Marinated Sea Bream Fillets Enriched with Lactiplantibacillus plantarum and Bifidobacterium animalis subsp. lactis: Brine Optimization and Product Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Brine Optimization
2.2.1. Brine Optimization: Experimental Design
2.2.2. Sampling
- (a)
- Salt (vinegar and citric acid were fixed to the coded level 0, that is, 40% and 0%);
- (b)
- Citric acid (vinegar and salt at 40% and 0%, respectively).
2.3. Product Optimization
2.3.1. Sample Preparation
2.3.2. Microbiological Analyses
2.3.3. Sensory Analyses
3. Results
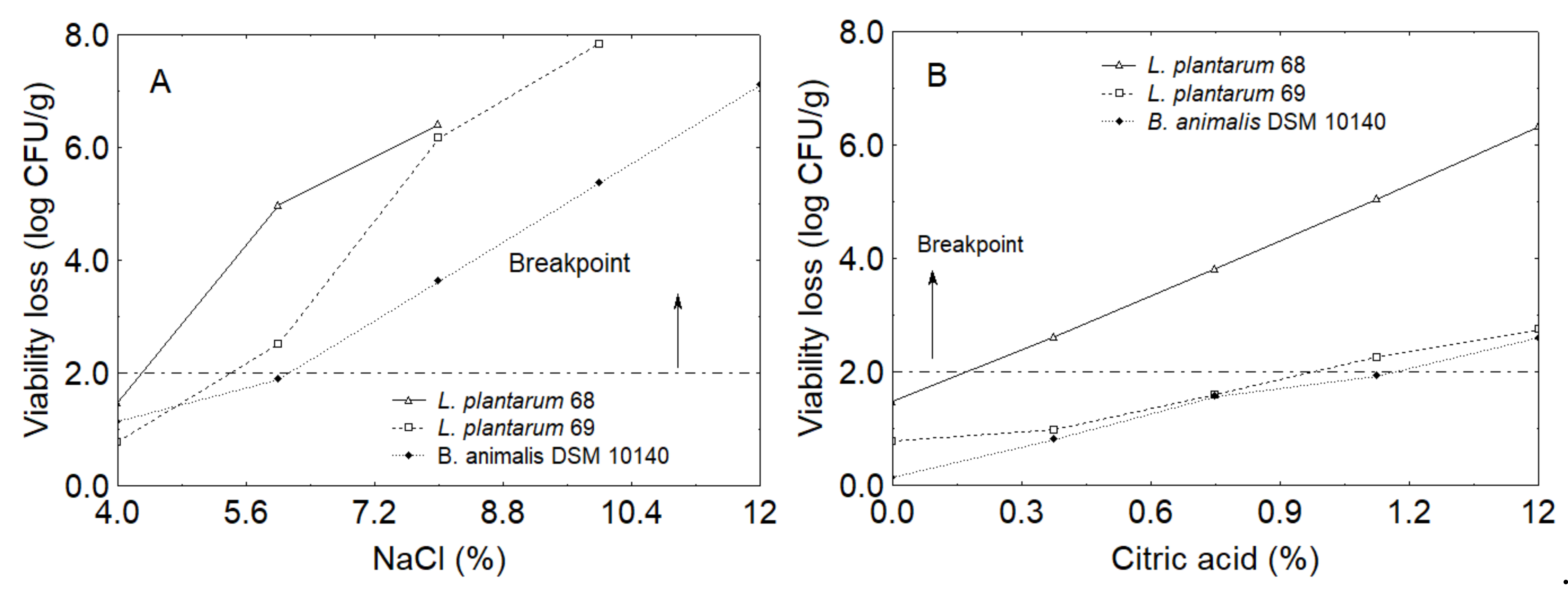
3.1. Brine Optimization
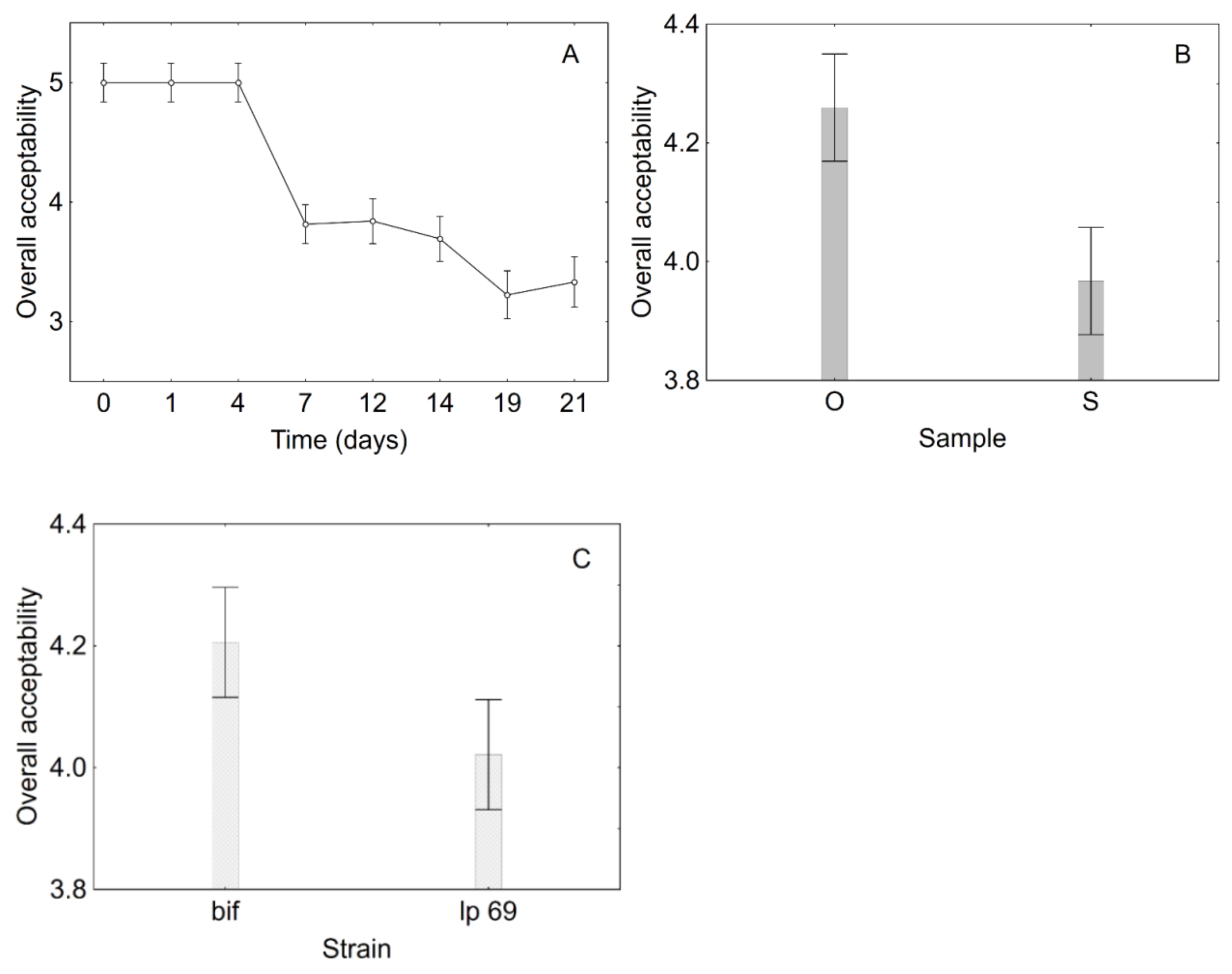
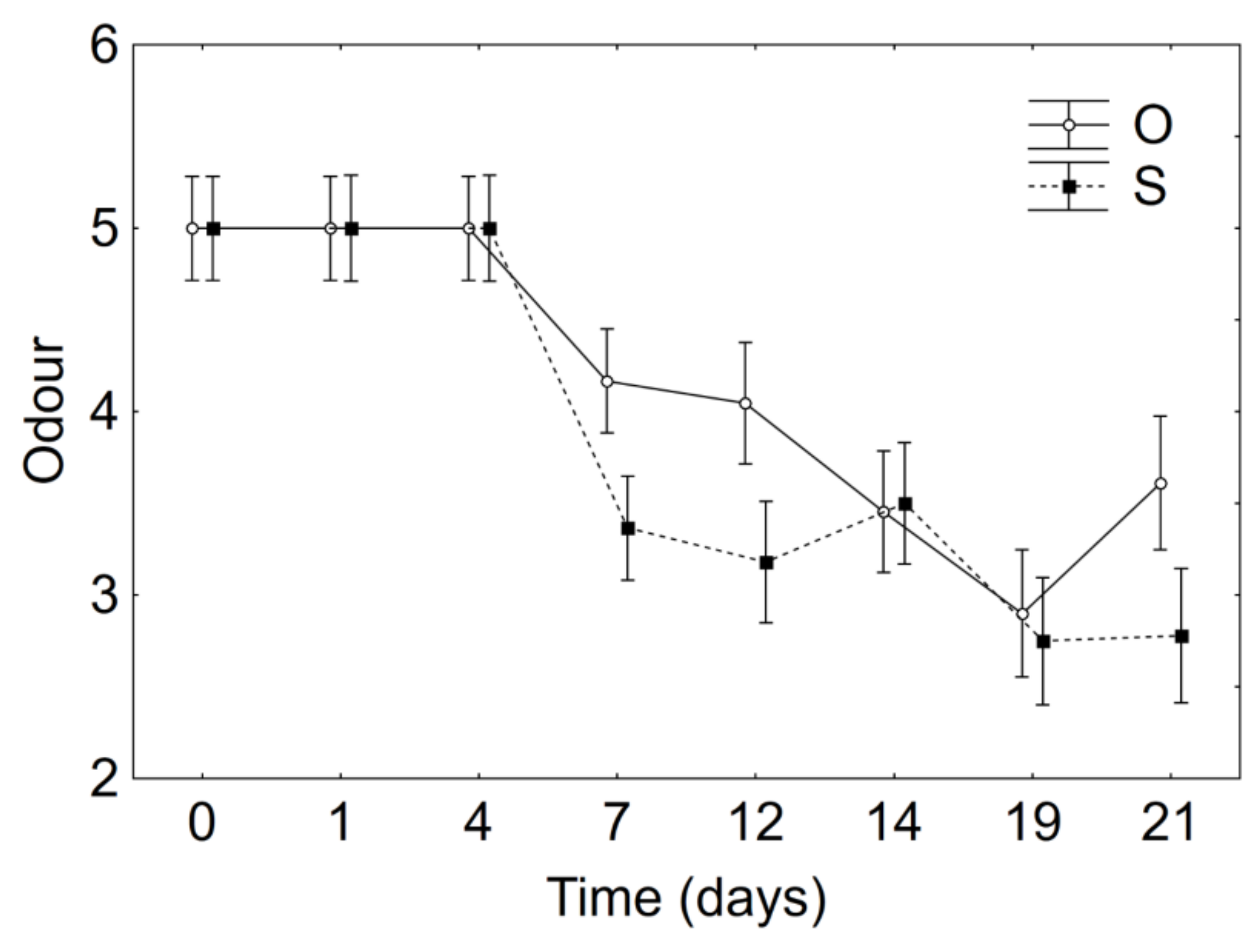
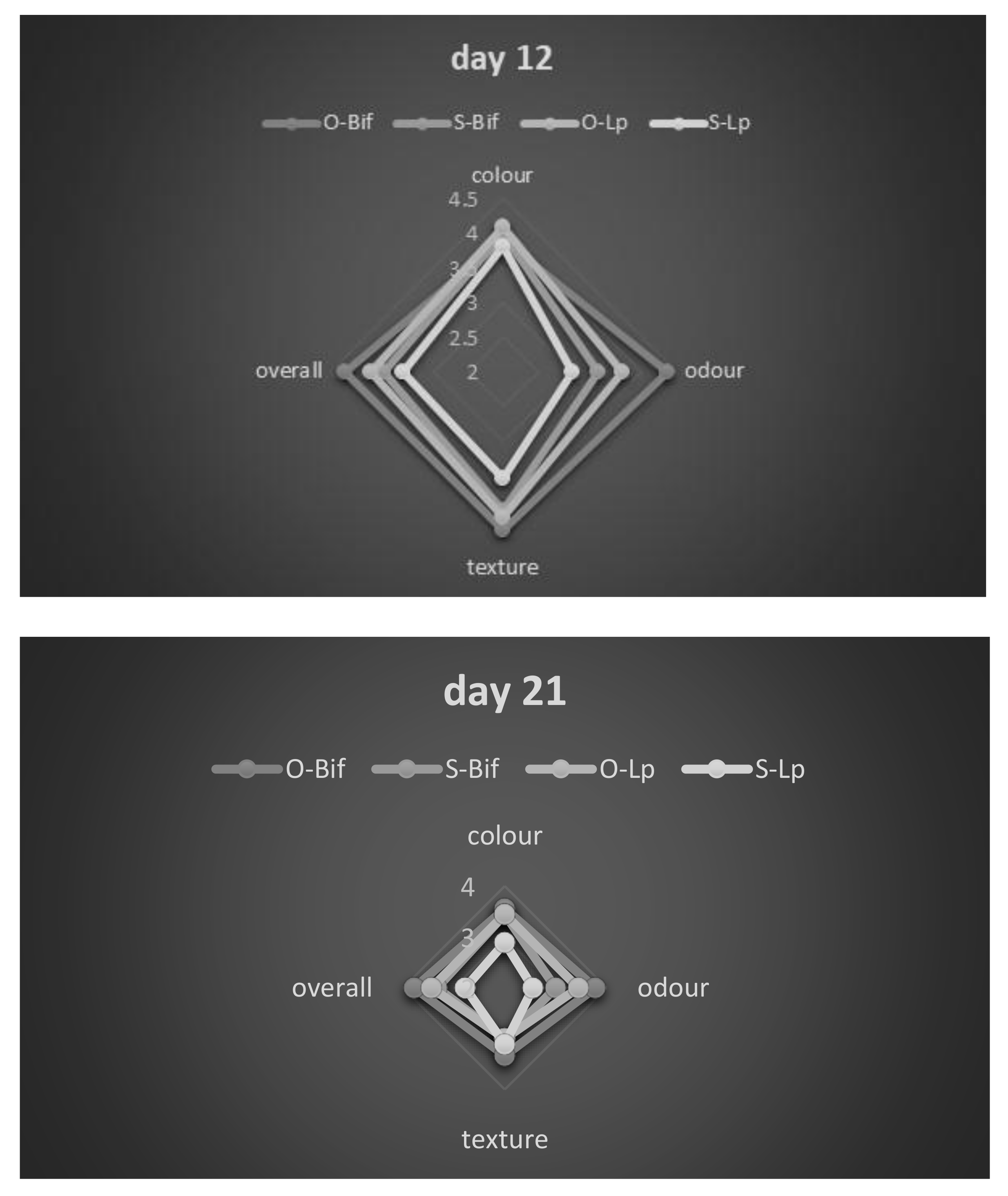
3.2. Product Optimization
Author Contributions
Funding
Conflicts of Interest
References
- Dinkçi, N.; Akdeniz, V.; Akalin, A.S. Survival of probiotics in functional foods during shelf life. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Academic Press: Philadelphia, PA, USA, 2019; pp. 201–233. [Google Scholar]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastro. Hepat. 2010, 7, 503–514. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Probiotic fermentation of plant based products: Possibilities and opportunities. Crit. Rev. Food Sci. 2012, 52, 183–199. [Google Scholar] [CrossRef]
- Peres, C.M.; Peres, C.; Hernández-Mendoza, A.; Malcata, F.X. Review on fermented plant materials as carriers and sources of potentially probiotic LAB—With an emphasis on table olives. Trends Food Sci. Tech. 2012, 26, 31–42. [Google Scholar] [CrossRef]
- Vijaya Kumar, B.; Sreedharamurthy, M.; Reddy, O.V.S. Physicochemical analysis of fresh and probioticated fruit juices with Lactobacillus casei. Int. J. App. Sci. Biotech. 2013, 1, 127–131. [Google Scholar] [CrossRef]
- Vijayendra, S.V.N.; Halami, P.M. Fermented vegetables-health benefits. In Fermented Vegetables; Tamang, J.P., Ed.; CRC Taylor & Francis Publisher: Boca Raton, FL, USA, 2015; pp. 327–344. [Google Scholar]
- Sidira, M.; Karapetsas, A.; Galanis, A.; Kanellaki, M.; Kourkoutas, Y. Effective survival of immobilized Lactobacillus casei during ripening and heat treatment of probiotic dry-fermented sausages and investigation of the microbial dynamics. Meat Sci. 2014, 96, 948–955. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-dairy prebiotic products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.J.; Metian, M. Food matters: Fish, income, and food supply—A comparative analysis. Rev. Fish. Sci. Aquac. 2017, 26, 15–28. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Jiang, Q.; Yang, F.; Wang, B. Phospholipid molecular species composition of Chinese traditional low-salt fermented fish inoculated with different starter cultures. Food Res. Int. 2018, 111, 87–96. [Google Scholar] [CrossRef]
- Vilavert, L.; Borrell, F.; Nadal, M.; Jacobs, S.; Minnens, F.; Verbeke, W.; Marques, A.; Domingo, J.L. Health risk/benefit information for consumers of fish and shellfish: Fish choice, a new online tool. Food Chem. Toxicol. 2017, 104, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.; Clifton, P.; Colquhoun, D.; Noakes, M.; Mori, T.A.; Sullivan, D. Indications for Omega-3 long chain polyunsaturated fatty acid in the prevention and treatment of cardiovascular disease. Heart Lung Circ. 2015, 24, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Baik, I.; Abbott, R.D.; Curb, J.D.; Shin, C. Intake of fish and n-3 fatty acids and future risk of metabolic syndrome. J. Am. Diet. Assoc. 2010, 110, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.Y.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. American heart association nutrition committee of the council on lifestyle and cardiometabolic health, council on epidemiology and prevention, council on cardiovascular disease in the young, council on cardiovascular and stroke nursing, & council on clinical cardiology. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American Heart Association. Circulation 2017, 135, 867–884. [Google Scholar]
- Tacon, A.G.J.; Metian, M. Fish matters: Importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Semjonovs, P.; Auzina, L.; Upite, D.; Grube, M.; Shvirksts, K.; Linde, R.; Denina, I.; Bormanis, A.; Upitis, A.; Ruklisha, M.; et al. Application of Bifidobacterium animalis subsp. lactis as starter culture for fermentation of Baltic herring (Clupea harengus membras) mince. Am. J. Food Tech. 2015, 10, 184–194. [Google Scholar]
- Speranza, B.; Racioppo, A.; Beneduce, L.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Autochthonous lactic acid bacteria with probiotic aptitudes as starter cultures for fish-based products. Food Microbiol. 2017, 65, 244–253. [Google Scholar] [CrossRef]
- Speranza, B.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Shelf life definition for Italian anchovies inoculated with Lactobacillus plantarum and Bifidobacterium animalis subsp. lactis. Innov. Food Sci. Emerg. Tech. 2012, 16, 171–180. [Google Scholar] [CrossRef]
- Giribaldi, M.; Gai, F.; Peiretti, P.G.; Ortoffi, M.F.; Lavermicocca, P.; Lonigro, S.L.; Valerio, F.; Cavallarin, L. Quality of ready-to-eat swordfish fillets inoculated with Lactobacillus paracasei IMPC 2.1. J. Sci. Food Agr. 2019, 99, 199–209. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evolut. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Speranza, B.; Racioppo, A.; Bevilacqua, A.; Beneduce, L.; Sinigaglia, M.; Corbo, M.R. Selection of autochthonous strains as starter cultures for fermented fish products. J. Food Sci. 2015, 80, M151–M160. [Google Scholar] [CrossRef]
- FAO. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO; FAO: Rome, Italy, 2013. [Google Scholar]
- Ray, B.; Bhunia, A. Fundamental Food Microbiology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2008; Chapter 16. [Google Scholar]
- Šimat, V.; Mićunović, A.; Bogdanović, T.; Listeš, I.; Generalić Mekinić, I.; Hamed, I.; Skroza, D. The impact of lemon juice on the marination of anchovy (Engraulis Encrasicolus): Chemical, microbiological and sensory changes. Ital. J. Food Sci. 2019, 31, 604–617. [Google Scholar]
- Sanchez, B.; Champomier-Verges, M.C.; Collado, M.C.; Anglade, P.; Baraige, F.; Sanz, Y.; de Los Reys-Gavillán, C.G.; Margolles, A.; Zagorec, M. Low-pH adaptation and the acid tolerance response of Bifidobacterium longum biotype longum. Appl. Environ. Microb. 2007, 73, 6450–6459. [Google Scholar] [CrossRef] [PubMed]
- Neffe-Skocińska, K.; Rzepkowska, A.; Szydłowska, A.; Kołożyn-Krajewska, D. Trends and possibilities of the use of probiotics in food production. In Handbook of Food Bioengineering, Alternative and Replacement Foods; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 65–94. [Google Scholar]
- Gokoglu, N.; Ucak, I. Effect of freshness grade of anchovy (Engraulis encrasicolus) on the quality of marinated product stored at 4 °C. Aquat. Sci. 2020, 2, 54–59. [Google Scholar] [CrossRef]
- Valerio, F.; Lonigro, S.L.; Giribaldi, M.; Di Biase, M.; De Bellis, P.; Cavallarin, L.; Lavermicocca, P. Probiotic Lactobacillus paracasei IMPC 2.1 strain delivered by ready-to-eat swordfish fillets colonizes the human gut after alternate-day supplementation. J. Funct. Foods 2015, 17, 468–475. [Google Scholar] [CrossRef]
- Cadun, A.; Cackli, S.; Kisla, D. A study of marination of deepwater pink shrimp (Parapenaeus longirostris, Lucas, 1846) and its shelf life. Food Chem. 2005, 90, 53–59. [Google Scholar] [CrossRef]
- Esaiassen, M.; Nilsen, H.; Joensen, S.; Skjerdal, T.; Carlehog, M.; Eilertsen, G.; Gundersen, B.; Elvevoll, E. Effects of catching method on quality changes during storage of cod (Gadhus morhua). Lebensm. Wiss. Technol. 2004, 37, 643–648. [Google Scholar] [CrossRef]
- Gokoglu, N.; Cengiz, E.; Yerlikaya, P. Determination of the shelf life of marinated sardine (Sardina pilchardus) stored at 4 °C. Food Control 2004, 15, 1–4. [Google Scholar] [CrossRef]
- Gokoglu, N.; Topuz, O.K.; Yerlikaya, P. Effects of pomegranate sauce on quality of marinated anchovy during refrigerated storage. LWT Food Sci. Technol. 2009, 42, 113–118. [Google Scholar] [CrossRef]
- Kilinc, B.; Cakli, S. Determination of the shelf life of sardine (Sardina pilchardus) marinades in tomato sauce stored at 4 °C. Food Control 2005, 16, 639–644. [Google Scholar] [CrossRef]
- Sallam, K.I.; Ahmed, A.M.; Elgazzar, M.M.; Eldaly, E.A. Chemical quality and sensory attributes of marinated Pacific saury (Cololabis saira) during vacuum-packaged storage at 4 °C. Food Chem. 2007, 102, 1061–1070. [Google Scholar] [CrossRef]
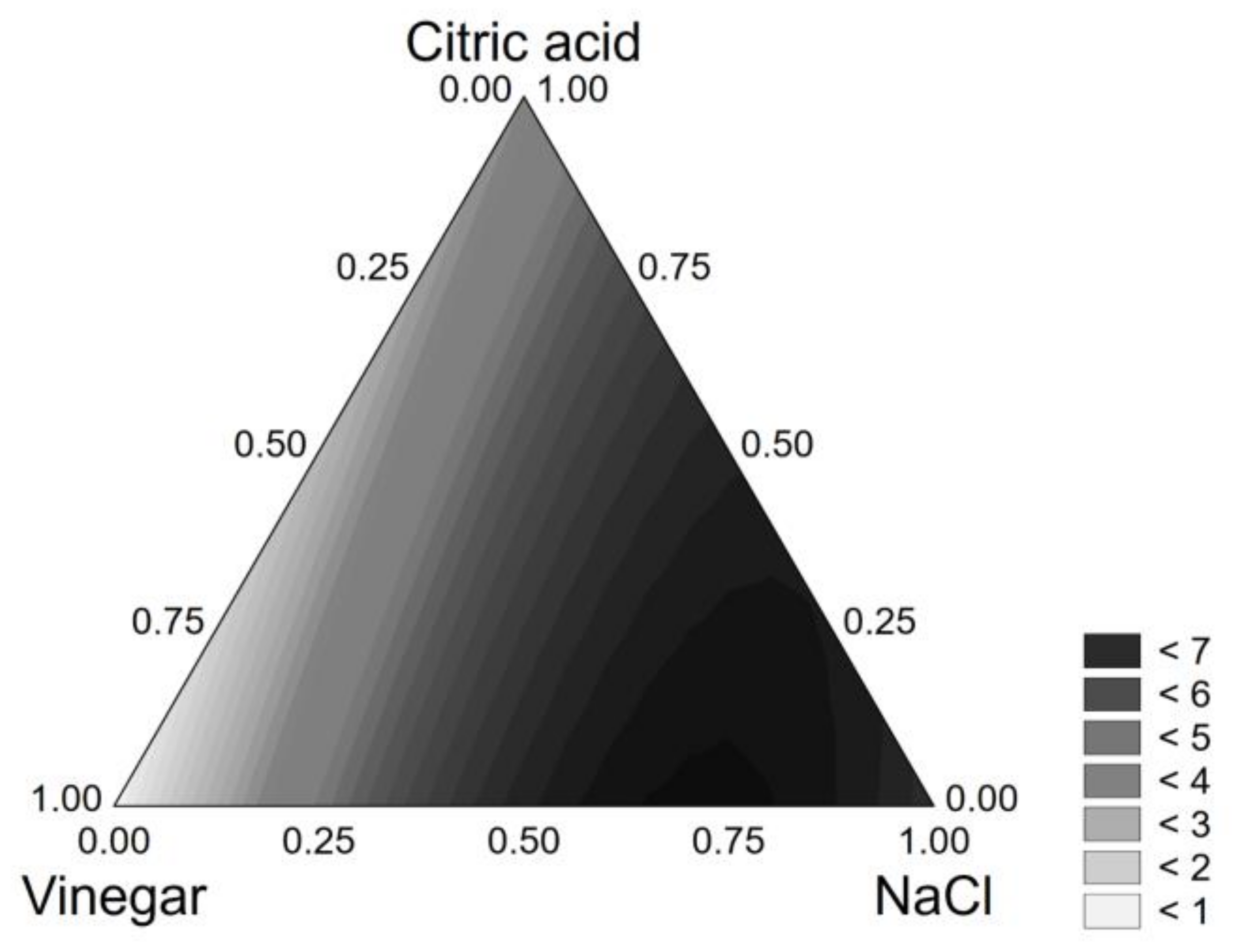
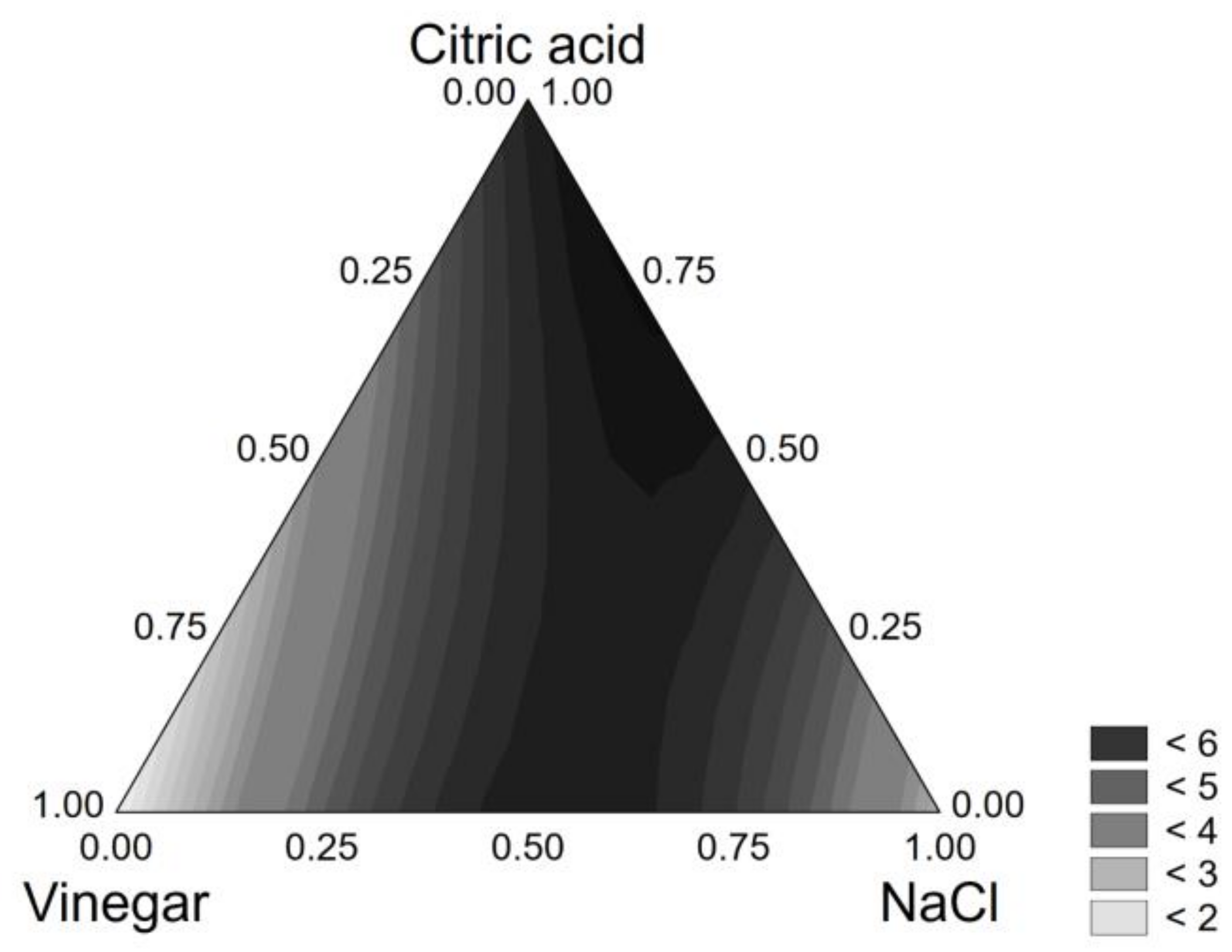
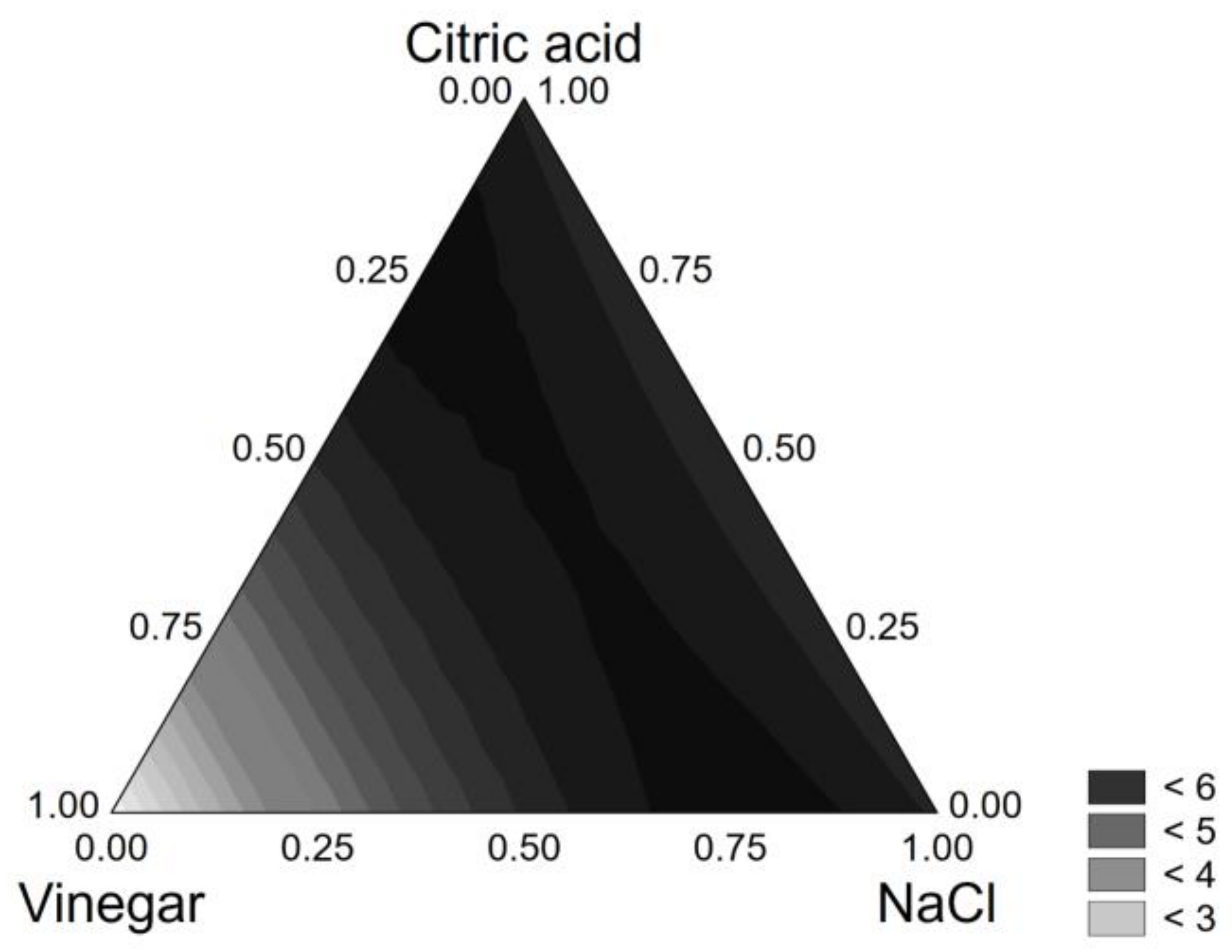
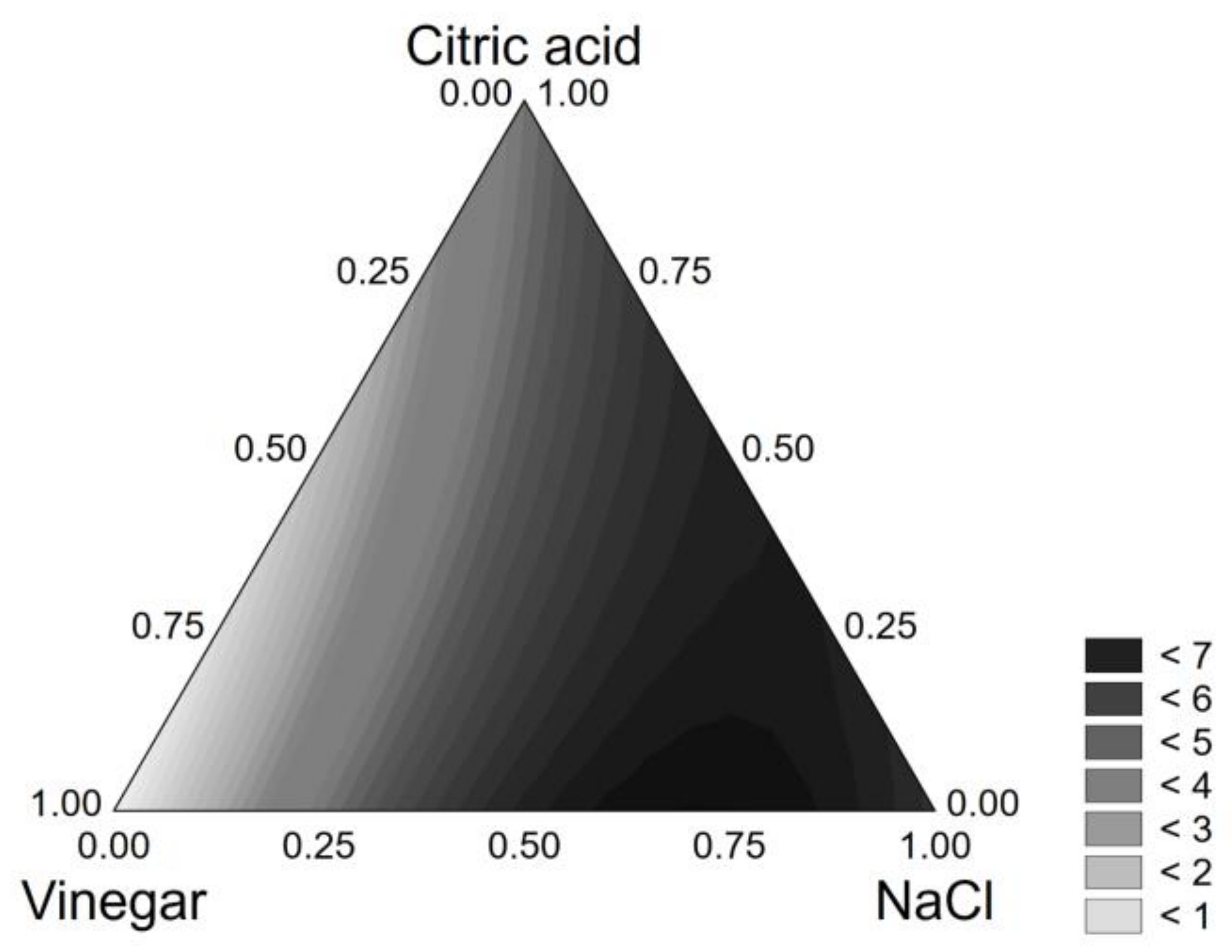
| Coded Values | Real Values | |||||
|---|---|---|---|---|---|---|
| Vinegar | NaCl | Citric Acid | Vinegar (%) | NaCl (%) | Citric Acid (%) | |
| 1 | 1 | 0 | 0 | 70 | 0 | 0 |
| 2 | 0 | 1 | 0 | 40 | 12 | 0 |
| 3 | 0 | 0 | 1 | 40 | 0 | 1.50 |
| 4 | 0.50 | 0.50 | 0 | 55 | 6 | 0 |
| 5 | 0.50 | 0 | 0.50 | 55 | 0 | 0.75 |
| 6 | 0 | 0.50 | 0.50 | 40 | 6 | 0.75 |
| 7 | 0.33 | 0.33 | 0.33 | 50 | 4 | 0.50 |
| Control | - | - | - | - | - | - |
| Quality Parameter | Description | Score |
|---|---|---|
| Colour | Glossy and bright surface | 5 |
| Slight glossy and bright surface | 4 | |
| Slight glossy and dull surface | 3 | |
| No glossy appearance, 1st discoloration | 2 | |
| No glossy appearance, a little yellow surface | 1 | |
| No glossy appearance, yellow surface | 0 | |
| Odour | No fishiness and no earthy smell | 5 |
| Little fishiness, no off-odour | 4 | |
| Little freshness, no off-odour | 3 | |
| Little freshness, first off-odour | 2 | |
| Distinct freshness and off-odour | 1 | |
| Strong freshness and strong off-odour | 0 | |
| Texture | Finger mark disappears immediately | 5 |
| Finger leaves mark less than 3 s | 4 | |
| Finger leaves mark longer than 3 s | 3 | |
| Muscle returns more than half way | 2 | |
| Very soft, no elasticity | 1 | |
| Highly soft, no elasticity, no connectivity | 0 | |
| Overall acceptance | Fresh, totally acceptable | 5 |
| Little fresh, acceptable | 4 | |
| Little fresh, marginally acceptable | 3 | |
| Not fresh, unacceptable | 2 | |
| Not fresh, totally unacceptable | 1 | |
| Clearly spoiled | 0 |
| NaCl | Citric Acid | |||
|---|---|---|---|---|
| Coded Values | Real Values | Coded Values | Real Values | |
| L. plantarum 69 | 0.23 | 2.76% | 0.63 | 0.95% |
| L. plantarum 68 | 0.04 | 0.50% | 0.11 | 0.17% |
| B. animalis 10140 | 0.25 | 3% | 0.50 | 0.75% |
| Time (Days) | L. plantarum 69 | B. animalis 10140 | ||
|---|---|---|---|---|
| O | S | O | S | |
| 0 | 7.85 ± 0.20 a | 7.78 ± 0.16 a | 7.75 ± 0.15 a | 7.80 ± 0.19 a |
| 2 | 7.18 ± 0.08 d | 7.20 ± 0.20 d | 7.75 ± 0.24 a | 7.68 ± 0.12 a |
| 7 | 7.65 ± 0.18 a | 7.72 ± 0.30 a | 7.77 ± 0.21 a | 7.34 ± 0.14 c |
| 10 | 7.33 ± 0.15 c | 7.50 ± 0.25 b | 7.43 ± 0.19 b | 7.50 ± 0.20 b |
| 14 | 7.30 ± 0.22 c | 7.37 ± 0.19 c | 7.29 ± 0.08 c,d | 7.30 ± 0.18 c |
| 21 | 7.20 ± 0.10 d | 7.35 ± 0.15 c | 7.35 ± 0.20 c | 7.45 ± 0.25 b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speranza, B.; Bevilacqua, A.; Racioppo, A.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Marinated Sea Bream Fillets Enriched with Lactiplantibacillus plantarum and Bifidobacterium animalis subsp. lactis: Brine Optimization and Product Design. Foods 2021, 10, 661. https://doi.org/10.3390/foods10030661
Speranza B, Bevilacqua A, Racioppo A, Campaniello D, Sinigaglia M, Corbo MR. Marinated Sea Bream Fillets Enriched with Lactiplantibacillus plantarum and Bifidobacterium animalis subsp. lactis: Brine Optimization and Product Design. Foods. 2021; 10(3):661. https://doi.org/10.3390/foods10030661
Chicago/Turabian StyleSperanza, Barbara, Antonio Bevilacqua, Angela Racioppo, Daniela Campaniello, Milena Sinigaglia, and Maria Rosaria Corbo. 2021. "Marinated Sea Bream Fillets Enriched with Lactiplantibacillus plantarum and Bifidobacterium animalis subsp. lactis: Brine Optimization and Product Design" Foods 10, no. 3: 661. https://doi.org/10.3390/foods10030661
APA StyleSperanza, B., Bevilacqua, A., Racioppo, A., Campaniello, D., Sinigaglia, M., & Corbo, M. R. (2021). Marinated Sea Bream Fillets Enriched with Lactiplantibacillus plantarum and Bifidobacterium animalis subsp. lactis: Brine Optimization and Product Design. Foods, 10(3), 661. https://doi.org/10.3390/foods10030661









