1. Introduction
Barley (
Hordeum vulgare L.) is the fourth most cultivated cereal in the world after maize, wheat and rice [
1]. It is widely used for feed and malt, with limited consumption as human food in some specific regions, such as the Maghreb and the high plateaus of the Himalayas. However, in recent years, it has attracted growing interest worldwide due to the health promoting properties of its bioactive compounds. In terms of health, several reports have demonstrated the positive effect of barley on the glycemic index, cholesterol and heart diseases [
2]. This is mainly due to the presence of β-glucans (2–11% d.w.), a dietary fiber component for which health claims have been approved by the US Food and Drug Administration and the European Food Safety Authority [
3,
4]. Additional beneficial effects have been described for β-glucans. These include their properties as enhancers of the immune system against infectious diseases and some types of cancer, and also as a key modulator of the composition and activity of human microbiota [
5,
6,
7]. Arabinoxylans are the second major component of barley dietary fiber (2–9% d.w.). Their proven health benefits include effects on the postprandial glucose response, cholesterol metabolism and immune response [
8,
9]. In barley, arabinoxylans are particularly associated with antioxidant activity due to the presence of phenolic acids linked to their structure [
10]. Barley is also a significant source of antioxidant compounds [
11,
12]. It contains more vitamin E than most cereals (17–49 μg/g d.w.), this being in the form of α-tocopherol and α-tocotrienol. Furthermore, barley contains high levels of phenolic compounds such as phenolic acids and flavan 3-ols which can be found free or ester-bound to the fiber. Flavan 3-ols like catechins, procyanidins and prodelphinidins are the most abundant free phenols, while phenolic acids such as ferulic,
p-coumaric and vanillic are the major constituents within the bounds. Anthocyanins are also present in considerable concentrations in some barley genotypes with colored kernels. All these phenolic compounds are considered potent antioxidants, free radical scavengers and inhibitors of lipid peroxidation [
13,
14]. In addition, preclinical studies and clinical trials have shown that polyphenols could greatly modulate the gut microbiota, thus favoring the growth of potential beneficial organisms and simultaneously inhibiting pathogenic bacteria [
15].
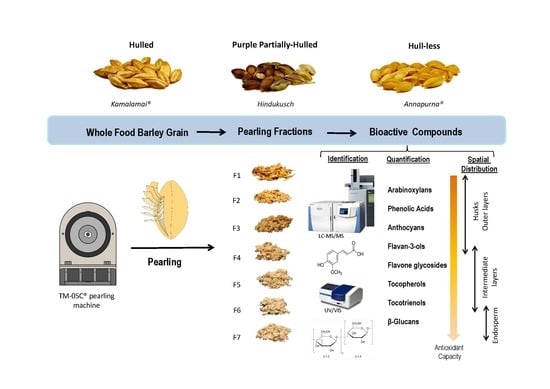
Barley grains may differ in important morphological characters. These include being hulled (covered), when the husks adhere to the caryopsis, partially hull-less (skinned), when a partial loss of the husks occurs, and hull-less (naked), when the husks are freely threshed at harvest, the latter being what is preferred for human consumption; grains from two or six rowed spikes or grains with colors such black, blue or purple, alternative to yellow. Based on the grain composition, barley is further classified as normal, high amylopectin (waxy) or high amylose starch types, high β-glucan, high lysine, and proanthocyanidin-free. All these types of grain differ widely in their physical and compositional characteristics and, accordingly, are processed differently and used for different commercial purposes.
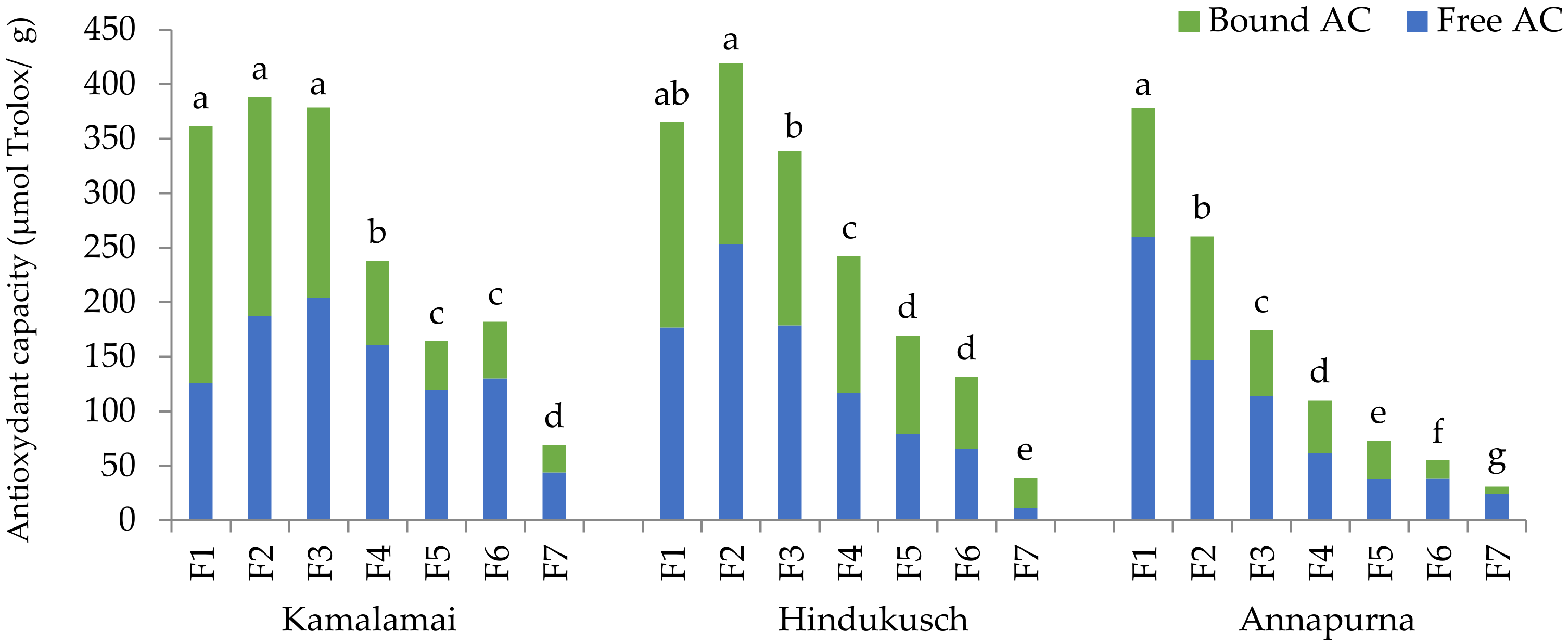
The bioactive compounds are not uniformly distributed across the barley grains. It is known that arabinoxylans, tocotrienols and phenolics are mainly located in the outer layers, tocopherols in the germ, and β-glucans in the endosperm [
16]. Thus, physical processes like pearling, an abrasive technique that gradually removes grain layers to obtain polished grain and by-products, allow favorable separation of fractions enriched in specific compounds and these can be used as functional ingredients. Several fold enrichment of antioxidant compounds has been described in barley pearled fractions [
17,
18] that have been used to improve the nutritional value of such wheat-based products as cookies, pasta and bread [
19,
20,
21,
22].
In recent years, increased efforts have been carried in a few countries to release new specific barley varieties for human consumption and for the food industry. In our food barley breeding program, we aim to produce varieties rich in β-glucans as well as antioxidant compounds adapted to the Spanish agro-climatic conditions. Three distinct high β-glucan barley genotypes from our program, differing in the type of grain (Kamalamai, hulled; Hindukusch, partially hull-less; Annapurna, hull-less) were selected to identify specific potential ingredients for the functional food industry. Thus, the main objectives of this work were as follows: (1) to analyze β-glucans, arabinoxylans, tocols, phenolic compounds and antioxidant capacity in the whole flours and pearling fractions of different types of barley grains; (2) to identify pearling fractions to be used potentially as functional ingredients. This research could provide further knowledge about the spatial distribution of a large number of bioactive compounds in barley pearling fractions, since most published works have focused on changes in various bioactive compounds separately [
16,
17,
23]. To the best of our knowledge, this is the first time that such an integrative study of the major health-promoting components in barley genotypes specifically bred for human food has been explored.
2. Materials and Methods
2.1. Plant Material
Kamalamai: registered Spanish variety, hulled, two rowed, normal endosperm (semillas Batlle SA).
Hindukusch: Afghan landrace, naked but often suffering from grain skinning, two rowed, normal endosperm and purple grain used as parent in our crosses.
Annapurna: registered Spanish variety, hull-less, two rowed, waxy endosperm (semillas Batlle SA).
The three genotypes were cultivated under similar conditions in Bell-lloc d’Urgell, Lleida (Spain) during the 2018–2019 growing season.
2.2. Whole Flours and Pearling Fractions
Grain with size above 2.5 mm was screened for this study using a stainless-steel mesh. Six pearling fractions from each genotype were obtained by sequential processing of grain using theTM-05C pearling machine (Satake Corporation, Hiroshima, Japan) at 1060 rpm. The grains were initially pearled to remove the 5% of the original grain weight that resulted in the first fraction F1 (0–5% w/w). The remained grains were pearled to remove the second fraction F2 (5–10%), and then the process was repeated to get fractions F3 (10–15%), F4 (15–20%), F5 (20–25%), F6 (25–30%), and the residual 70% pearled grain F7 (30–100% w/w). After each pearling session, the pearling machine was cleaned to avoid mixtures between fractions. Fractions, pearled grains and whole grains were ground in a Foss Cyclotec 1093™ mill equipped with a 0.5-mm screen (Foss Iberia, Barcelona, Spain) prior to chemical analyses.
2.3. Protein and Ash Content
The protein content was done according to the Kjeldahl method in a Kjeltec system I (Foss Tecator AB, Höganäs, Sweden) using the conversion factor of 5.7. The ash content was determined in a muffle furnace according to the AOAC Official Method 942.05 [
24].
2.4. β-Glucan and Arabinoxylan Content
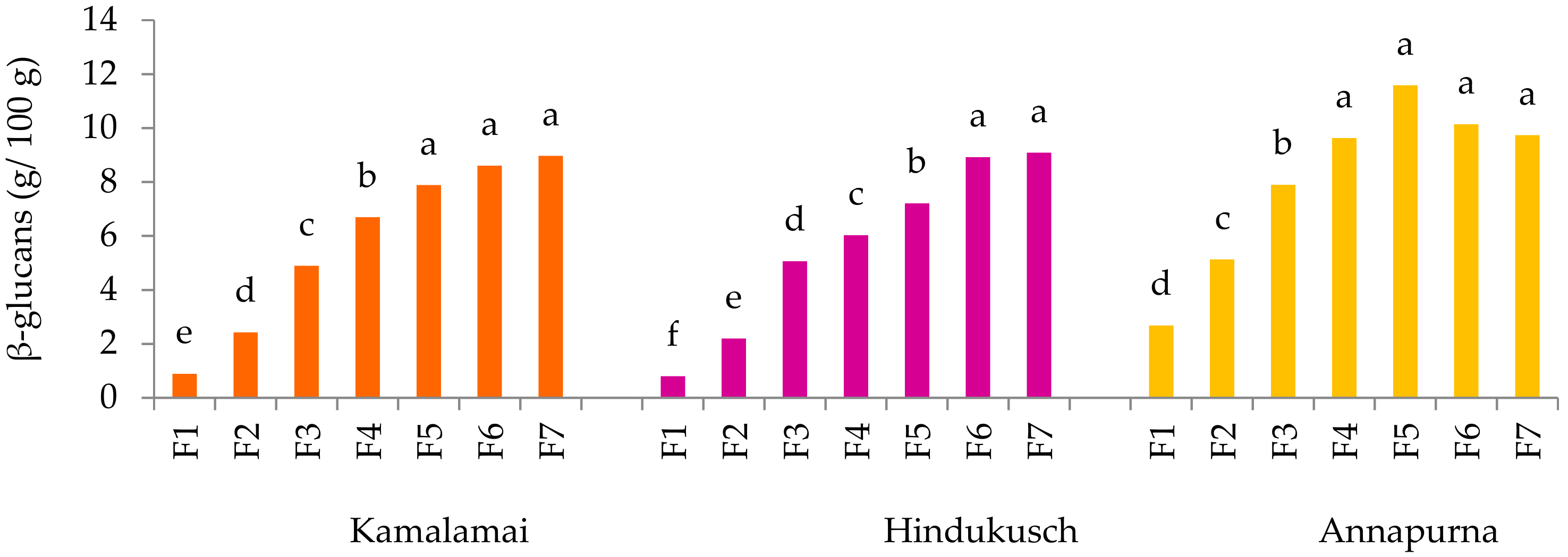
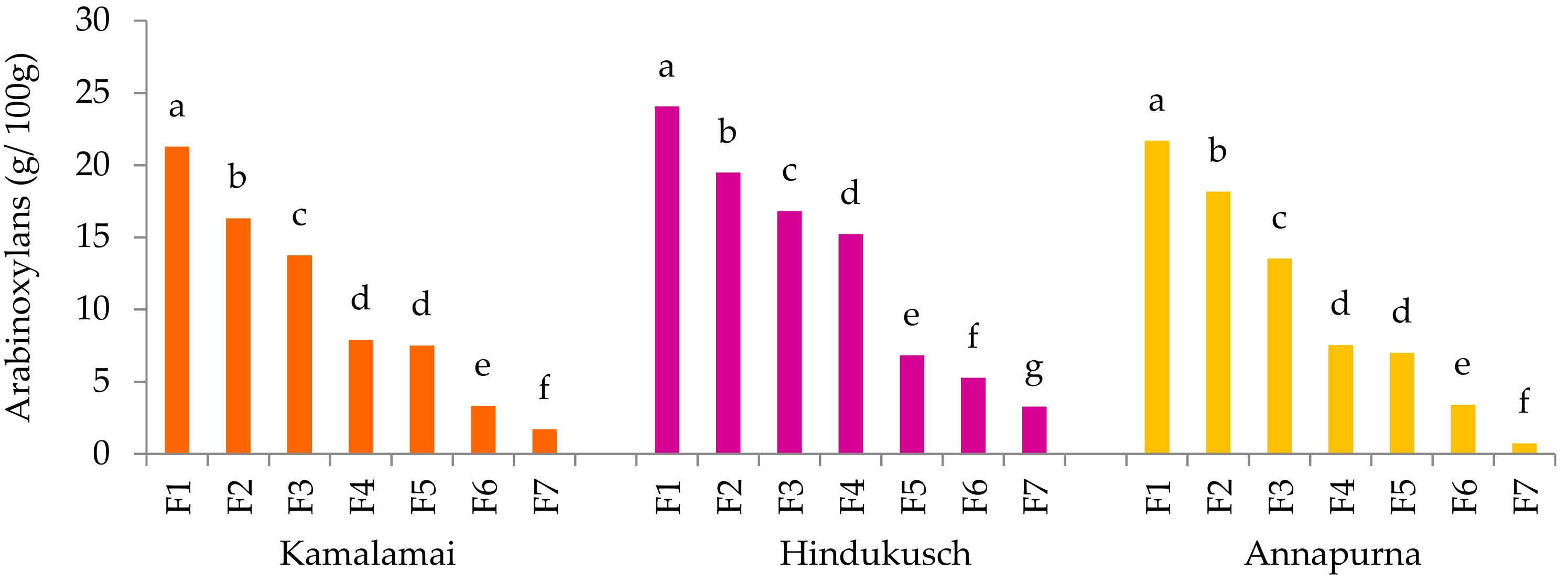
The β-glucan and arabinoxylan contents were determined by means of the mixed-linkage β-glucan assay (K-BGLU) and D-xylose assay (K-XYLOSE) kits from Megazyme (Wicklow, Ireland).
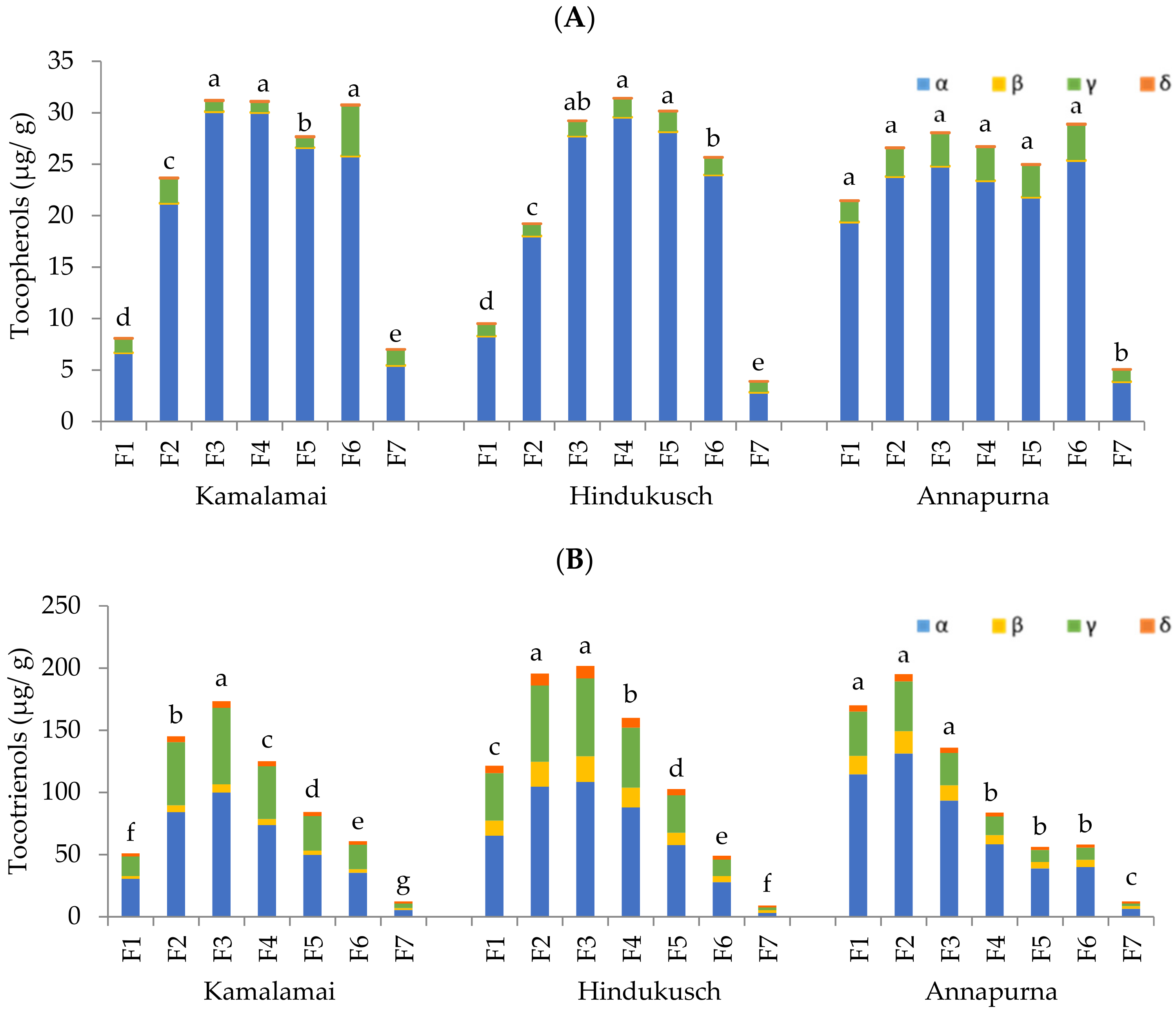
2.5. Tocols Content
Tocopherols and tocotrienols (α-, β-, γ-, and δ-isomers) were quantified by high performance liquid chromatography (HPLC) coupled to a fluorescence detector. One gram of each barley genotype was extracted three times with 10 mL n-hexane and the extract collected after centrifuging at 9000×
g for 10 min. The supernatants were pooled, reduced to dryness under a flow of nitrogen, and reconstituted in 1 mL of n-hexane. Normal phase HPLC with fluorescence detection (excitation 292 nm, emission 325 nm) was used to analyze tocopherols and tocotrienols. Aliquots of 50 μL were injected into the HPLC system following the chromatographic conditions described by Martínez-Subirà et al. [
12]. Tocopherols and tocotrienols isomers were quantified with external standard curves. Results were expressed as μg/g dry sample.
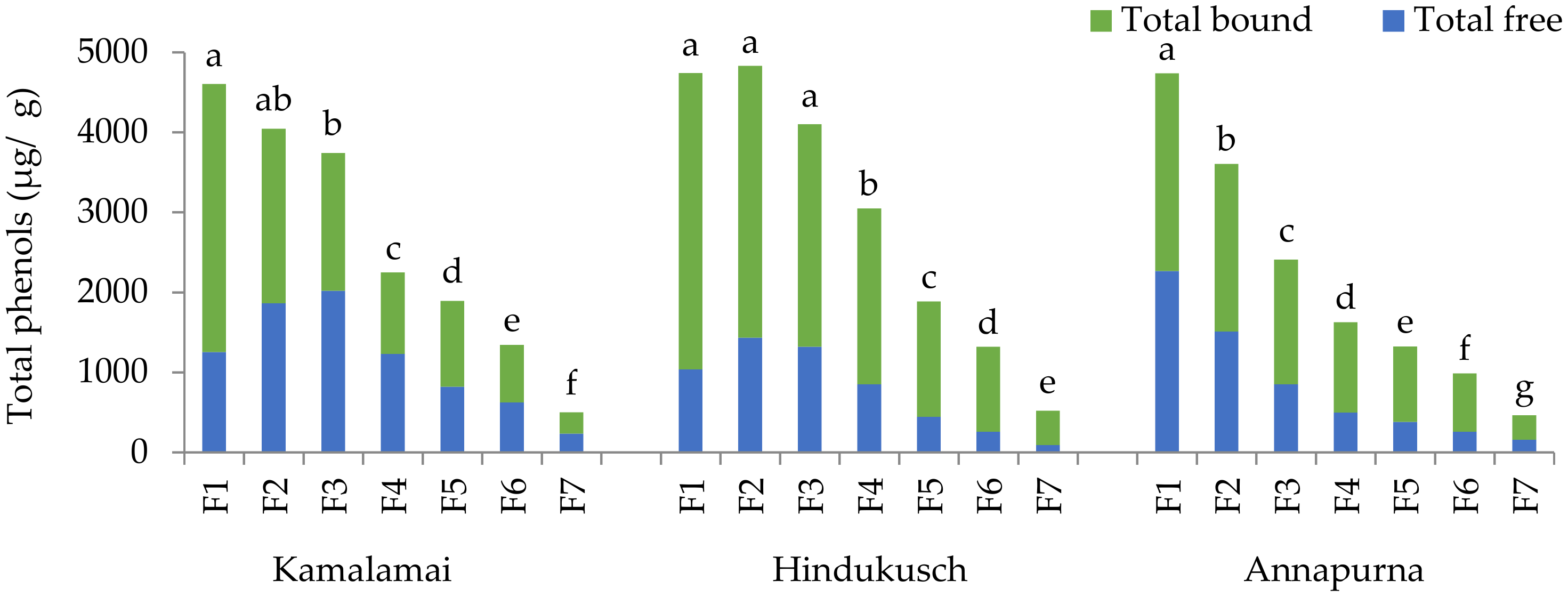
2.6. Phenolic Compounds (PCs) and Anthocyanin Analysis by UPLC-MS/MS
Free phenolic compounds were extracted three times by adding 1 mL of 79.5% methanol, 19.5% Milli Q water, and 1% formic acid solution to 150 mg of barley flours. The samples were sonicated for 30 s and centrifuged at 9000×
g for 10 min. The supernatants from each extraction were pooled and filtered through 0.22 μm polyvinylidene fluoride (PVDF) filter discs before chromatographic analysis. The residue was subjected to alkaline hydrolysis by adding 6 mL of 2 mol/L NaOH to obtain bound phenols. The samples were left over-night at room temperature for complete hydrolysis. Then, they were sonicated for 1 min and centrifuged at 9000×
g for 10 min; the supernatant was acidified with HCl 37% (
w/w) to pH 2. A total of 350 μL of supernatant was mixed with phosphoric acid 10 min, centrifuged at 9000×
g, and subjected to μSPE clean-up according to Serra et al. [
25]. Briefly, the micro-cartridges were pre-conditioned with acidified water (pH 2) and methanol. The samples were loaded onto the μSPE and subsequently washed with water and water/methanol 95/5 (
v/v). The PCs were eluted with methanol, and 2.5 μL of the eluate was directly analyzed by liquid chromatography. The extracts were analyzed by Ac Quity Ultra-Performance
TM liquid chromatography coupled to a tandem mass spectrometer (UPLC-MS/MS), equipped with the analytical column Ac Quity BEH C18 (100 mm × 2.1 mm i.d., 1.7 μm) and the Van Guard TM Pre-Column Ac Quity BEH C 18 (5 mm × 2.1 mm, 1.7 μm), all from Waters, Milford, MA, USA. The mobile phase was 0.2% (
v/v) acetic acid and acetonitrile for the phenolic compounds (PCs), and 10% acetic acid (
v/v) and acetonitrile for the anthocyanins. The UPLC system was coupled to a triple quadrupole detector mass spectrometer from Waters equipped with a Z-spray electrospray interface for ionization, operating in the negative mode [M−H]
− for the PCs and the positive mode [M−H]
+ for the anthocyanins. Quantification was based on a 0.02–25 ng calibration curve of commercially available standards, and the results were expressed as μg/g dry sample. A linear response was obtained for all standards and tested by linear regression analysis. The limits of detection (LOD) ranged from 0.007 to 0.09 ng and the limits of quantification (LOQ) from 0.02 to 0.30 ng.
2.7. Antioxidant Capacity (AC) Analysis
The Oxygen Radical Absorbance Capacity (ORAC) of both the free and bound extracts was measured as described Huang et al. [
26]. The antioxidant capacity (AC) was determined using the FLUO star OPTIMA fluorescence reader (BMG Labtech, Offenburg, Germany) in a 96-well polystyrene microplate controlled by the OPTIMA 2.10 R2 software. Changes in fluorescence were measured under controlled temperature (37 °C) in a reader with fluorescence filters with 485 nm excitation and 520 nm emission wavelengths. Trolox (6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid) was used as control, with one ORAC unit being equal to the antioxidant protection given by 1 μmol Trolox. The results were expressed as μmols of Trolox-equivalents per 100 g of dry sample.
2.8. Statistical Analysis
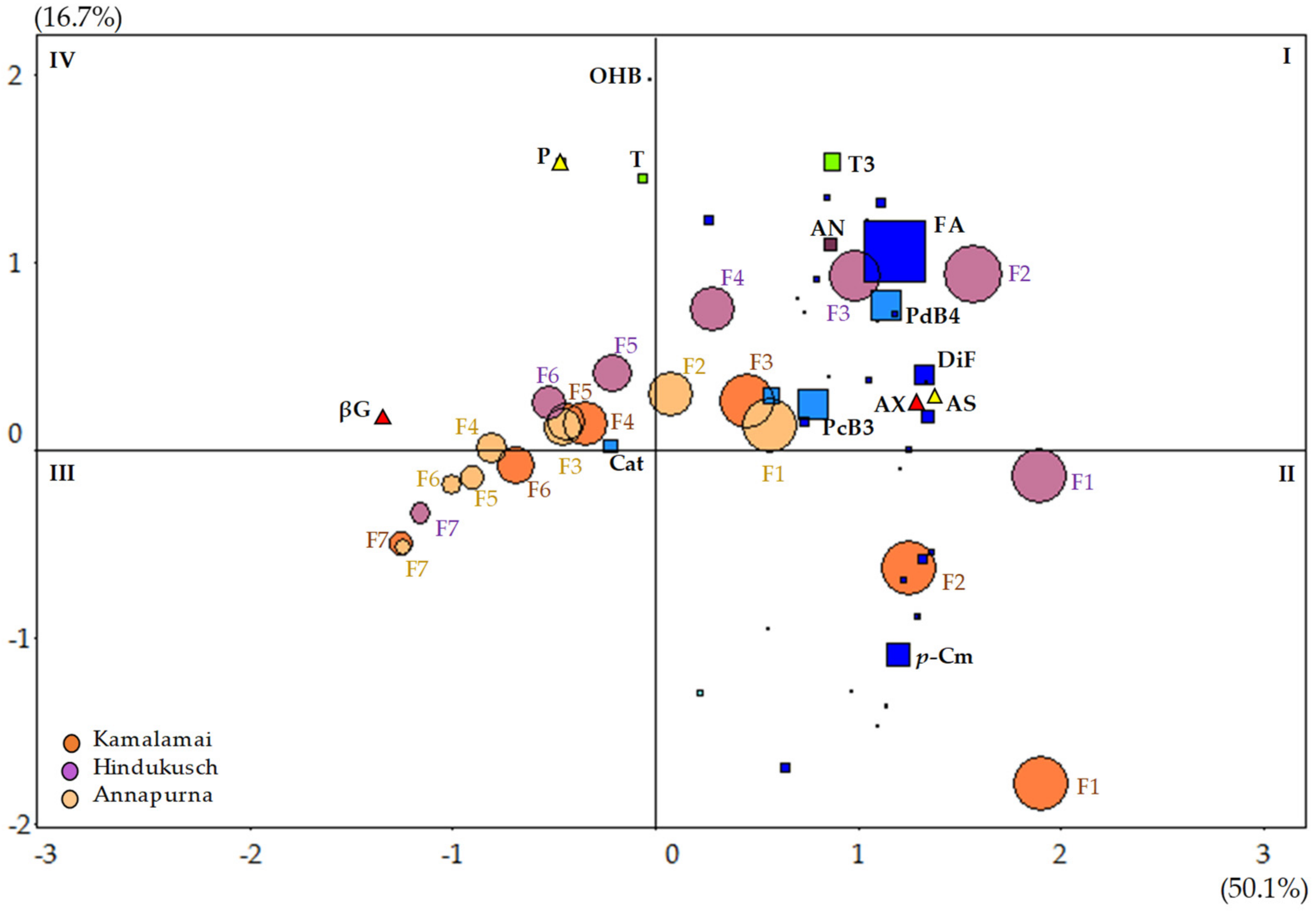
All statistical analyses were conducted using JMP®Pro14 (SAS institute Inc., Cary, NC, USA). Analytical measurements were carried out in triplicate and the results were presented as mean values. Data was checked for normality and for homoscedasticity of variances based on a number of diagnostic tools, such as residual plots, Box-Cox′s Lambda and Levene’s test of equality of variances, provided by JMP. When needed, a logarithmic transformation was used and indicated in the text. For multiple comparisons, Tukey–Kramer’s honestly-significant-difference tests (HSD) (α = 0.05) were conducted once the corresponding ANOVA F-tests were found significant. Principal Component Analysis (PCA) was used to graphically represent the association between fractions, genotypes and bioactive compounds using standardized data.