.94 ± 11.00 e,A | 88.32 ± 1.51 c,A | 90.90 ± 2.09 c,A |
| PM4-MAE-CO | 26.98 ± 6.12 c,A | 30.78 ± 2.78 c,A | 510.69 ± 5.50 c,A | 535.58 ± 3.84 d,B | 89.38 ± 4.51 c,A | 92.32 ± 1.43 c,A |
| PM2-CE | 15.01 ± 2.44 b,A | 19.21 ± 4.39 b,A | 269.50 ± 2.17 b,A | 318.46 ± 6.60 c,B | 54.29 ± 3.64 b,A | 57.79 ± 2.09 b,A |
| PM4-CE | 12.33 ± 1.90 b,A | 16.21 ± 2.52 b,A | 279.91 ± 4.53 b,A | 297.76 ± 2.14 b,B | 50.61 ± 1.44 b,A | 55.95 ± 4.62 b,A |
| Corn oil | 0.48 ± 0.007 a,A | 0.48 ± 0.02 a,A | 3.45 ± 0.21 a,A | 3.45 ± 0.21 a,A | 14.51 ± 3.17 a,A | 14.51 ± 3.17 a,A |
| Sample Names | Colour Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| PULP | PEEL | |||||||
| L* | a* | b* | ΔE* | L* | a* | b* | ΔE* | |
| PM2-UAE-CO | 3.90 ± 0.33 a | 7.09 ± 0.71 b | 5.67 ± 0.21 a | 60.23 ± 0.08 c,A | 4.52 ± 2.26 b | 2.92 ± 0.61a | 3.17 ± 1.43a | 62.30 ± 0.55 c,B |
| PM2-MAE-CO | 1.84 ± 0.29 a | 13.85 ± 2.28c | 3.78 ± 1.17 a | 43.65 ± 1.20 b,A | 1.37 ± 0.27 a | 7.47 ± 6.90 a | 2.16 ± 0.51a | 45.08 ± 0.29 b,A |
| PM4-UAE-CO | 1.79 ± 0.56 a | 5.01 ± 1.49 a,b | 2.84 ± 0.99 a | 63.87 ± 1.24 d,A | 1.87 ± 0.59 a | 5.48 ± 1.98 a | 3.01 ± 1.03a | 63.67 ± 1.29 c,A |
| PM4-MAE-CO | 1.43 ± 0.11 a | 3.76 ± 0.38 a | 2.50 ± 0.29 a | 44.55 ± 0.20 b,A | 1.54 ± 0.50 a | 4.15 ± 1.74 a | 2.41 ± 0.85a | 44.56 ± 1.09 b,A |
| PM2-CE | 18.73 ± 2.57 b | 16.69 ± 1.16 d | 32.35 ± 3.78 b | 35.98 ± 1.56 a,A | 16.74 ± 1.08 c | 15.43 ± 3.08 b | 16.71 ± 8.71b | 36.69 ± 1.34 a,A |
| PM4-CE | 19.89 ± 3.47 b | 19.59 ± 2.27 e | 34.02 ± 5.88 b | 36.81 ± 0.22 a,A | 27.68 ± 1.64 d | 22.24 ± 1.40 c | 47.01 ± 2.57c | 38.49 ± 1.24 a,A |
| Sample Names | Protection Factor (PF) of Carotenoids Extracts of Pumpkin in Corn Oil | |
|---|---|---|
| Pulp | Peel | |
| UAE_control | - | - |
| MAE_control | - | - |
| PM2-UAE-CO | 1.61 ± 0.00 a,A | 1.81 ± 0.05 a,B |
| PM2-MAE-CO | 1.59 ± 0.02 a,A | 1.79 ± 0.05 a,B |
| PM4-UAE-CO | 1.59 ± 0.01 a,A | 1.74 ± 0.03 a,B |
| PM4-MAE-CO | 1.59 ± 0.01 a,A | 1.71 ± 0.06 a,A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, M.; Bhat, R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). Foods 2021, 10, 787. https://doi.org/10.3390/foods10040787
Sharma M, Bhat R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). Foods. 2021; 10(4):787. https://doi.org/10.3390/foods10040787
Chicago/Turabian StyleSharma, Minaxi, and Rajeev Bhat. 2021. "Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil)" Foods 10, no. 4: 787. https://doi.org/10.3390/foods10040787
APA StyleSharma, M., & Bhat, R. (2021). Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). Foods, 10(4), 787. https://doi.org/10.3390/foods10040787