Bisphenol A and Metabolites in Meat and Meat Products: Occurrence, Toxicity, and Recent Development in Analytical Methods
Abstract
1. Introduction
2. Regulatory Aspects
2.1. Europe—Food Contact Materials
2.2. European BPA Limits in Foods
2.3. USA
2.4. Canada
2.5. Rest of the World
3. Toxic Effects of BPA on Human Health
4. BPA Contamination in Canned Meat Products
5. BPA Levels in Raw/Non-Canned Meat Products
6. Recent Advancements in Analytical Methods
6.1. Sample Pre-Treatment
6.2. Extraction
6.3. Clean-up
6.4. Instrumental Analysis
6.4.1. HPLC-UV
6.4.2. HPLC-FLD
6.4.3. HPLC-MS
6.4.4. GC-MS
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brock, J.W.; Yoshimura, Y.; Barr, J.R.; Maggio, V.L.; Graiser, S.R.; Nakazawa, H.; Needham, L.L. Measurement of bisphenol A levels in human urine. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 323–328. [Google Scholar] [CrossRef]
- Mirmira, P.; Evans-Molina, C. Bisphenol A, obesity, and type 2 diabetes mellitus: Genuine concern or unnecessary preoccupation? Transl. Res. 2014, 164, 13–21. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Determination of free and conjugated forms of bisphenol A in human urine and serum by liquid chromatography–tandem mass spectrometry. Environ. Sci. Technol. 2012, 46, 5003–5009. [Google Scholar] [CrossRef] [PubMed]
- Andra, S.S.; Austin, C.; Yang, J.; Patel, D.; Arora, M. Recent advances in simultaneous analysis of bisphenol A and its conjugates in human matrices: Exposure biomarker perspectives. Sci. Total Environ. 2016, 572, 770–781. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Bemrah, N.; Jean, J.; Rivière, G.; Sanaa, M.; Leconte, S.; Bachelot, M.; Deceuninck, Y.; Le Bizec, B.; Dauchy, X.; Roudot, A.-C.; et al. Assessment of dietary exposure to bisphenol A in the French population with a special focus on risk characterisation for pregnant French women. Food Chem. Toxicol. 2014, 72, 90–97. [Google Scholar] [CrossRef]
- Deceuninck, Y.; Bichon, E.; Gény, T.; Veyrand, B.; Grandin, F.; Viguié, C.; Marchand, P.; Le Bizec, B. Quantitative method for conjugated metabolites of bisphenol A and bisphenol S determination in food of animal origin by Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2019, 1601, 232–242. [Google Scholar] [CrossRef]
- Sajiki, J.; Miyamoto, F.; Fukata, H.; Mori, C.; Yonekubo, J.; Hayakawa, K. Bisphenol A (BPA) and its source in foods in Japanese markets. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2007, 24, 103–112. [Google Scholar] [CrossRef]
- Thomson, B.M.; Grounds, P.R. Bisphenol A in canned foods in New Zealand: An exposure assessment. Food Addit. Contam. 2005, 22, 65–72. [Google Scholar] [CrossRef]
- Braunrath, R.; Podlipna, D.; Padlesak, S.; Cichna-Markl, M. Determination of bisphenol A in canned foods by immunoaffinity chromatography, HPLC, and fluorescence detection. J. Agric. Food Chem. 2005, 53, 8911–8917. [Google Scholar] [CrossRef]
- Feshin, D.B.; Fimushkin, P.V.; Brodskii, E.S.; Shelepchikov, A.A.; Mir-Kadyrova, E.Y.; Kalinkevich, G.A. Determination of bisphenol A in foods as 2,2-bis-(4-(isopropoxycarbonyloxy)phenyl)propane by gas chromatography/mass spectrometry. J. Anal. Chem. 2012, 67, 460–466. [Google Scholar] [CrossRef]
- Goodson, A.; Summerfield, W.; Cooper, I. Survey of bisphenol A and bisphenol F in canned foods. Food Addit. Contam. 2002, 19, 796–802. [Google Scholar] [CrossRef]
- Geens, T.; Apelbaum, T.Z.; Goeyens, L.; Neels, H.; Covaci, A. Intake of bisphenol A from canned beverages and foods on the Belgian market. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 1627–1637. [Google Scholar] [CrossRef]
- Cao, X.-L.; Perez-Locas, C.; Dufresne, G.; Clement, G.; Popovic, S.; Beraldin, F.; Dabeka, R.; Feeley, M. Concentrations of bisphenol A in the composite food samples from the 2008 Canadian total diet study in Quebec City and dietary intake estimates. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 791–798. [Google Scholar] [CrossRef]
- Liao, C.Y.; Kannan, K. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, S.; Bemrah, N.; Roudot, A.-C.; Marchioni, E.; Le Bizec, B.; Faivre, F.; Kadawathagedara, M.; Botton, J.; Rivière, G. Human health risks related to the consumption of foodstuffs of animal origin contaminated by bisphenol A. Food Chem. Toxicol. 2017, 110, 333–339. [Google Scholar] [CrossRef]
- Lim, D.S.; Kwack, S.J.; Kim, K.-B.; Kim, H.S.; Lee, B.M. Risk Assessment of Bisphenol a Migrated from Canned Foods in Korea. J. Toxicol. Environ. Health Part A Curr. Issues 2009, 72, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Mariscal-Arcas, M.; Rivas, A.; Granada, A.; Monteagudo, C.; Murcia, M.; Olea-Serrano, F. Dietary exposure assessment of pregnant women to bisphenol-A from cans and microwave containers in Southern Spain. Food Chem. Toxicol. 2009, 47, 506–510. [Google Scholar] [CrossRef]
- Gyllenhammar, I.; Glynn, A.; Darnerud, P.O.; Lignell, S.; Van Delft, R.; Aune, M. 4-Nonylphenol and bisphenol A in Swedish food and exposure in Swedish nursing women. Environ. Int. 2012, 43, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Y.; Kannan, K. Concentrations and Profiles of Bisphenol A and Other Bisphenol Analogues in Foodstuffs from the United States and Their Implications for Human Exposure. J. Agric. Food Chem. 2013, 61, 4655–4662. [Google Scholar] [CrossRef]
- Agency, F.S. Survey of Bisphenols in Canned Foods; Food Surveillance Information Sheet; Number 13/01. April 2001; Food Standards Agency: London, UK, 2000.
- Wang, X.; Chang, F.; Bai, Y.; Chen, F.; Zhang, J.; Chen, L. Bisphenol A enhances kisspeptin neurons in anteroventral periventricular nucleus of female mice. J. Endocrinol. 2014, 221, 201–213. [Google Scholar] [CrossRef]
- Commission Directive 2002/72/EC of 6 August 2002 relating to plastic materials and articles intended to come into contact with foodstuffs. Off. J. L 2002, 220, 0018.
- Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and article intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. Off. J. L 2004, 338, 4–17.
- EFSA. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to 2,2-bis(4-hydroxyphenyl) propane (bisphenol A). EFSA J. 2006, 428, 1–75. [Google Scholar]
- Commission Directive 2011/8/EU of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of Bisphenol A in plastic infant feeding bottles. Off. J. L 2011, 26, 11–14.
- Commission Regulation (EU) 2018/213 of 12 February 2018 on the use of bisphenol A in varnishes and coatings intended to come into contact with food and amending Regulation (EU) No 10/2011 as regards the use of that substance in plastic food contact materials. Off. J. L 2018, 41, 6–12.
- Mead, R.N.; Seaton, P.J. GC–MS Quantitation and identification of bisphenol-A isolated from water. J. Chem. Educ. 2011, 88, 1130–1132. [Google Scholar] [CrossRef]
- Regulation No 1442/2012 of 24 December 2012 aiming at banning the manufacture, import, export and commercialisation of all forms of food packaging containing bisphenol A. Off. J. Fr. Repub. OJFR 2012.
- U.S. Food and Drug Administration. Draft Assessment of Bisphenol A for Use in Food Contact Applications; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2008.
- U.S. Food and Drug Administration. Science Board Sub-Committee on Bisphenol A 2013; Scientific Peer Review of the Draft Assessment of Bisphenol A for Use in Food Contact Applications; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2013.
- U.S. Food and Drug Administration. Updated Review of Literature and Data on Bisphenol A; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2014.
- National Conference of State Legislatures (NCSL) Policy Update: State Restrictions on Bisphenol A (BPA) in Consumer Products. 2015. Available online: http://www.ncsl.org/research/environment-and-natural-resources/policy-update-on-state-restrictions-on-bisphenol-a.aspx. (accessed on 9 January 2021).
- Health Canada. Government of Canada Acts to Protect Newborns and Infants from Bisphenol from Polycarbonate Plastic Bottles; Health Canada: Ottawa, ON, Canada, 2009.
- Health Canada. Health Canada’s Updated Assessment of Bisphenol A (BPA) Exposure from Food Sources; Health Canada: Ottawa, ON, Canada, 2012.
- Canada Gazette. Order Adding a Substance to Schedule 1 to the Canadian Environmental Protection Act, 1999; Canada Gazette: Ottawa, ON, Canada, 2010; Volume 144.
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- Golub, M.S.; Wu, K.L.; Kaufman, F.L.; Li, L.-H.; Moran-Messen, F.; Zeise, L.; Alexeeff, G.V.; Donald, J.M. Bisphenol A: Developmental Toxicity from Early Prenatal Exposure. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 441–466. [Google Scholar] [CrossRef]
- Negri-Cesi, P. Bisphenol A Interaction With Brain Development and Functions. Dose Response 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Macczak, A.; Bukowska, B.; Michalowicz, J. Comparative study of the effect of BPA and its selected analogues on hemoglobin oxidation, morphological alterations and hemolytic changes in human erythrocytes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 176, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; De Angelis, C.; Conforti, A.; Piscopo, M.; Auriemma, R.S.; et al. Bisphenol A: An emerging threat to female fertility. Reprod. Biol. Endocrinol. 2020, 18, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hafner, K.S.; Flaws, J.A. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol. Appl. Pharmacol. 2014, 276, 157–164. [Google Scholar] [CrossRef]
- Hunt, P.A.; Lawson, C.; Gieske, M.; Murdoch, B.; Smith, H.; Marre, A.; Hassold, T.J.; Vandevoort, C.A. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc. Natl. Acad. Sci. USA 2012, 109, 17525–17530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Q.; Zhang, X.-F.; Zhang, L.-J.; Chao, H.-H.; Pan, B.; Feng, Y.-M.; Li, L.; Sun, X.-F.; Shen, W. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol. Biol. Rep. 2012, 39, 5651–5657. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E. Prenatal Exposure to Bisphenol A at Environmentally Relevant Doses Adversely Affects the Murine Female Reproductive Tract Later in Life. Environ. Health Perspect. 2009, 117, 879–885. [Google Scholar] [CrossRef]
- Vigezzi, L.; Bosquiazzo, V.L.; Kass, L.; Ramos, J.G.; Muñoz-De-Toro, M.; Luque, E.H. Developmental exposure to bisphenol A alters the differentiation and functional response of the adult rat uterus to estrogen treatment. Reprod. Toxicol. 2015, 52, 83–92. [Google Scholar] [CrossRef]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod. Toxicol. 2007, 24, 253–258. [Google Scholar] [CrossRef]
- Xiao, S.; Diao, H.; Smith, M.A.; Song, X.; Ye, X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod. Toxicol. 2011, 32, 434–441. [Google Scholar] [CrossRef]
- Berger, R.G.; Shaw, J.; Decatanzaro, D. Impact of acute bisphenol-A exposure upon intrauterine implantation of fertilized ova and urinary levels of progesterone and 17 beta-estradiol. Reprod. Toxicol. 2008, 26, 94–99. [Google Scholar] [CrossRef]
- George, J.T.; Seminara, S.B. Kisspeptin and the Hypothalamic Control of Reproduction: Lessons from the Human. Endocrinology 2012, 153, 5130–5136. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Lee, C.; Yeung, W.; Giesy, J.P.; Wong, M.; Zhang, X.; Hecker, M.; Wong, C.K. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod. Toxicol. 2011, 31, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Cabaton, N.J.; Wadia, P.R.; Rubin, B.S.; Zalko, D.; Schaeberle, C.M.; Askenase, M.H.; Gadbois, J.L.; Tharp, A.P.; Whitt, G.S.; Sonnenschein, C.; et al. Perinatal Exposure to Environmentally Relevant Levels of Bisphenol A Decreases Fertility and Fecundity in CD-1 Mice. Environ. Health Perspect. 2011, 119, 547–552. [Google Scholar] [CrossRef]
- Maffini, M.V.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol. Cell. Endocrinol. 2006, 254, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Nah, W.H.; Park, M.J.; Gye, M.C. Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice. Clin. Exp. Reprod. Med. 2011, 38, 75–81. [Google Scholar] [CrossRef]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; Saal, F.S.V. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef]
- Wozniak, A.L.; Bulayeva, N.N.; Watson, C.S. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ. Health Perspect. 2005, 113, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Chattopadhyay, S.; Gong, E.-Y.; Ahn, R.S.; Lee, K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol. Sci. 2003, 75, 40–46. [Google Scholar] [CrossRef]
- Kim, J.-C.; Shin, H.-C.; Cha, S.-W.; Koh, W.-S.; Chung, M.-K.; Han, S.-S. Evaluation of developmental toxicity in rats exposed to the environmental estrogen bisphenol A during pregnancy. Life Sci. 2001, 69, 2611–2625. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Fortino, A.E.; Polston, E.K. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol. Teratol. 2006, 28, 111–118. [Google Scholar] [CrossRef]
- Kawai, K.; Nozaki, T.; Nishikata, H.; Aou, S.; Takii, M.; Kubo, C. Aggressive behavior and serum testosterone concentration during the maturation process of male mice: The effects of fetal exposure to bisphenol A. Environ. Health Perspect. 2003, 111, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.C.; Vandenbergh, J.G. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm. Behav. 2006, 50, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-H.; Baek, J.-H.; Lee, S.-Y.; Jang, C.-G. Prenatal and Postnatal Exposure to Bisphenol A Induces Anxiolytic Behaviors and Cognitive Deficits in Mice. Synapse 2010, 64, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, K.; Narita, M.; Narita, M.; Akama, H.; Suzuki, T. Memory impairment associated with a dysfunction of the hippocampal cholinergic system induced by prenatal and neonatal exposures to bisphenol-A. Neurosci. Lett. 2007, 418, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Porrini, S.; Belloni, V.; Seta, D.D.; Farabollini, F.; Giannelli, G.; Dessi-Fulgheri, F. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res. Bull. 2005, 65, 261–266. [Google Scholar] [CrossRef]
- Olsen, C.M.; Meussen-Elholm, E.T.; Samuelsen, M.; Holme, J.A.; Hongslo, J.K. Effects of the environmental oestrogens bisphenol A, tetrachlorobisphenol A, tetrabromobisphenol A, 4-hydroxybiphenyl and 4,4’-dihydroxybiphenyl on oestrogen receptor binding, cell proliferation and regulation of oestrogen sensitive proteins in the human breast cancer cell line MCF-7. Pharmacol. Toxicol. 2003, 92, 180–188. [Google Scholar]
- Tarapore, P.; Ying, J.; Ouyang, B.; Burke, B.; Bracken, B.; Ho, S.-M. Exposure to Bisphenol A Correlates with Early-Onset Prostate Cancer and Promotes Centrosome Amplification and Anchorage-Independent Growth In Vitro. PLoS ONE 2014, 9, e90332. [Google Scholar] [CrossRef]
- Menale, C.; Mita, D.G.; Diano, N.; Diano, S. Adverse effects of bisphenol A exposure on glucose metabolism regulation. Open Biotechnol. J. 2016, 10, 122–130. [Google Scholar] [CrossRef]
- Saal, F.S.V.; Nagel, S.C.; Coe, B.L.; Angle, B.M.; Taylor, J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 2012, 354, 74–84. [Google Scholar] [CrossRef]
- Gao, X.Q.; Wang, H.S. Impact of Bisphenol A on the Cardiovascular System—Epidemiological and Experimental Evidence and Molecular Mechanisms. Int. J. Environ. Res. Public Health 2014, 11, 8399–8413. [Google Scholar] [CrossRef] [PubMed]
- Michalowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V. Migration phenomenon in food packaging. Food–package interactions, mechanisms, types of migrants, testing and relative legislation—A review. Food Bioprocess Technol. 2014, 7, 21–36. [Google Scholar] [CrossRef]
- Noonan, G.O.; Ackerman, L.K.; Begley, T.H. Concentration of Bisphenol A in Highly Consumed Canned Foods on the U.S. Market. J. Agric. Food Chem. 2011, 59, 7178–7185. [Google Scholar] [CrossRef]
- Goodson, A.; Robin, H.; Summerfield, W. Migration of bisphenol A from can coatings—Effects of damage, storage conditions and heating. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2004, 21, 1015–1026. [Google Scholar] [CrossRef]
- Munguía-López, E.M.; Gerardo-Lugo, S.; Peralta, E.; Bolumen, S.; Soto-Valdez, H. Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2005, 22, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, B.; Radović, L.; Natić, D.; Dodevska, M.; Vraštanović-Pavičević, G.; Balaban, M.; Stojanović, Z.; Antić, V. Migration of bisphenol a into food simulants and meat rations during initial time of storage. Packag. Technol. Sci. 2020, 33, 75–82. [Google Scholar] [CrossRef]
- Cao, X.-L.; Kosarac, I.; Popovic, S.; Zhou, S.; Smith, D.; Dabeka, R. LC-MS/MS analysis of bisphenol S and five other bisphenols in total diet food samples. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1740–1747. [Google Scholar] [CrossRef]
- Adeyi, A.A.; Babalola, B.A. Bisphenol-A (BPA) in Foods commonly consumed in Southwest Nigeria and its Human Health Risk. Sci. Rep. 2019, 9, 17458. [Google Scholar] [CrossRef]
- Alabi, A.; Caballero-Casero, N.; Rubio, S. Quick and simple sample treatment for multiresidue analysis of bisphenols, bisphenol diglycidyl ethers and their derivatives in canned food prior to liquid chromatography and fluorescence detection. J. Chromatogr. A 2014, 1336, 23–33. [Google Scholar] [CrossRef]
- Bendito, M.D.P.; Bravo, S.R.; Reyes, M.L.L.; Prieto, A.G. Determination of bisphenol A in canned fatty foods by coacervative microextraction, liquid chromatography and fluorimetry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Lorber, M.; Schecter, A.; Paepke, O.; Shropshire, W.; Christensen, K.; Birnbaum, L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ. Int. 2015, 77, 55–62. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Guilhermino, L. Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination. Exposure and risk to human consumers. J. Hazard. Mater. 2020, 393, 122419. [Google Scholar] [CrossRef] [PubMed]
- Waechter, J.; Thornton, C.; Markham, D.; Domoradzki, J. Factors affecting the accuracy of bisphenol a and bisphenol a-monoglucuronide estimates in Mammalian tissues and urine samples. Toxicol. Mech. Methods 2007, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, A.K.; Lillegaard, I.T.L.; Voorspoels, S.; Carlsen, M.H.; Løken, E.B.; Brantsæter, A.L.; Haugen, M.; Meltzer, H.M.; Thomsen, C. Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ. Int. 2014, 73, 259–269. [Google Scholar] [CrossRef]
- Shao, B.; Han, H.; Li, N.; Ma, Y.; Tu, X.; Wu, Y. Analysis of alkylphenol and bisphenol A in meat by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry. Food Chem. 2007, 105, 1236–1241. [Google Scholar] [CrossRef]
- Fernandez, M.F.; Arrebola, J.; Taoufiki, J.; Navalón, A.; Ballesteros, O.; Pulgar, R.; Vilchez, J.; Olea, N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 2007, 24, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Podlipna, D.; Cichna-Markl, M. Determination of bisphenol A in canned fish by sol-gel immunoaffinity chromatography, HPLC and fluorescence detection. Eur. Food Res. Technol. 2007, 224, 629–634. [Google Scholar] [CrossRef]
- Sun, C.; Leong, L.P.; Barlow, P.J.; Chan, S.H.; Bloodworth, B.C. Single laboratory validation of a method for the determination of Bisphenol A, Bisphenol A diglycidyl ether and its derivatives in canned foods by reversed-phase liquid chromatography. J. Chromatogr. A 2006, 1129, 145–148. [Google Scholar] [CrossRef]
- Cunha, S.; Inácio, T.; Almada, M.; Ferreira, R.; Fernandes, J. Gas chromatography-mass spectrometry analysis of nine bisphenols in canned meat products and human risk estimation. Food Res. Int. 2020, 135, 109293. [Google Scholar] [CrossRef]
- Deceuninck, Y.; Bichon, E.; Durand, S.; Bemrah, N.; Zendong, Z.; Morvan, M.; Marchand, P.; Dervilly-Pinel, G.; Antignac, J.; Leblanc, J.; et al. Development and validation of a specific and sensitive gas chromatography tandem mass spectrometry method for the determination of bisphenol A residues in a large set of food items. J. Chromatogr. A 2014, 1362, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Basheer, C.; Lee, H.K.; Tan, K.S. Endocrine disrupting alkylphenols and bisphenol-A in coastal waters and supermarket seafood from Singapore. Mar. Pollut. Bull. 2004, 48, 1161–1167. [Google Scholar] [CrossRef]
- Pedersen, S.N.; Lindholst, C. Quantification of the xenoestrogens 4-tert.-octylphenol and bisphenol A in water and in fish tissue based on microwave assisted extraction, solid-phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A 1999, 864, 17–24. [Google Scholar] [CrossRef]
- Tavazzi, S.; Benfenati, E.; Barcelo, D. Accelerated solvent extraction then liquid chromatography coupled with mass Spectrometry for determination of 4-t-octyl phenol, 4-nonylphenols, and bisphenol ain fish liver. Chromatographia 2002, 56, 463–467. [Google Scholar] [CrossRef]
- Lapviboonsuk, J.; Leepipatpiboon, N. A simple method for the determination of bisphenol A diglycidyl ether and its derivatives in canned fish. Anal. Methods 2014, 6, 5666–5672. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Hu, J.Y. Selective removal of estrogenic compounds by molecular imprinted polymer (MIP). Water Res. 2008, 42, 4101–4108. [Google Scholar]
- Phenomenex, Extraction of Bisphenol A from Water Using the Polymeric SPE Sorbent Strata-X. Technical Notes, TN-0040. 2010. Available online: https://phenomenex.blob.core.windows.net/documents/1285c130-c17f-43a7-838cc230535afc48 (accessed on 12 March 2019).
- Cao, X.L. Recent Development on Analytical Methods for Determination of Bisphenol a in Food and Biological Samples. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 2795–2829. [Google Scholar] [CrossRef]
- Aristiawan, Y.; Aryana, N.; Putri, D.; Styarini, D. Analytical Method Development for Bisphenol a in Tuna by Using High Performance Liquid Chromatography-UV. Procedia Chem. 2015, 16, 202–208. [Google Scholar] [CrossRef]
- Gallart-Ayala, H.; Nunez, O.; Lucci, P. Recent advances in LC-MS analysis of food-packaging contaminants. TrAC Trends Anal. Chem. 2013, 42, 99–124. [Google Scholar] [CrossRef]
- Yonekubo, J.; Hayakawa, K.; Sajiki, J. Concentrations of bisphenol A, bisphenol A diglycidyl ether, and their derivatives in canned foods in Japanese markets. J. Agric. Food Chem. 2008, 56, 2041–2047. [Google Scholar] [CrossRef]
- Gritti, F.; Guiochon, G. Accurate measurements of the true column efficiency and of the instrument band broadening contributions in the presence of a chromatographic column. J. Chromatogr. A 2014, 1327, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Battal, D.; Cok, I.; Unlusayin, I.; Tunctan, B. Development and validation of an LC-MS/MS method for simultaneous quantitative analysis of free and conjugated bisphenol A in human urine. Biomed. Chromatogr. 2014, 28, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Ayala, H.; Moyano, E.; Galceran, M.T. Analysis of bisphenols in soft drinks by on-line solid phase extraction fast liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2011, 683, 227–233. [Google Scholar] [CrossRef]
- Hassan, N.H.; Al Othman, H.I.A.; Malek, N.R.A.; Zulkurnain, M.; Saad, B.; Wong, Y.F. Simultaneous Quantitative Assessment of Ochratoxin A, Patulin, 5-Hydroxymethylfurfural, and Bisphenol A in Fruit Drinks Using HPLC with Diode Array-Fluorimetric Detection. Foods 2020, 9, 1633. [Google Scholar] [CrossRef] [PubMed]
- Takino, A.; Tsuda, T.; Kojima, M.; Harada, H.; Muraki, K.; Wada, M. Development of analytical method for bisphenol A in canned fish and meat by HPLC. J. Food Hyg. Soc. Jpn. 1999, 40, 325–333. [Google Scholar] [CrossRef]
- Babu, S.; Uppu, S.N.; Martin, B.; Agu, O.A.; Uppu, R.M. Unusually high levels of bisphenol A (BPA) in thermal paper cash register receipts (CRs): Development and application of a robust LC-UV method to quantify BPA in CRs. Toxicol. Mech. Methods 2015, 25, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.P.; Zhuang, Y.F.; Liu, B.L. Simultaneous Determination of Bisphenol A and Bisphenol S in Environmental Water using Ratio Derivative Ultraviolet Spectrometry. S. Afr. J. Chem. 2014, 67, 99–103. [Google Scholar]
- Watabe, Y.; Kondo, T.; Imai, H.; Morita, M.; Tanaka, N.; Haginaka, J.; Hosoya, K. Improved detectability with a polymer-based trapping device in rapid HPLC analysis for ultra-low levels of bisphenol A (BPA) in environmental samples. Anal. Sci. 2004, 20, 133–137. [Google Scholar] [CrossRef]
- Grumetto, L.; Montesano, D.; Seccia, S.; Albrizio, S.; Barbato, F. Determination of Bisphenol A and Bisphenol B Residues in Canned Peeled Tomatoes by Reversed-Phase Liquid Chromatography. J. Agric. Food Chem. 2008, 56, 10633–10637. [Google Scholar] [CrossRef]
- Del Olmo, M.; Zafra, A.; Jurado, A.; Vilchez, J. Determination of bisphenol A (BPA) in the presence of phenol by first-derivative fluorescence following micro liquid-liquid extraction (MLLE). Talanta 2000, 50, 1141–1148. [Google Scholar] [CrossRef]
- Vilarinho, F.; Lestido-Cardama, A.; Sendón, R.; De Quirós, A.R.B.; Vaz, M.D.F.; Sanches-Silva, A. HPLC with Fluorescence Detection for Determination of Bisphenol A in Canned Vegetables: Optimization, Validation and Application to Samples from Portuguese and Spanish Markets. Coatings 2020, 10, 624. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Assessment of bisphenol A and bisphenol B in canned vegetables and fruits by gas chromatography-mass spectrometry after QuEChERS and dispersive liquid -liquid microextraction. Food Control 2013, 33, 549–555. [Google Scholar] [CrossRef]
- Jurek, A.; Leitner, E. Comparing different gas chromatographic methods for the quantification of bisphenol A (BPA) trace levels in paper and cardboard products from the market. Food Addit. Contam. Part A 2015, 32, 1331–1342. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.; Martínez-Castro, I. Derivatization of carbohydrates for GC and GC-MS analyses. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1226–1240. [Google Scholar] [CrossRef]
- Ronderos-Lara, J.G.; Saldarriaga-Noreña, H.; Murillo-Tovar, M.A.; Vergara-Sánchez, J. Optimization and application of a GC-MS Method for the determination of endocrine disruptor compounds in natural water. Separations 2018, 5, 33. [Google Scholar] [CrossRef]
- Munguia-Lopez, E.M.; Peralta, E.; Gonzalez-Leon, A.; Vargas-Requena, C.; Soto-Valdez, H. Migration of bisphenol A (BPA) from epoxy can coatings to jalapeno peppers and an acid food simulant. J. Agric. Food Chem. 2002, 50, 7299–7302. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-W.; Ding, W.-H. Trace determination of bisphenol A and phytoestrogens in infant formula powders by gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1027, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xiang, L.; Li, J.; Yang, Z.; Fang, J.; Zhao, C.; Xu, S.; Cai, Z. Investigation on fragmentation pathways of bisphenols by using electrospray ionization Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
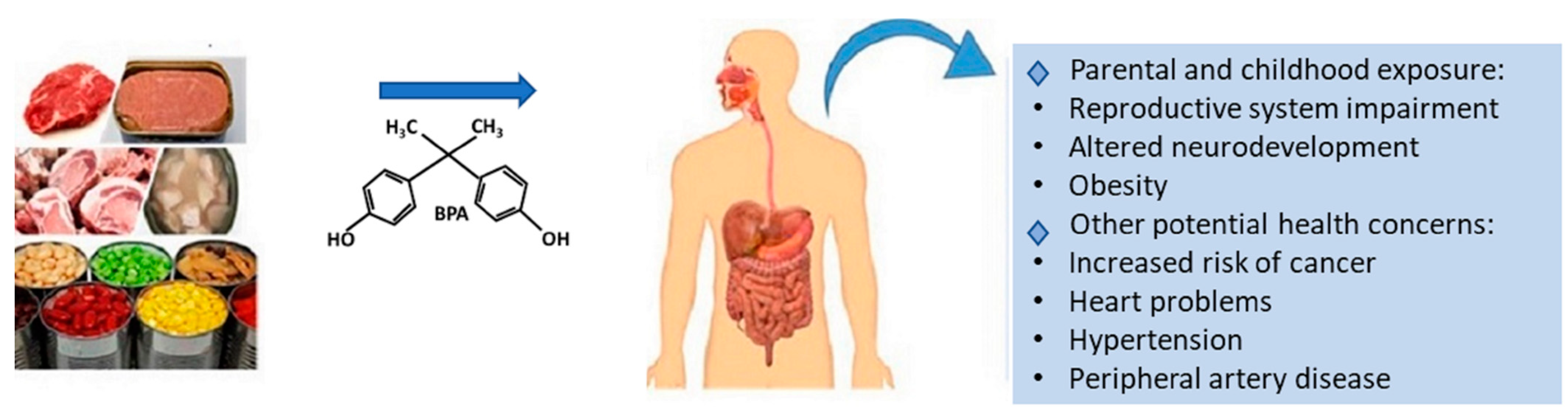
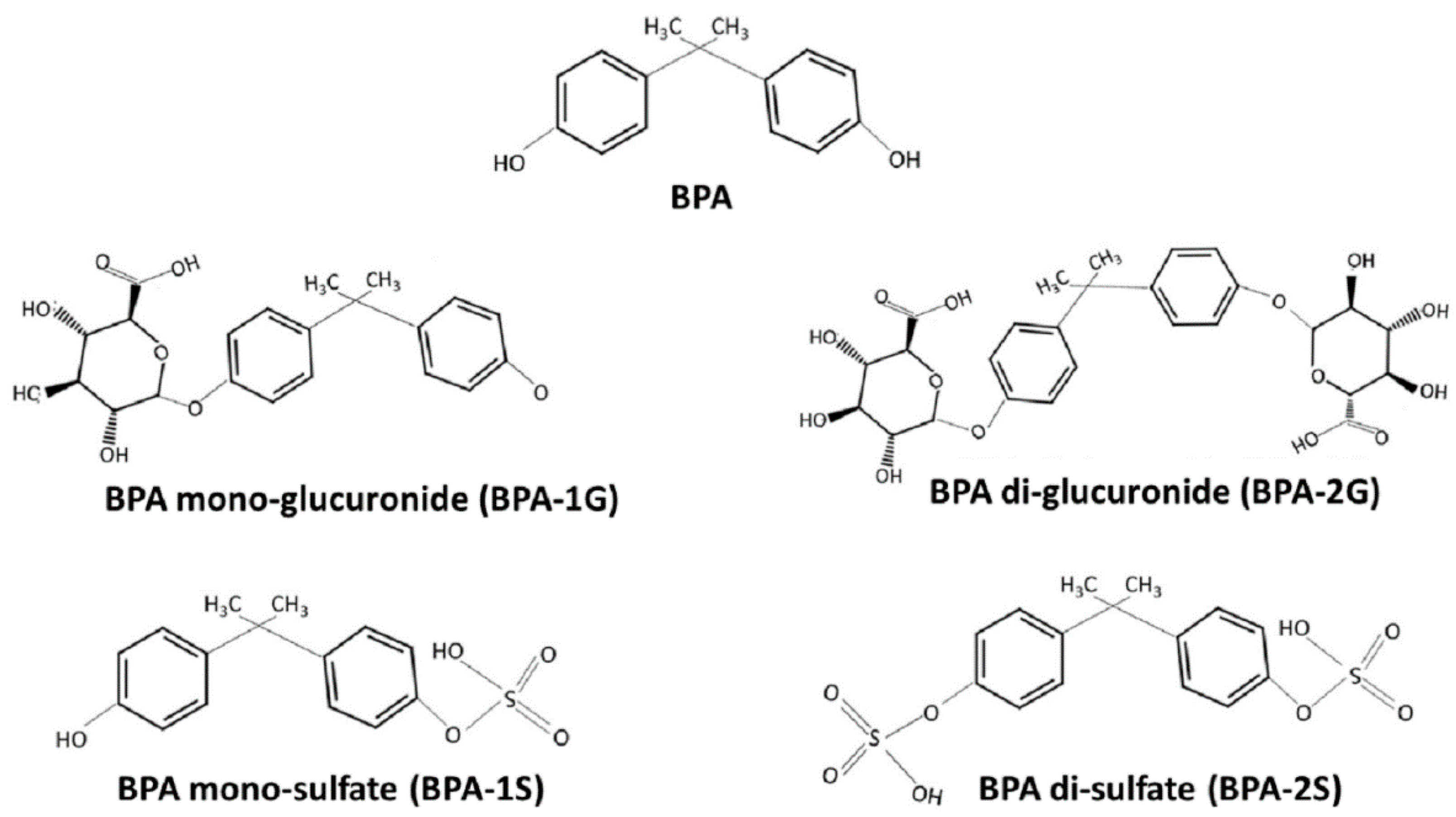
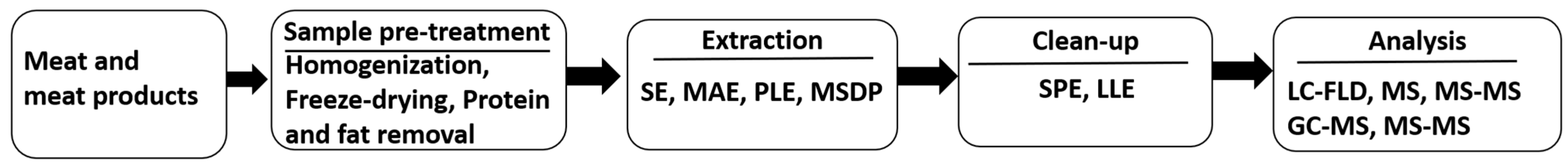
| Country | Selected Food/Food Groups Analysed | Population Groups | Dietary Exposure of BPA(µg/kg bW/Day) | Reference | |
|---|---|---|---|---|---|
| Mean ± Sd * | Range (Min–Max) | ||||
| Belgium | Canned beverages and foods | Adults | 0.015 | - | [13] |
| Canada (Quebec city) | Dairy, meat, fish, soup, bread and cereal, vegetable, fruit, beverages, baby food and fast food | Infants Children (1–19 years) Adults | - | 0.17–0.33 0.082–0.23 0.052–0.081 | [14] |
| China | Cereal products, meat and meat products, fish and seafood, dairy products, bean products, vegetables, snacks, and beverages | Adult men Adult women | 0.484 0.494 | - | [15] |
| France | Bread and cereals, dairy and egg products, meat, poultry and game, fish and seafood, fruits and vegetables, beverages, and fast foods | Infants Children and adolescents Adults Pregnant women | - | 0.12–0.14 0.05–0.06 0.038–0.040 0.05–0.06 | [6] |
| France | non-canned foods from animal origin | Children and adolescents Adults Pregnant women | - | 0.048–0.050 0.034–0.035 0.047–0.049 | [16] |
| Korea | Vegetables, fruits, fish, meat, tea, and coffee (canned) | Adults | 1.509 | - | [17] |
| New Zealand | Fruits and vegetables, fish, soup and sauces, canned meat, spaghetti and baked beans, infant foods, and beverages | Adult (60 kg) Adult (75 kg) | 0.078 0.063 | - | [9] |
| Spain (Southern) | Fish, meat, vegetables, pulses, and soft drinks (canned and microwave containers) | Pregnant women | 1.1 ± 0.84 | - | [18] |
| Sweden | Cereal products, fish, dairy and products, fruits and vegetables, and beverages | Adults (17–79 years) | - | 0.04–0.07 | [19] |
| United states | Solid foods, oil, beverages, and dairy products | Toddlers Infants Children Teenagers Adults | 0.243 0.142 0.117 0.0636 0.0586 | - | [20] |
| Figure | Sample Description | Concentration of BPA (µg/kg) | Reference | |
|---|---|---|---|---|
| Mean ± Sd * | Range (Min–Max) | |||
| Beef Chicken | Three different samples were collected for each category at three different local markets. | 12.7 ± 7.7 4.42 ± 1.5 | 5.88–21.3 2.94–6.36 | [77] |
| Meat balls Tripe | Samples were collected from local markets and kept at room temperatures before opening. | 82 ± 3 62 ± 2 | - | [78] |
| Lean pork | Lean pork cooked in its own juice. Cans stored at room temperature. | 37 ± 5 | _ | [79] |
| Goulash | N/A | 27 ± 4 | 9.6–22.0 | [10] |
| Luncheon meats Meat soup | Foods were prepared and combined into food composites according to established procedures. | 10.5 29.1 | - | [14] |
| Canned meat Infant meat puree | N/A | 19.39 35.22 | - | [11] |
| Sausages | Samples were randomly chosen from local supermarket. Stored at room temperature and analyzed within seven days after purchase. | 26.7 | - | [13] |
| Hot dogs Chopped pork and ham Corned beef | Three cans of each samples were purchased from retail outlets. Collected samples were stored at room temperature. | - | 21–33 16–17 59–70 | [12] |
| Minced Beef | Empty cans were filled with foods processed at 121 °C for 90 min, sealed, and either stored at 5 and 20 °C for up to 9 months or at 40 °C for 3 months | 53.8 ± 7.6 | - | [73] |
| Cooked pork (Spam) Beef boiled in soya sauce | Food items with different brands were purchased from local markets and stored at room temperature. | - | 38.7–51.04 9.11–26.58 | [17] |
| 98% fat free chicken breast Premium quality corned beef Premium quality Deviled ham spread Corned beef Pork (Spam classic) Chunk white chicken | Three cans of particular foods were collected from local supermarket. | 5.70 3.48 2.36 0.78 0.26 | 1.64–1.73 | [80] |
| Beef Chicken Pork Meat sauce/soup | 5 meat and 18 soup or sauces cans were purchased from supermarket. | 4 | 9–10 10–20 11–13 | [8] |
| Canned meat | Single cans of different brands were purchased from major supermarkets. | - | 29–98 | [9] |
| Figure | Sample Description | BPA Concentration (µg/kg) | Reference | |
|---|---|---|---|---|
| Mean ± Sd * | Range (Min–Max) | |||
| Beef steak Pork chop Mutton Roast pork Veal | Overall, 20,280 food items were purchased from French territory at regional scale, and prepared as typically consumed by the population. | 3.40 ± 6.66 16.95 ± 10.34 7.76 ± 6.43 12.44 ± 17.38 34.41 ± 58.73 | 0.11–26.91 4.09–40.09 1.71–22.74 2.20–68.92 3.68–223.52 | [6] |
| Beef steak Pork chop Mutton Roast pork Veal | 322 non-canned foods of animal origin was collected with two types of packing- pre-packaged and cut-to-order. | 2.93 ± 5.51 1.61 ± 2.8 3.19 ± 5.92 3.45 ± 9.04 1.16 ± 1.65 | 0.09–25.18 0.09–7.03 0.09–18.92 0.09–43.58 0.09–5.72 | [16] |
| Minced meat Chicken fillet Sausages Hamburgers Sliced salami Liver paté Sliced ham Sliced turkey | Food items in plastic packages were collected from grocery store and stored in a refrigerator or a freezer according to specifications written on the label. | 0.19 <0.10 2.1 0.17 0.29 3.2 <0.10 0.88 | - | [83] |
| Pork Beef Chicken Mutton Duck | Whole of chicken and duck was purchased, and the skin and internal organs were removed. For pork, thin meat was purchased. Samples were stored at 4 °C until analysis. | 0.33 0.73 0.54 | 0.9–7.08 0.49–0.85 | [84] |
| Meat Samples | Type of Bisphenols | Extraction Method | Brief Description of Extraction Method | Reference |
|---|---|---|---|---|
| Canned chicken | BPA | QuEChERS | Homogenized samples were mixed with acetonitrile, NaCl, MgSO4 and extracted with QuEChERS extraction kit, and derivatized. | [77] |
| Tripe Meat ball Fish and Seafood | BPA, BPB, BPF, BPE, BADGEs | SUPRAS-based microextraction | Solid content of the canned food was homogenized, an aliquot mixed with supramolecular solvent, vortexed, centrifuged. Extract was obtained with glass syringe and used for chromatographic analysis. | [78] |
| Goulash, caned | BPA | Sol-gel immunoaffinity chromatography | Gel was formed by mixing 1 mL of Phosphate-buffered saline containing 1 mg of BPA antibody with 1 mL of prehydrolyzed tetramethoxysilane. The resulting silica glass was ground in an achate mortar and packed into a 3 mL glass column equipped with a polytetrafluoroethylene frit. Sample was homogenized with acetonitrile and hexane, centrifuged, extracted with acetonitrile, filtered before placed into column, and eluted with acetonitrile/water (40:60, v/v) | [10] |
| Luncheon meats, canned Soups, meat, canned | BPA | SPE | Sample mixed with internal standards (BPA-d16) were extracted with acetonitrile, cleaned-up through C18 SPE cartridge, and eluted with 50% acetonitrile/water. | [14] |
| Beef, steak Beef, roast Beef, ground Pork, fresh Veal, cutlets Lamb Luncheon meats, cold cuts Organ meats Wieners and sausages | BPS, BPB, BPAF | Internally spiked samples were mixed with acetonitrile, cleaned-up with Strata-X SPE cartridge, the cartridge was rinsed with 10 mL of 20% acetonitrile in water, and eluted with 10 mL of methanol. | [76] | |
| Meat pates and sausages | BPA, BPB, BPF, BPAF, and BPZ | QuEChERS | Homogenized sample were taken into glass vials containing n-heptane and water. Vials were vortexed after adding acetonitrile, MgSO4, and NaCl. An aliquot of the supernatant was added to Z-sep + and C18, mixed and vortexed. | [88] |
| Meat, poultry and game, offal, delicatessen meats | BPA | SPE | Two successive solid phase extractions (SPE) were performed. The first SPE was carried out using polystyrene-divinyl benzene polymer. After loading the sample, the stationary phase was washed with water, water/methanol (90:10, v/v) and water/methanol (40:60, v/v). Analyte elution was done with methanol and load into specific Molecularly Imprinted Polymer (MIP) stationary phase after evaporation and resubmission into acetonitrile. After following the conditioning and washing steps the analytes were eluted with methanol. | [89] |
| Bovine muscle Cut of bovine meat Roast Pork Raw ham Parma Ham Turkey breast Chicken breast Swine muscle Ovine meat Bovine liver Chipolata sausage | BPA, BPA-G, BPA-2G, BPA-S, BPA-2S | SPE | Sample mixed with internal standards, extracted with water/acetonitrile (50:50) and purified with two successive SPE columns of polystyrene-divinylbenzene polymer and quaternary ammonium SPE SAX cartridge. | [16] |
| Meat (beef, pork, chicken, duck, sausages) | BPA, BPS, BPF | SPE | Solid samples spiked with internal standards were extracted twice with acetonitrile, purified with NH2 cartridges (Strata), and eluted with 80% methanol/acetone. | [15,20] |
| Beef chicken Pork Meat sauce | BPA | SPE | Homogenized samples were extracted with acetonitrile, passed through solid extraction column (OASIS), eluted with ethyl acetate, dried under N2, and dissolved in acetonitrile before analysis. | [8] |
| Beef Pork Mutton Chicken | BPA | SPE | Sample mixed with celite, ground into powder, packed into a stainless-steel ASE cells containing activated alumina. Acetone was used for the extraction and cleaned-up with amino-propyl SPE cartridge. | [84] |
| Corned beef, canned | BPA | SLE | Homogenized sample was extracted with acetonitrile, derivatized with acetic anhydride. Sample containing more than 1% fat acetonitrile and trimethylpentane was used. | [9] |
| Chromatographic Analysis | Types of Column (Phase Dimensions (Length × ID; Particle Size) Manufacturer) | Mobile Phase for LC/Carrier Gas for GC | Sensitivity | Linearity and Range | Mean Recovery (%) | Reference |
|---|---|---|---|---|---|---|
| HPLC UV | 5 µm Waters C18 column, 250 × 4.6 mm Wakosil 5C18 4.6 mm × 150 mm | Water/acetonitrile (40:60, v/v); Isocratic conditions 60% Methanol; Isocratic conditions | LOQ: 1.5 mg/kg LOD: 0.8 mg/kg LOD: 25 µg/kg | 89.84 89.9 | [97,104] | |
| HPLC-FLD | Ultrabase C-18 column (particle size 5 µm, length 250 mm, i.d.4.6 mm) Hypersil ODS C18 column (5 mm, 4.6 × 150 mm) | Water and acetonitrile; Gradient conditions Water and acetonitrile; Gradient conditions | MDL: 0.8 µg/kg MQL: 2.9 µg/kg MQL: 15–113 ng/g | 0.9995 | 80–110 90–99 | [78,79] |
| C18 column, 150 × 3 mm i.d., 3µm | 50 mM sodium acetate buffer (pH 4.8, adjusted with acetic acid) and acetonitrile; Gradient conditions | LOQ: 0.4 to 1.5 ng/mL; LOD: 0.2 to 0.8 ng/mL | 0.9993; 0.2–50 ng/ mL | 27–103 | [10] | |
| HPLC-MS/MS | Waters (1.7 μm, 2.1 mm x 100 mm) attached to a Waters Van Guard BEH phenyl pre-column (1.7 μm, 2.1 × 5 mm). | Water and acetonitrile; Gradient conditions | LOD: 0.18 ng/g | 0.99 | 92.4–102 | [76] |
| C- 18 column (150 mm × 2.1 mm ID, 3.5 µm) | Methanol and water with 0.1% ammonia; Gradient conditions | LOQ: 1 µg/kg | 0.99 | 91–99 | [84] | |
| Thermo Hypersil Gold column (100×2.1 mm, 1.9 μm) | 0.1% formic acid in water (MP A) and 0.1% formic acid in acetonitrile | LOD/LOQ: 0.02/0.06 μg/kg for BPA-G; 0.4/1.2 μg/kg for BPA-2G; 0.09/0.27 μg/kg for BPA-S. | [16] | |||
| Betasil C18 (2.1 × 100 mm, 5 μm) connected to a Javelin guard column (Betasil C18, 2.1 × 20 mm, 5 μm) | Methanol and water; Gradient conditions | LOQ: 0.01–3.14 ng/g | 0.99; 0.01–100ng/ml | 62–120 | [15,20] | |
| Shim-Pack VP-ODS column (150 × 4.6mm i.d., Shimadzu) | Acetonitrile–water–phosphor c acid (40:60:0.2); Isocratic conditions | 0.1 ng/ml (RSD 3.2) for LC-MS; 0.1 ng/ ml (RSD 1.2) for LC-MS/MS | - | 71.6–83.9 | [8] | |
| Symmetry C18 (3.5 µm, 150mm × 2.1 mm i.d., Waters) | Acetonitrile/water (40:60), Isocratic conditions | LOD: 0.3 ng/ml | - | 93 | [99] | |
| GC-MS | Agilent HP-5 ms (30 m × 0.25 mm × 0.25 µm (film thickness) | Helium | LOD: 0.00013 ng/g LOQ: 0.0004 ng/g | 0.998 | 80–99 | [77] |
| HP-5MS Capillary column (30 m × 0.25 mm × 1.0 µm) | Helium | LOD: 1 ng/g | - | - | [14] | |
| DB-5MS column (30 m × 0.25 mm I.D. × 0.25 µm film thickness | Helium | LOD: 0.15 µg/kg LOQ: 0.5 µg/kg | 0.99; 2.5–200 µg/kg | 75–95 | [88] | |
| ZB-5MS (Phenomenex) 30 m × 0.25 mm i.d., 0.25 µm film thickness | Helium | LOD: 0.01 to 0.03 µg/kg LOOQ: 0.03 to 0.08 µg/kg | 0.9990; 0–100 µg/kg | 100 | [89] | |
| J&W DB5ms, 30m × 0.25mm i.d, 0.25 µm film thickness | Helium | LOQ: 10 µg/kg for <1% fat containing sample; 20 µg/kg for >1% fat containing | - | 42–112 | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddique, M.A.b.; Harrison, S.M.; Monahan, F.J.; Cummins, E.; Brunton, N.P. Bisphenol A and Metabolites in Meat and Meat Products: Occurrence, Toxicity, and Recent Development in Analytical Methods. Foods 2021, 10, 714. https://doi.org/10.3390/foods10040714
Siddique MAb, Harrison SM, Monahan FJ, Cummins E, Brunton NP. Bisphenol A and Metabolites in Meat and Meat Products: Occurrence, Toxicity, and Recent Development in Analytical Methods. Foods. 2021; 10(4):714. https://doi.org/10.3390/foods10040714
Chicago/Turabian StyleSiddique, Md Abu bakar, Sabine M. Harrison, Frank J. Monahan, Enda Cummins, and Nigel P. Brunton. 2021. "Bisphenol A and Metabolites in Meat and Meat Products: Occurrence, Toxicity, and Recent Development in Analytical Methods" Foods 10, no. 4: 714. https://doi.org/10.3390/foods10040714
APA StyleSiddique, M. A. b., Harrison, S. M., Monahan, F. J., Cummins, E., & Brunton, N. P. (2021). Bisphenol A and Metabolites in Meat and Meat Products: Occurrence, Toxicity, and Recent Development in Analytical Methods. Foods, 10(4), 714. https://doi.org/10.3390/foods10040714







