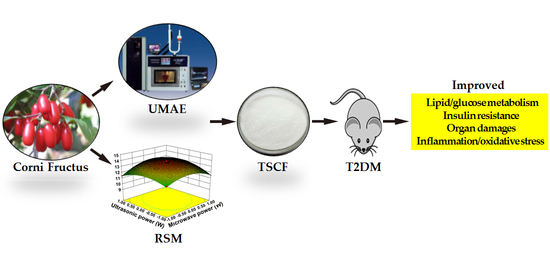
Total Saponins Isolated from Corni Fructus via Ultrasonic Microwave-Assisted Extraction Attenuate Diabetes in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Standard Curve of TS
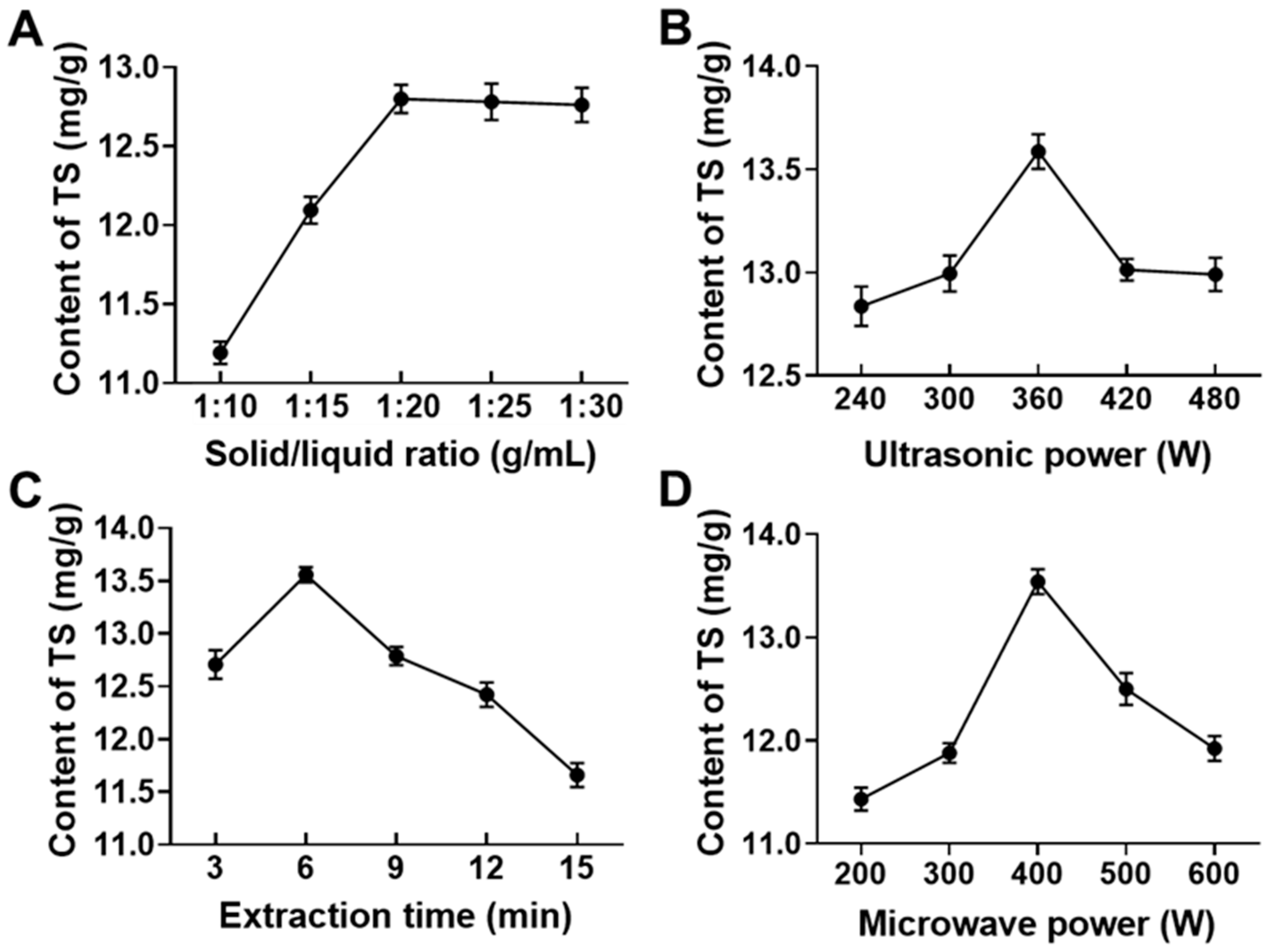
2.4. Single Factor Experimental Design for Extraction of Total Saponins from CF (TSCF) with Ultrasonic-Microwave Assisted Extraction (UMAE)
2.5. Optimization of TSCF Extraction by RSM
2.6. Purification of Crude TSCF
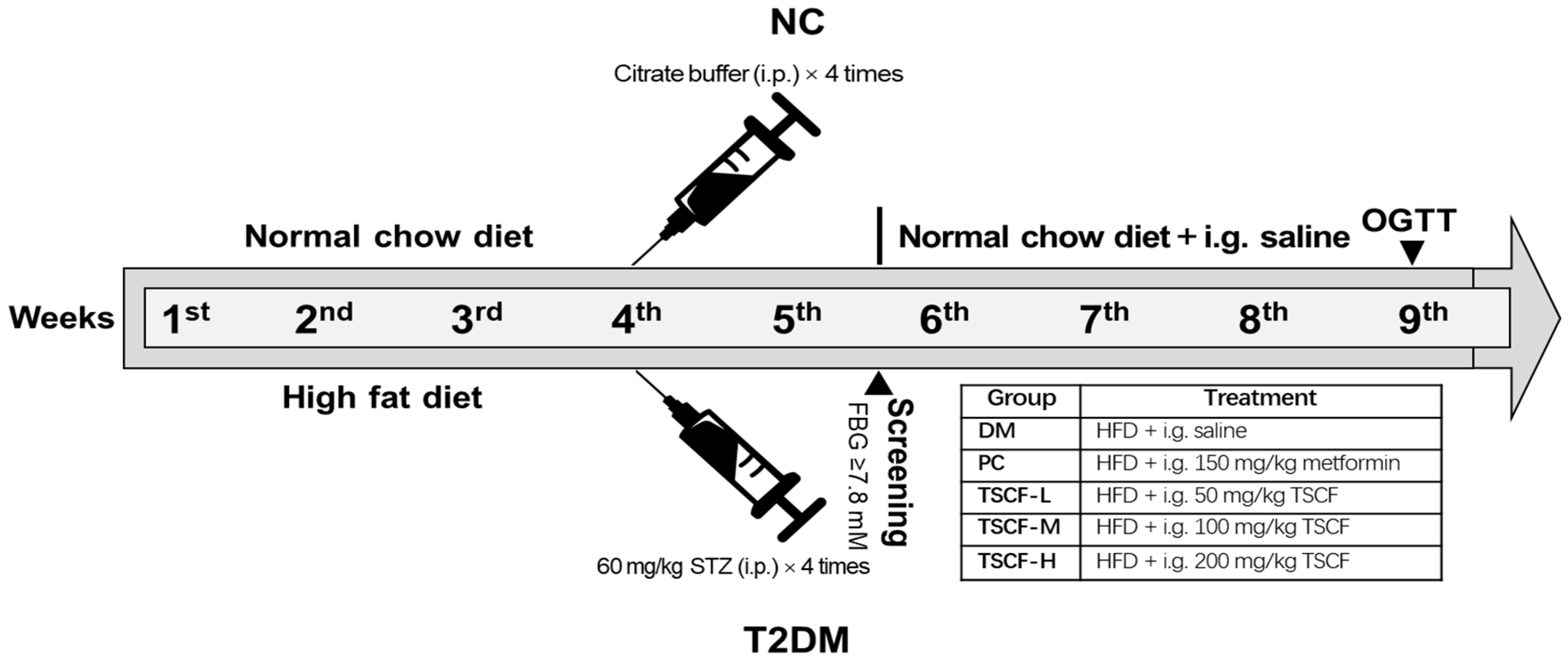
2.7. Establishment of T2DM Model in Mice
2.8. Treat T2DM Mice with TSCF
2.9. Fasting Blood Glucose (FBG) and Oral Glucose Tolerance Test (OGTT)
2.10. Biochemical Assay
2.11. Histopathological Examinations
2.12. RNA Quantitation by Real-Time qPCR
2.13. Statistical Analysis
3. Results and Discussion
3.1. Optimization of UMAE Conditions by RSM for TSCF Extraction
3.2. Purification of Crude TSCF
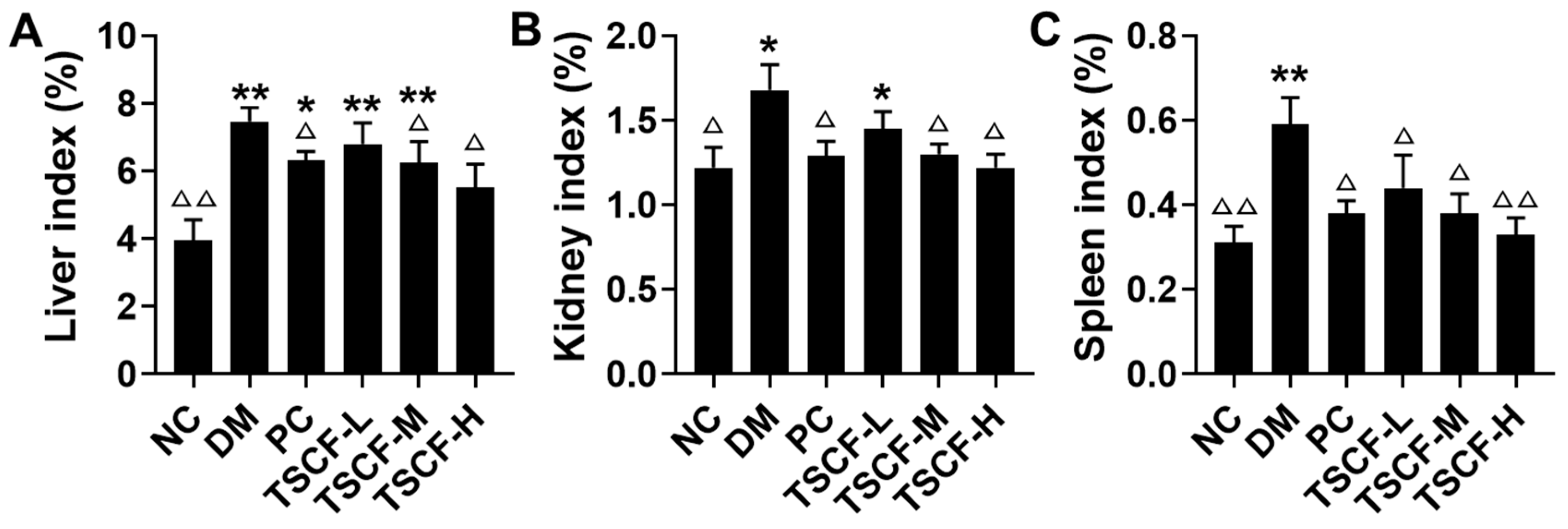
3.3. The Influence of TSCF on Body Weight and Organ Index of Diabetic Mice
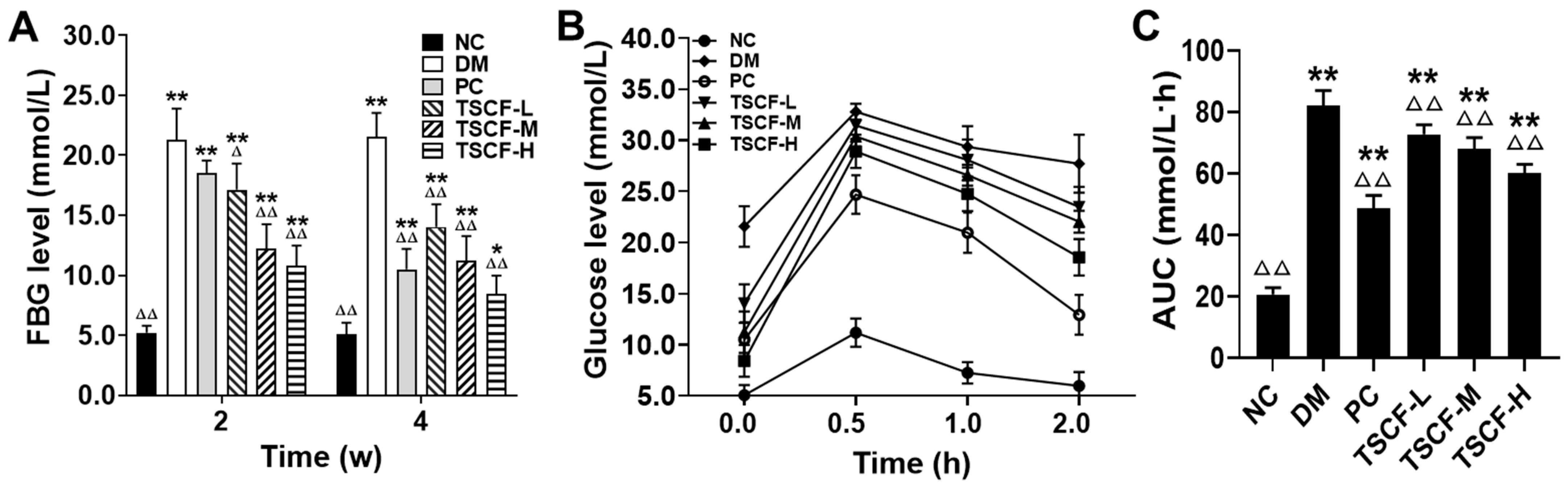
3.4. The Effects of TSCF on Glucose Metabolism of T2DM Mice
3.5. The Effects of TSCF on Lipid Metabolism in T2DM Mice
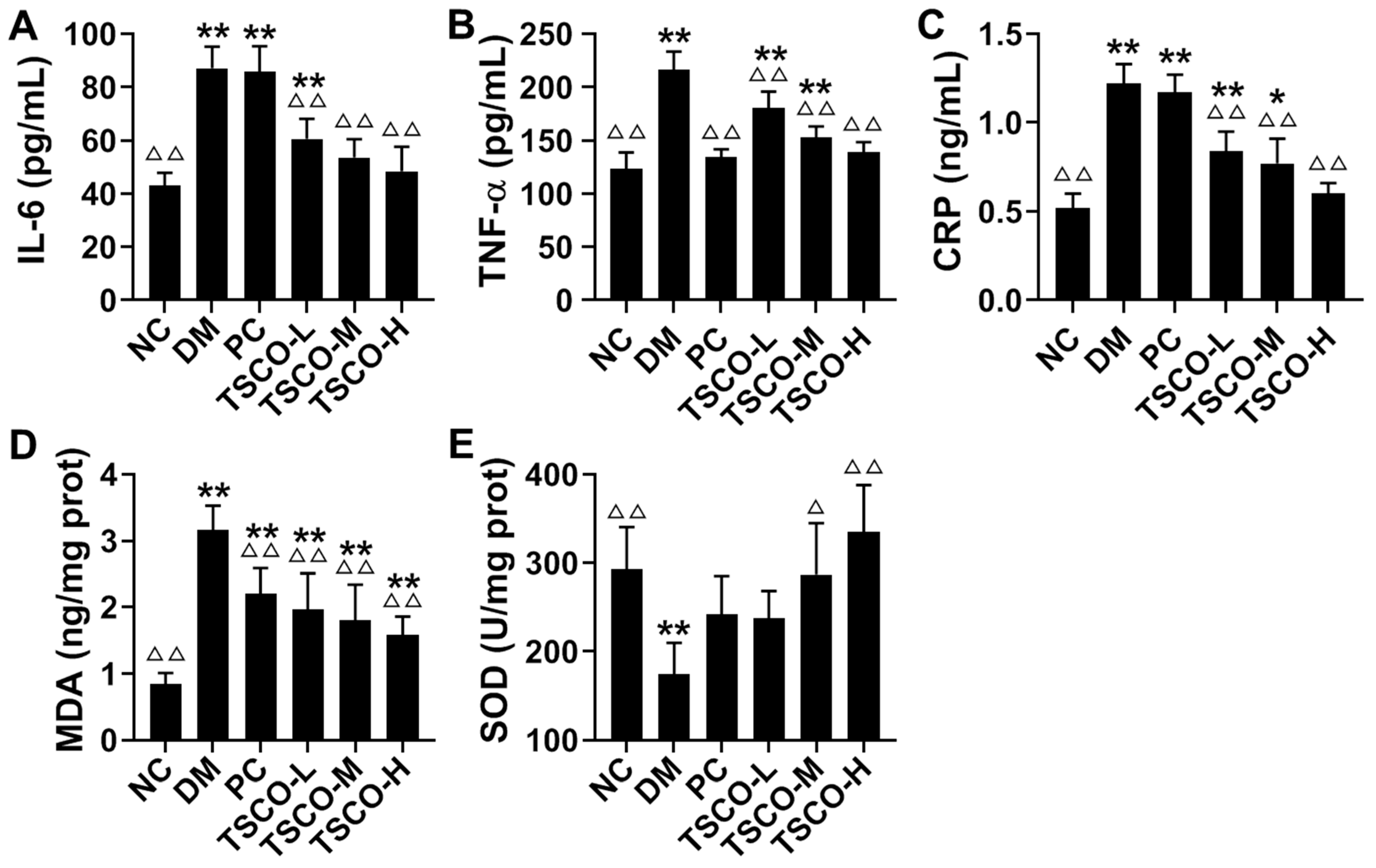
3.6. Effects of TSCF on Inflammation and Oxidative Stress in T2DM Mice
3.7. TSCF Ameliorated Pancreas and Liver Damages in T2DM Mice
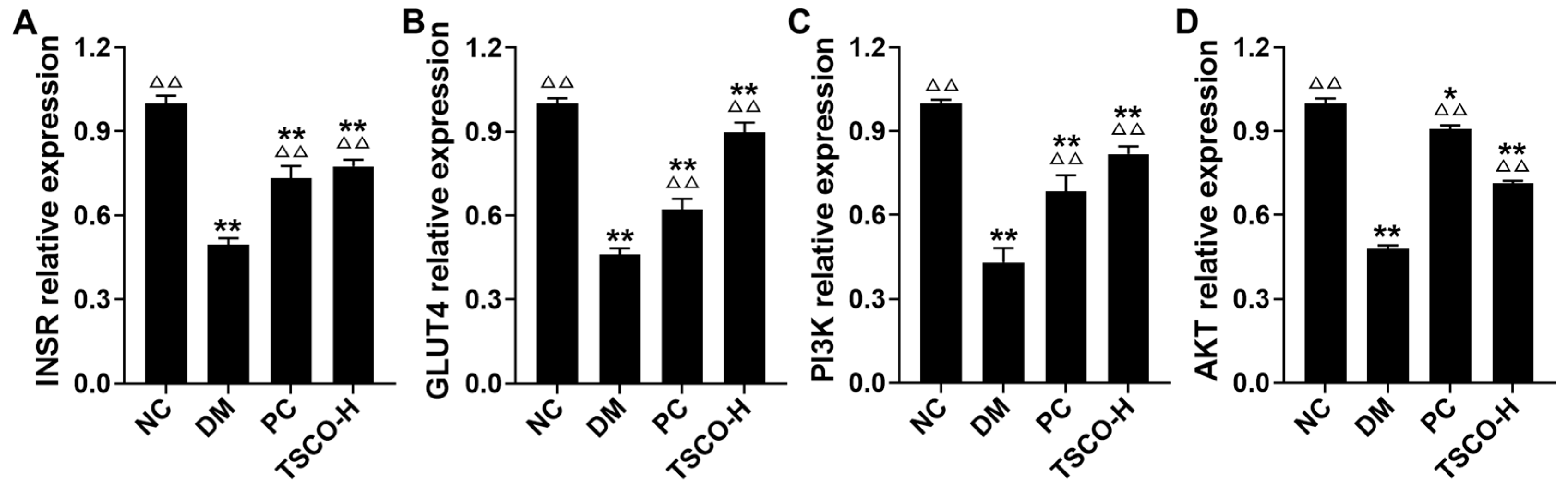
3.8. TSCF Regulates INSR, GLUT4, PI3K and AKT Signaling Pathways in Skeletal Muscle of T2DM Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, C.H.; Noh, J.S.; Kim, J.H.; Tanaka, T.; Zhao, Q.; Matsumoto, K.; Shibahara, N.; Yokozawa, T. Evaluation of Morroniside, Iridoid Glycoside from Corni Fructus, on Diabetes-Induced Al-terations such as Oxidative Stress, Inflammation, and Apoptosis in the Liver of Type 2 Diabetic db/db Mice. Biol. Pharm. Bull. 2011, 34, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, C.; Fan, W.; Yi, T.; Wei, A.; Ma, Y. Hypoglycemic and hypolipidemic effects of a polysaccharide from Fructus Corni in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2019, 133, 420–427. [Google Scholar] [CrossRef]
- Lee, N.H.; Seo, C.S.; Lee, H.Y.; Jung, D.Y.; Lee, J.K.; Lee, J.A.; Song, K.Y.; Shin, H.K.; Lee, M.Y.; Seo, Y.B.; et al. Hepatoprotective and Antioxidative Activities of Cornus officinalis against Acetamino-phen-Induced Hepatotoxicity in Mice. Evid.-Based Compl. Alt. 2012, 2012, 804924. [Google Scholar]
- Nouska, C.; Kazakos, S.; Mantzourani, I.; Alexopoulos, A.; Bezirtzoglou, E.; Plessas, S. Fermentation of Cornus Mas L. Juice for Functional Low Alcoholic Beverage Production. Curr. Res. Nutr. Food Sci. J. 2016, 4, 119–124. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Piórecki, N. Bioactive Compounds in Cornelian Cherry Vinegars. Molecules 2018, 23, 379. [Google Scholar] [CrossRef]
- Xu, M.; Wu, H.Y.; Liu, H.; Gong, N.; Wang, Y.R.; Wang, Y.X. Morroniside, a secoiridoid glycoside from Cornus officinalis, attenu-ates neuropathic pain by activation of spinal glucagon-like peptide-1 receptors. Brit. J. Pharmacol. 2017, 174, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; An, S.; Chen, X.; Bi, H.; Zhang, Q.; Wang, T.; Han, B.; Zhang, H.; Kang, J. Corni Fructus as a Natural Resource Can Treat Type 2 Diabetes by Regulating Gut Microbiota. Am. J. Chin. Med. 2020, 48, 1–23. [Google Scholar] [CrossRef]
- Chen, C.C.; Hsu, C.Y.; Chen, C.Y.; Liu, H.K. Fructus Corni suppresses hepatic gluconeogenesis related gene transcription, en-hances glucose responsiveness of pancreatic beta-cells, and prevents toxin induced beta-cell death. J. Ethnopharmacol. 2008, 117, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.-D.; Xue, X.-C.; Gao, M.-L.; Wang, X.-F.; Shu, Z.; Mu, N.; Gao, Y.; Wang, Z.-L.; Hao, Q.; Li, W.-N.; et al. Therapeutic Effects of PADRE-BAFF Autovaccine on Rat Adjuvant Arthritis. BioMed. Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Kayukawa, C.T.M.; Oliveira, M.A.S.; Kaspchak, E.; Sanchuki, H.B.S.; Igarashi-Mafra, L.; Mafra, M.R. Quillaja bark saponin effects on Kluyveromyces lactis beta-galactosidase activity and structure. Food Chem. 2020, 303, 125388. [Google Scholar] [CrossRef]
- Weng, Y.; Yu, L.; Cui, J.; Zhu, Y.-R.; Guo, C.; Wei, G.; Duan, J.-L.; Yin, Y.; Guan, Y.; Wang, Y.-H.; et al. Antihyperglycemic, hypolipidemic and antioxidant activities of total saponins extracted from Aralia taibaiensis in experimental type 2 diabetic rats. J. Ethnopharmacol. 2014, 152, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, S.; Feng, T.; Chen, Y.; Yang, G. Hypoglycemic and hypolipidemic effects of total saponins from Stauntonia chinensis in diabetic db/db mice. J. Cell. Mol. Med. 2018, 22, 6026–6038. [Google Scholar] [CrossRef]
- Liu, B.; Miao, J.; Peng, M.; Liu, T.; Miao, M. Effect of 3:7 ratio of Astragalus total saponins and Curcumin on the diabetic nephropathy rats model. Saudi J. Biol. Sci. 2019, 26, 188–194. [Google Scholar] [CrossRef]
- Lin, M.-H.; Liu, H.-K.; Huang, W.-J.; Huang, C.-C.; Wu, T.-H.; Hsu, F.-L. Evaluation of the Potential Hypoglycemic and Beta-Cell Protective Constituents Isolated from Corni Fructus To Tackle Insulin-Dependent Diabetes Mellitus. J. Agric. Food Chem. 2011, 59, 7743–7751. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Dong, L.; Gao, Q.; Yin, L.; Quan, H.; Chen, R.; Fu, X.; Lin, D. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J. Ethnopharmacol. 2018, 213, 280–301. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Tang, H.; Long, N.; Qiu, M.; Gao, M.; Sun, F.; Dai, M. Assessment of the anti-virulence potential of extracts from four plants used in traditional Chinese medicine against multidrug-resistant pathogens. BMC Complement. Med. Ther. 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.H.; Li, C.S.; Li, D.D. Progress in research of the active chemical components of Cornus officinalis. Chin. J. New. Drugs 2001, 10, 808–812. [Google Scholar]
- Jarzębski, M.; Siejak, P.; Smułek, W.; Fathordoobady, F.; Guo, Y.; Pawlicz, J.; Trzeciak, T.; Kowalczewski, P.Ł.; Kitts, D.D.; Singh, A.; et al. Plant Extracts Containing Saponins Affects the Stability and Biological Activity of Hempseed Oil Emulsion System. Molecules 2020, 25, 2696. [Google Scholar] [CrossRef]
- Fu, S.Y.; Li, Z.J.; Wang, Z.T. Study on the gross saponinsin natural beverage of Cornus officinalis. Zhejiang Linxueyuan Xuebao 1998, 15, 105–107. [Google Scholar]
- Shi, G.Q.; Ma, T.T.; Liu, K.; Liu, Y.Q. Purification of total saponins of Cornus officinalis by membrane separations technology. Henan Chem. Ind. 2015, 9, 19–23. [Google Scholar]
- Zheng, Q.X.; Liu, H.; Li, J.R. The technology of extraction process saponin from Cornus officinalis Sieb. et Zucc. at ultra high pressure. Food Sci. Tech. 2011, 36, 183–186. [Google Scholar]
- You, Q.H.; Yin, X.L.; Zhang, S.N.; Jiang, Z.H. Extraction, purification, and antioxidant activities of polysaccharides from Tricholo-ma mongolicum Imai. Carbohyd. Polym. 2014, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Niu, D.; Wang, T.; An, S.; Wang, Y.; Chen, X.; Bi, H.; Xue, X.; Kang, J. Ultrasonic–microwave assisted extraction of total triterpenoid acids from Corni Fructus and hypoglycemic and hypolipidemic activities of the extract in mice. Food Funct. 2020, 11, 10709–10723. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin re-sistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Mu, J.; Xin, G.; Zhang, B.; Wang, Y.; Ning, C.; Meng, X. Beneficial effects of Aronia melanocarpa berry extract on hepatic insulin resistance in type 2 diabetes mellitus rats. J. Food Sci. 2020, 85, 1307–1318. [Google Scholar] [CrossRef]
- Tang, W.; Li, S.; Liu, Y.; Huang, M.-T.; Ho, C.-T. Anti-diabetic activity of chemically profiled green tea and black tea extracts in a type 2 diabetes mice model via different mechanisms. J. Funct. Foods 2013, 5, 1784–1793. [Google Scholar] [CrossRef]
- Tian, Y.M.; Liu, Y.; Wang, S.; Dong, Y.; Su, T.; Ma, H.J.; Zhang, Y. Anti-diabetes effect of chronic intermittent hypobaric hypoxia through improving liver insu-lin resistance in diabetic rats. Life Sci. 2016, 150, 1–7. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jin, G.Y.; Li, G.Z.; Yan, G.H. Cornuside suppresses lipopolysaccharide-induced inflammatory mediators by inhibiting nuclear factor-kappa B activation in RAW 264.7 macrophages. Biol. Pharm. Bull. 2011, 34, 959–966. [Google Scholar] [CrossRef]
- Gao, Y.-F.; Zhang, M.-N.; Wang, T.-X.; Wu, T.-C.; Ai, R.-D.; Zhang, Z.-S. Hypoglycemic effect of D-chiro-inositol in type 2 diabetes mellitus rats through the PI3K/Akt signaling pathway. Mol. Cell. Endocrinol. 2016, 433, 26–34. [Google Scholar] [CrossRef]
- Jiang, S.; Ren, D.; Li, J.; Yuan, G.; Li, H.; Xu, G.; Han, X.; Du, P.; An, L. Effects of compound K on hyperglycemia and insulin resistance in rats with type 2 diabetes mellitus. Fitoterapia 2014, 95, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Mayakrishnan, T.; Nakkala, J.R.; Jeepipalli, S.P.; Raja, K.; Chandra, V.K.; Mohan, V.K.; Sadras, S.R. Fenugreek seed extract and its phytocompounds- trigonelline and diosgenin arbitrate their hepatoprotective effects through attenuation of endoplasmic reticulum stress and oxidative stress in type 2 diabetic rats. Eur. Food Res. Technol. 2015, 240, 223–232. [Google Scholar] [CrossRef]


| No. | X1 (g/mL) | X2 (W) | X3 (W) | X4 (min) | Theoretical Yield of TS (mg/g) | Actual Yield of TS (mg/g) |
|---|---|---|---|---|---|---|
| 1 | 0 (1/20) | −1 (300 | 1 (420) | 0 (6) | 11.38 | 11.42 |
| 2 | 0 (1/20) | 1 (500) | −1 (300) | 0 (6) | 11.56 | 11.48 |
| 3 | 0 (1/20) | 1 (500) | 0 (360) | −1 (3) | 12.66 | 12.69 |
| 4 | 1 (1/25) | 0 (400) | 0 (360) | 1 (9) | 10.64 | 10.38 |
| 5 | 0 (1/20) | −1 (300) | 0 (360) | 1 (9) | 9.71 | 9.76 |
| 6 | −1 (1/15) | −1 (300) | 0 (360) | 0 (6) | 11.65 | 11.54 |
| 7 | 0 (1/20) | 0 (400) | 1 (420) | −1 (3) | 12.60 | 12.31 |
| 8 | 0 (1/ 20) | 0 (400) | 0 (360) | 0 (6) | 13.76 | 13.94 |
| 9 | 0 (1/ 20) | 0 (400) | 0 (360) | 0 (6) | 13.76 | 13.75 |
| 10 | 0 (1/ 20) | 0 (400) | −1 (300) | 1 (9) | 9.83 | 10.08 |
| 11 | 0 (1/ 20) | −1 (300) | 0 (360) | −1 (3) | 12.29 | 12.57 |
| 12 | −1 (1/15) | 1 (500) | 0 (360) | 0 (6) | 11.65 | 12.02 |
| 13 | −1 (1/15) | 0 (400) | 1 (420) | 0 (6) | 11.65 | 11.52 |
| 14 | 1 (1/ 25) | 1 (500) | 0 (360) | 0 (6) | 12.83 | 12.91 |
| 15 | 0 (1/ 20) | 0 (400) | −1 (300) | −1 (3) | 12.70 | 12.42 |
| 16 | 0 (1/ 20) | 0 (400) | 1 (420) | 1 (9) | 10.58 | 10.82 |
| 17 | 0 (1/ 20) | 1 (500) | 1 (420) | 0 (6) | 12.71 | 12.51 |
| 18 | 0 (1/ 20) | 0 (400) | 0 (360) | 0 (6) | 13.76 | 13.57 |
| 19 | 0 (1/ 20) | 0 (400) | 0 (360) | 0 (6) | 13.76 | 13.85 |
| 20 | 0 (1/ 20) | 0 (400) | 0 (360) | 0 (6) | 13.76 | 13.71 |
| 21 | 1 (1/ 25) | 0 (400) | 1 (420) | 0 (6) | 13.01 | 13.34 |
| 22 | 0 (1/ 20) | −1 (300) | −1 (300) | 0 (6) | 11.89 | 12.05 |
| 23 | 1 (1/ 25) | 0 (400) | −1 (300) | 0 (6) | 12.00 | 12.21 |
| 24 | −1 (1/15) | 0 (400) | −1 (300) | 0 (6) | 12.00 | 11.75 |
| 25 | −1 (1/15) | 0 (400) | 0 (360) | 1 (9) | 9.98 | 9.89 |
| 26 | 0 (1/ 20) | 1 (500) | 0 (360) | 1 (9) | 10.35 | 10.15 |
| 27 | 1 (1/ 25) | −1 (300) | 0 (360) | 0 (6) | 11.83 | 11.43 |
| 28 | 1 (1/ 25) | 0 (400) | 0 (360) | −1 (3) | 13.11 | 13.15 |
| 29 | −1 (1/15) | 0 (400) | 0 (360) | −1 (3) | 12.40 | 12.62 |
| Group | Body Weight (g) | ||||
|---|---|---|---|---|---|
| 0 w | 1 w | 2 w | 3 w | 4 w | |
| NC | 33.33 ± 2.78 | 33.68 ± 2.70 | 34.04 ± 1.80 △ | 33.66 ± 1.85 △ | 32.71 ± 1.63 △ |
| DM | 33.29 ± 2.69 | 31.35 ± 2.76 | 30.89 ± 1.89 * | 29.01 ± 2.13 ** | 28.65 ± 1.48 * |
| PC | 32.81 ± 1.74 | 32.18 ± 2.35 | 32.41 ± 2.28 | 32.73 ± 2.32 △ | 32.42 ± 1.56 △ |
| TSCF-L | 33.23 ± 1.98 | 32.04 ± 2.28 | 32.47 ± 0.81 | 32.47 ± 0.81 △ | 32.15 ± 1.57 △ |
| TSCF-M | 33.65 ± 2.67 | 33.07 ± 2.65 | 32.54 ± 1.46 | 33.26 ± 2.22 △△ | 32.41 ± 1.17 △ |
| TSCF-H | 32.48 ± 2.38 | 32.58 ± 1.66 | 32.27 ± 1.10 | 33.33 ± 1.77 △△ | 32.25 ± 1.63 △ |
| Group | FINS (mIU/L) | HOMA-IS | HOMA-IR |
|---|---|---|---|
| NC | 9.46 ± 1.71 △△ | –3.84 ± 0.25 △△ | 2.13 ± 0.55 △△ |
| DM | 5.89 ± 0.73 ** | –4.93 ± 0.15 ** | 6.19 ± 1.02 ** |
| PC | 8.40 ± 1.92 △△ | –4.46 ± 0.22 **,△△ | 3.92 ± 0.90 **,△△ |
| TSCF-L | 7.03 ± 0.97 * | –4.53 ± 0.19 **,△△ | 4.38 ± 0.61 **,△△ |
| TSCF-M | 8.26 ± 0.69 △△ | –4.45 ± 0.15 **,△△ | 3.84 ± 0.57 **,△△ |
| TSCF-H | 9.43 ± 1.53 △△ | –4.31 ± 0.08 **,△△ | 3.52 ± 0.81 *,△△ |
| Group | TC | TG | HDL-C | LDL-C |
|---|---|---|---|---|
| NC | 2.49 ± 0.11 △△ | 0.67 ± 0.08 △△ | 1.68 ± 0.13 △△ | 2.98 ± 0.79 △△ |
| DM | 2.89 ± 0.28 ** | 0.93 ± 0.11 ** | 1.01 ± 0.96 ** | 4.81 ± 0.71 ** |
| PC | 2.66 ± 0.13 | 0.68 ± 0.11 △△ | 1.66 ± 0.11 △△ | 4.48 ± 0.71 ** |
| TSCF-L | 2.50 ± 0.23 △△ | 0.73 ± 0.09 △△ | 1.35 ± 0.09 **,△△ | 3.70 ± 0.30 *,△△ |
| TSCF-M | 2.69 ± 0.16 | 0.70 ± 0.06 △△ | 1.39 ± 0.12 **,△△ | 3.45 ± 0.25 △△ |
| TSCF-H | 2.68 ± 0.23 | 0.67 ± 0.10 △△ | 1.46 ± 0.11 *,△△ | 3.21 ± 0.43 △△ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.; Niu, D.; Wang, T.; Han, B.; He, C.; Yang, X.; Sun, H.; Zhao, K.; Kang, J.; Xue, X. Total Saponins Isolated from Corni Fructus via Ultrasonic Microwave-Assisted Extraction Attenuate Diabetes in Mice. Foods 2021, 10, 670. https://doi.org/10.3390/foods10030670
An S, Niu D, Wang T, Han B, He C, Yang X, Sun H, Zhao K, Kang J, Xue X. Total Saponins Isolated from Corni Fructus via Ultrasonic Microwave-Assisted Extraction Attenuate Diabetes in Mice. Foods. 2021; 10(3):670. https://doi.org/10.3390/foods10030670
Chicago/Turabian StyleAn, Shujing, Dou Niu, Ting Wang, Binkai Han, Changfen He, Xiaolin Yang, Haoqiang Sun, Ke Zhao, Jiefang Kang, and Xiaochang Xue. 2021. "Total Saponins Isolated from Corni Fructus via Ultrasonic Microwave-Assisted Extraction Attenuate Diabetes in Mice" Foods 10, no. 3: 670. https://doi.org/10.3390/foods10030670
APA StyleAn, S., Niu, D., Wang, T., Han, B., He, C., Yang, X., Sun, H., Zhao, K., Kang, J., & Xue, X. (2021). Total Saponins Isolated from Corni Fructus via Ultrasonic Microwave-Assisted Extraction Attenuate Diabetes in Mice. Foods, 10(3), 670. https://doi.org/10.3390/foods10030670