Assessment of Antimicrobial Activity of Pomegranate, Cranberry, and Black Chokeberry Extracts against Foodborne Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Preparation of Bacterial Strains
2.3. Preparation of Extracts
2.4. Determination of Antimicrobial Activity
2.5. Preparation of Raw Meatballs
2.6. Microbiological Analysis of Meatball Portions
2.7. pH Measurement
2.8. Statistical Analysis
3. Results
3.1. Application of Cranberry Extracts
3.2. Application of Black Chokeberry Extracts
3.3. Application of Pomegranate Extracts
3.4. Effectiveness of the Solvent Type per Strain
3.5. Effectiveness of Solvent Concentration
3.6. Effectiveness of Extract Concentration
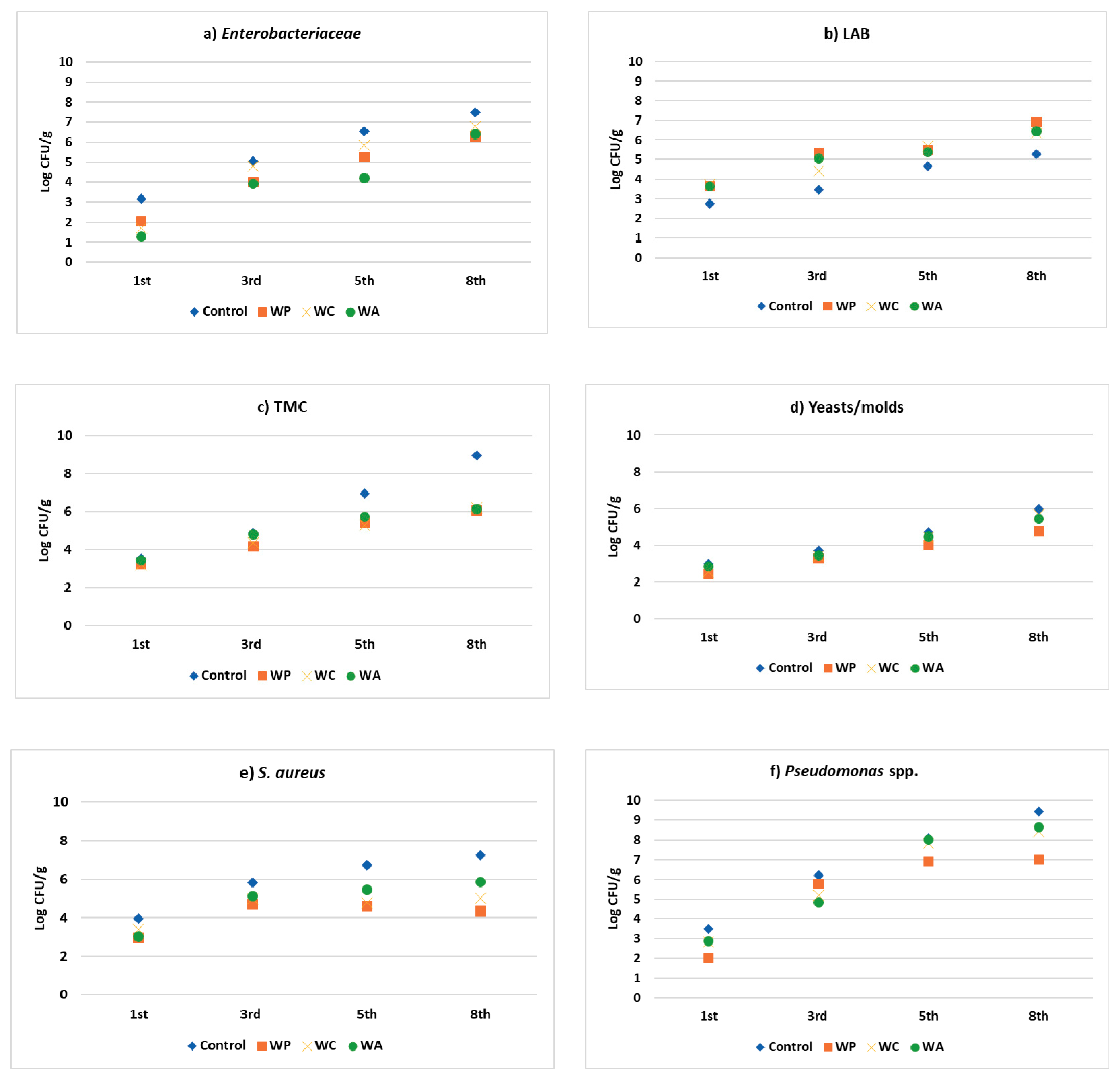
3.7. Application of Aqueous Extracts in Raw Meatballs
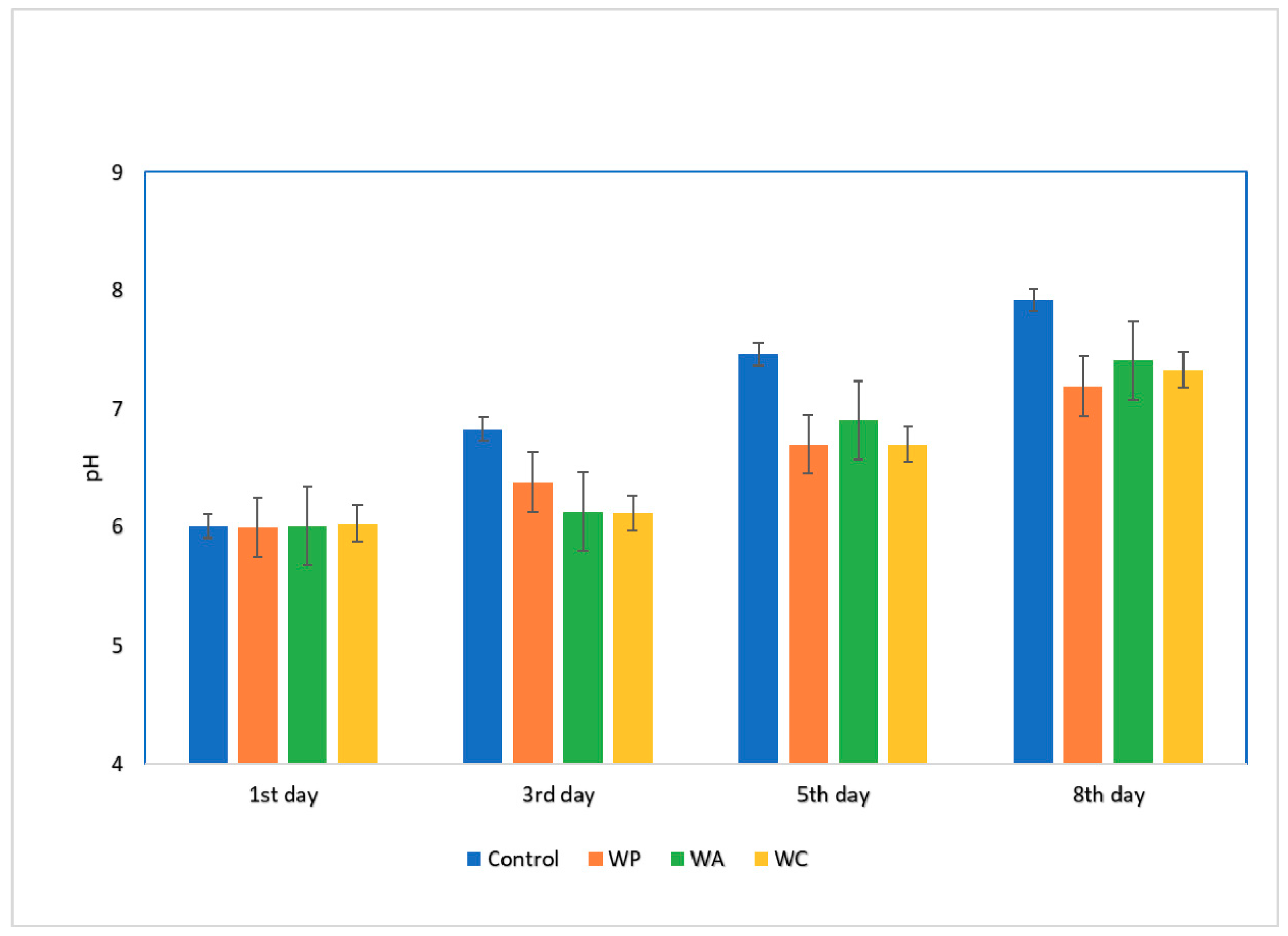
3.8. pH Values of Raw Meatballs during Refrigerated Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial and antioxidant effects of five spice and herb extracts as natural preservatives of raw pork. J. Sci. Food Agric. 2009, 89, 1879–1885. [Google Scholar] [CrossRef]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Ciz, M.; Lojek, A.; Vasicek, O.; Blazheva, D.; Nedelcheva, P.; Vojtek, L.; Hyrsl, P. Antioxidant, antimicrobial and neutrophil-modulating activities of herb extracts. Acta Biochim. Pol. 2014, 61, 359–367. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-ΚB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-ΚB activation along with their inhibitory effect on INOS expression and NO production in Activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Wu, V.C.-H.; Qiu, X.; Bushway, A.; Harper, L. Antibacterial effects of American cranberry (Vaccinium macrocarpon) concentrate on foodborne pathogens. LWT-Food Sci. Technol. 2008, 41, 1834–1841. [Google Scholar] [CrossRef]
- Al-Zoreky, N.S. Antimicrobial activity of pomegranate (Punica Granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Caillet, S.; Côté, J.; Sylvain, J.-F.; Lacroix, M. Antimicrobial effects of fractions from cranberry products on the growth of seven pathogenic bacteria. Food Control 2012, 23, 419–428. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Määttä-Riihinen, K.; Oksman-Caldentey, K.-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef]
- Machado, T.B.; Pinto, A.V.; Pinto, M.; Leal, I.C.R.; Silva, M.G.; Amaral, A.C.F.; Kuster, R.M.; Netto-dosSantos, K.R. In vitro activity of Brazilian medicinal plants, naturally occurring naphthoquinones and their analogues, against methicillin-resistant Staphylococcus Aureus. Int. J. Antimicrob. Agents 2003, 21, 279–284. [Google Scholar] [CrossRef]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 53, 461–467. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In vitro gastrointestinal digestion of Pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Côté, J.; Caillet, S.; Doyon, G.; Dussault, D.; Sylvain, J.-F.; Lacroix, M. Antimicrobial effect of cranberry juice and extracts. Food Control 2011, 22, 1413–1418. [Google Scholar] [CrossRef]
- Tamkutė, L.; Gil, B.M.; Carballido, J.R.; Pukalskienė, M.; Venskutonis, P.R. Effect of cranberry pomace extracts isolated by pressurized ethanol and water on the inhibition of food pathogenic/spoilage bacteria and the quality of pork products. Food Res. Int. 2019, 120, 38–51. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, Á.A. Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef]
- Wafa, B.A.; Makni, M.; Ammar, S.; Khannous, L.; Hassana, A.B.; Bouaziz, M.; Es-Safi, N.E.; Gdoura, R. Antimicrobial effect of the tunisian nana variety Punica granatum L. extracts against Salmonella enterica (serovars kentucky and enteritidis) isolated from chicken meat and phenolic composition of its peel extract. Int. J. Food Microbiol. 2017, 241, 123–131. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- CLSI Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard M7-A7; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Lei, Y.; Jia, J.; Yuangang, Z. Study on antioxidant activities of extractions from some kinds of crataegus fruits. J. Chin. Inst. Food Sci. Technol. 2009, 4, 28–32. [Google Scholar]
- Isogai, E.; Isogai, H.; Hirose, K.; Hayashi, S.; Oguma, K. In vivo synergy between green tea extract and levofloxacin against enterohemorrhagic Escherichia coli O157 infection. Curr. Microbiol. 2001, 42, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Chamcha, V.; Jones, A.; Quigley, B.R.; Scott, J.R.; Amara, R.R. Oral immunization with a recombinant Lactococcus lactis–expressing HIV-1 antigen on group A Streptococcus pilus induces strong mucosal immunity in the gut. J. Immunol. 2015, 195, 5025–5034. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.; Munckhof, W.J. Antibiotics currently used in the treatment of infections caused by Staphylococcus aureus. Int. Med. J. 2005, 35, S3–S16. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs; European Commission: Luxembourg, 2005. [Google Scholar]
- Prakash, B.; Kumar, A.; Singh, P.P.; Songachan, L.S. Antimicrobial and antioxidant properties of phytochemicals: Current status and future perspective. In Functional and Preservative Properties of Phytochemicals; Academic Press: Cambridge, MA, USA, 2020; pp. 1–45. [Google Scholar]
- Wang, G.; Wang, H.; Han, Y.; Ye, T.K.; Xu, X.; Zhou, G. Evaluation of the spoilage potential of bacteria isolated from chilled chicken in vitro and in situ. Food Microbiol. 2017, 63, 139–146. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Hamilton, V.E.S.; Chapman, D.G.; Taylor, P.W.; Lamb, A.J. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J. Appl. Microbiol. 2007, 103, 1562–1567. [Google Scholar] [CrossRef]
- Liepiņa, I.; Nikolajeva, V.; Jākobsone, I. Antimicrobial activity of extracts from fruits of Aronia melanocarpa and Sorbus aucuparia. Environ. Exp. Biol. 2013, 11, 195–199. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef]
- Prashanth, D.; Asha, M.K.; Amit, A. Antibacterial activity of Punica granatum. Fitoterapia 2001, 72, 171–173. [Google Scholar] [CrossRef]
- McCarrell, E.M.; Gould, S.W.; Fielder, M.D.; Kelly, A.F.; El Sankary, W.; Naughton, D.P. Antimicrobial activities of pomegranate rind extracts: enhancement by addition of metal salts and vitamin C. BMC Complement. Altern. Med. 2008, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Voravuthikunchai, S.; Lortheeranuwat, A.; Jeeju, W.; Sririrak, T.; Phongpaichit, S.; Supawita, T. Effective medicinal plants against enterohaemorrhagic Escherichia Coli O157: H7. J. Ethnopharmacol. 2004, 94, 49–54. [Google Scholar] [CrossRef]
- Alzoreky, N.S.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Braga, L.C.; Shupp, J.W.; Cummings, C.; Jett, M.; Takahashi, J.A.; Carmo, L.S.; Chartone-Souza, E.; Nascimento, A.M.A. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J. Ethnopharmacol. 2005, 96, 335–339. [Google Scholar] [CrossRef]
- Fullerton, M.; Khatiwada, J.; Johnson, J.U.; Davis, S.; Williams, L.L. Determination of antimicrobial activity of sorrel (Hibiscus sabdariffa) on Esherichia coli O157: H7 isolated from food, veterinary, and clinical samples. J. Med. Food 2011, 14, 950–956. [Google Scholar] [CrossRef]
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Kovačević, D.B.; Putnik, P.; Mrkonjić, Ž.; Pavlić, B. Supercritical extracts of wild thyme (Thymus serpyllum L.) by-product as natural antioxidants in ground pork patties. LWT 2020, 130, 109661. [Google Scholar] [CrossRef]
- Ranucci, D.; Roila, R.; Andoni, E.; Braconi, P.; Branciari, R. Punica granatum and Citrus spp. extract mix affects spoilage microorganisms growth rate in vacuum-packaged cooked sausages made from pork meat, emmer wheat (Triticum dicoccum Schübler), almond (Prunus dulcis Mill.) and hazelnut (Corylus avellana L.). Foods 2019, 8, 664. [Google Scholar] [CrossRef]
- Lytou, A.E.; Nychas, G.J.E.; Panagou, E.Z. Effect of pomegranate based marinades on the microbiological, chemical and sensory quality of chicken meat: A metabolomics approach. Int. J. Food Microbiol. 2018, 267, 42–53. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Bi, X.; Duo, K.; Sun, Q.; Yun, X.; Han, J. Antimicrobial activity and mechanism of action of the Amaranthus tricolor crude extract against Staphylococcus aureus and potential application in cooked meat. Foods 2020, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Pilasombut, K.; Laosinwattana, C.; Tuyen Nguyen, T.K.; Ngamyeeesoon, N.; Teerarak, M. Antimicrobial properties of extracts from Carissa carandas L. fruits and its application in chilled and frozen ground pork. Int. J. Agric. Technol. 2019, 15, 91–102. [Google Scholar]
- Moorthy, K.; Vinodhhini, T.P.R.; Sureshkumar, B.T.; Vijayalakshmi, P.; Thajuddin, N. Antimicrobial activity and qualitative phytochemical analysis of Punica granatum Linn. (pericarp). J. Med. Plant Res. 2012, 7, 474–479. [Google Scholar]
- Sadeghian, A.; Ghorbani, A.; Mohamadi-Nejad, A.; Rakhshandeh, H. Antimicrobial activity of aqueous and methanolic extracts of pomegranate fruit skin. Avicenna J. Phytomed. 2011, 1, 67–73. [Google Scholar]
- Topçu, K.C.; Kaya, M.; Kaban, G. Probiotic properties of lactic acid bacteria strains isolated from pastırma. LWT 2020, 134, 110216. [Google Scholar] [CrossRef]
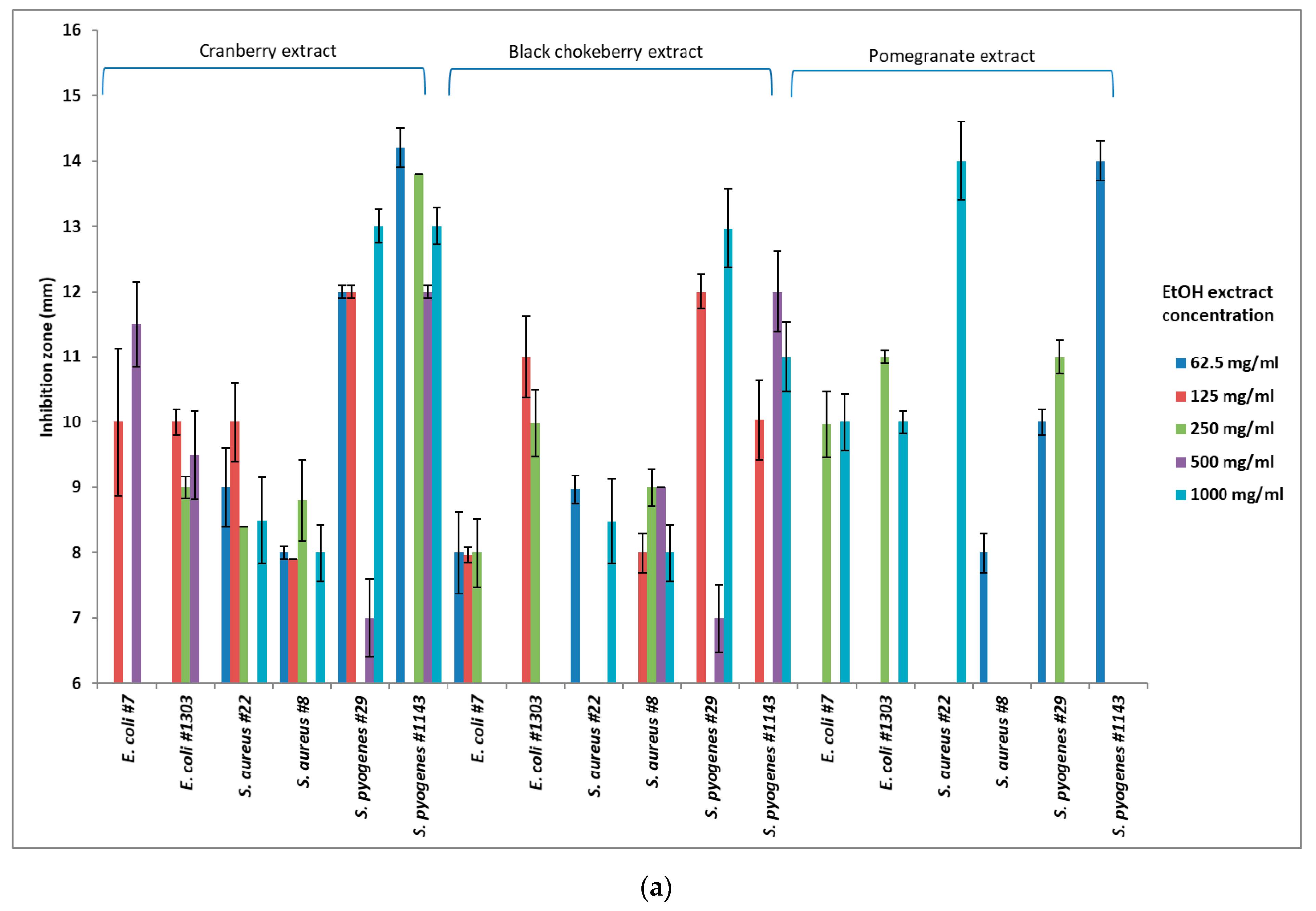
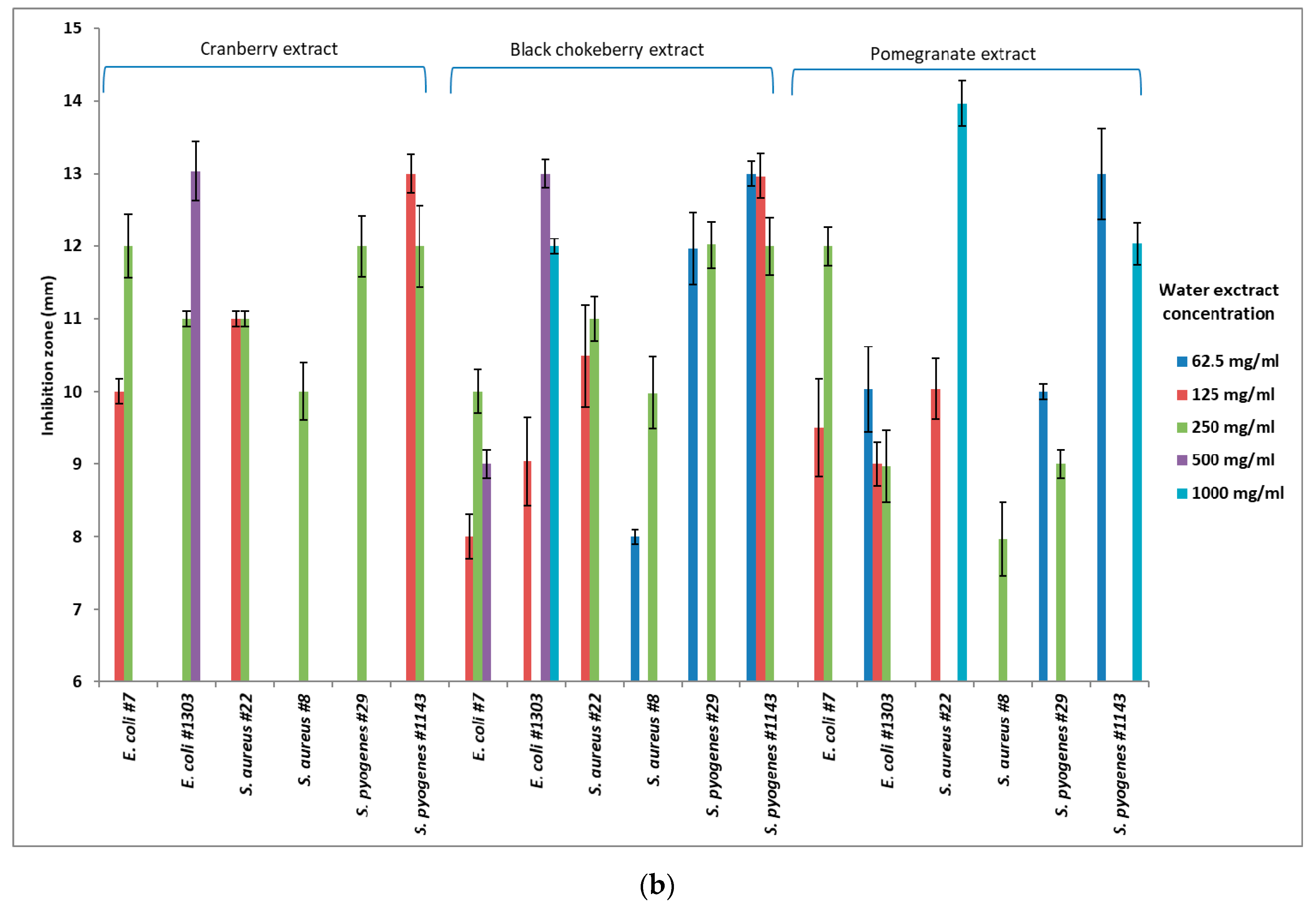
| Solvents | Concentration of Solvents | E. coli | S. aureus | S. pyogenes | ||||
|---|---|---|---|---|---|---|---|---|
| 7 | 1303 | 22 | 8 | 29 | 1143 | |||
| EtOH | 5% | Diameter of ZOI in mm | 11 ± 0.4 b | 9 ± 0.2 a | 9 ± 0.6 a | 9 ± 0.6 a | 12 ± 0.1 c | 14 ± 0.3 d |
| Extract Concentration (mg/mL) | 125 | 250 | 62.5 | 250 | 125 | 62.5 | ||
| 10% | Diameter of ZOI in mm | 12 ± 0.5 a | 10 ± 0.2 b | 9 ± 0.3 b | 8 ± 0.1 d | 7 ± 0.6 c | 12 ± 0.1 a | |
| Extract Concentration (mg/mL) | 500 | 500 | 1000 | 125 | 500 | 500 | ||
| 20% | Diameter of ZOI in mm | 11 ± 0.2 c | 9 ± 0.6 b | 8 ± 0.5 a | 8 ± 0.4 a | 13 ± 0.3 d | 13 ± 0.3 d | |
| Extract Concentration (mg/mL) | 500 | 500 | 1000 | 1000 | 1000 | 1000 | ||
| Water | 5% | Diameter of ZOI in mm | 9 ± 0.1 b | 10 ± 0.2 c | 10 ± 0.6 c | 8 ± 0.1 a | 12 ± 0.1 d | 14 ± 0.4 e |
| Extract Concentration (mg/mL) | 125 | 125 | 125 | 62.5 | 62.5 | 62.5 | ||
| 10% | Diameter of ZOI in mm | 10 ± 0.2 a | 11 ± 0.1 b | 11 ± 0.1 b | 10 ± 0.1 a | 12 ± 0.6 c | 13 ± 0.3 d | |
| Extract Concentration (mg/mL) | 125 | 250 | 125 | 250 | 250 | 125 | ||
| 20% | Diameter of ZOI in mm | 12 ± 0.4 c | 13 ± 0.4 d | 11 ± 0.1 b | 10 ± 0.6 a | 12 ± 0.3 c | 12 ± 0.6 c | |
| Extract Concentration (mg/mL) | 250 | 500 | 250 | 250 | 250 | 250 | ||
| Penicillin | - | - | 12 ± 1.7 | 10.0 ± 0.0 | - | - | ||
| Levofloxacin | 32.3 ± 0.6 | 31.7 ± 2.9 | - | - | - | - | ||
| Vancomycin | - | - | - | - | 10.7 ± 0.6 | 14 ± 1.7 | ||
| Solvents | Concentration of Solvents | E. coli | S. aureus | S. pyogenes | ||||
|---|---|---|---|---|---|---|---|---|
| 7 | 1303 | 22 | 8 | 29 | 1143 | |||
| EtOH | 5% | Diameter of ZOI in mm | 8 ± 0.6 a | 11 ± 0.6 d | 9 ± 0.2 b | 9 ± 0.2 b | 12 ± 0.3 e | 10 ± 0.6 c |
| Extract Concentration (mg/mL) | 62.5 | 125 | 62.5 | 250 | 125 | 125 | ||
| 10% | Diameter of ZOI in mm | 8 ± 0.1 b | 10 ± 0.6 d | 9 ± 0.3 c | 8 ± 0.3 b | 7 ± 0.5 a | 12 ± 0.6 e | |
| Extract Concentration (mg/mL) | 125 | 250 | 1000 | 125 | 500 | 500 | ||
| 20% | Diameter of ZOI in mm | 8 ± 0.5 a | 10 ± 0.5 b | 8 ± 0.4 a | 8 ± 0.4 a | 13 ± 0.6 d | 11 ± 0.5 c | |
| Extract Concentration (mg/mL) | 250 | 250 | 1000 | 1000 | 1000 | 1000 | ||
| Water | 5% | Diameter of ZOI in mm | 8 ± 0.3 a | 9 ± 0.6 b | 10 ± 0.5 c | 8 ± 0.1 a | 12 ± 0.5 d | 13 ± 0.2 e |
| Extract Concentration (mg/mL) | 125 | 125 | 125 | 62.5 | 62.5 | 62.5 | ||
| 10% | Diameter of ZOI in mm | 10 ± 0.3 a | 13 ± 0.2 d | 11 ± 0.5 b | 10 ± 0.6 a | 12 ± 0.4 c | 13 ± 0.3 d | |
| Extract Concentration (mg/mL) | 250 | 500 | 125 | 250 | 250 | 125 | ||
| 20% | Diameter of ZOI in mm | 9 ± 0.2 a | 12 ± 0.1 d | 11 ± 0.3 c | 10 ± 0.5 b | 12 ± 0.3 d | 12 ± 0.4 d | |
| Extract Concentration (mg/mL) | 500 | 1000 | 250 | 250 | 250 | 250 | ||
| Penicillin | - | - | 12 ± 1.7 | 10.0 ± 0.0 | - | - | ||
| Levofloxacin | 32.3 ± 0.6 | 31.7 ± 2.9 | - | - | - | - | ||
| Vancomycin | - | - | - | - | 10.7 ± 0.6 | 15 ± 1.7 | ||
| Solvents | Concentration of Solvents | E. coli | S. aureus | S. pyogenes | ||||
|---|---|---|---|---|---|---|---|---|
| 7 | 1303 | 22 | 8 | 29 | 1143 | |||
| EtOH | 5% | Diameter of ZOI in mm | 10 ± 0.3 b | 11 ± 0.1 c | 15 ± 0.2 e | 8 ± 0.3 a | 10 ± 0.2 b | 14 ± 0.3 d |
| Extract Concentration (mg/mL) | 500 | 250 | 500 | 62.5 | 62.5 | 62.5 | ||
| 10% | Diameter of ZOI in mm | 10 ± 0.4 a | 10 ± 0.2 a | 10 ± 0.4 a | 12 ± 0.1 c | 11 ± 0.1 b | 12 ± 0.6 c | |
| Extract Concentration (mg/mL) | 1000 | 1000 | 500 | 500 | 250 | 500 | ||
| 20% | Diameter of ZOI in mm | 10 ± 0.5 a | 11 ± 0.2 b | 14 ± 0.6 d | 10 ± 0.2 a | 11 ± 0.4 b | 12 ± 0.3 c | |
| Extract Concentration (mg/mL) | 250 | 500 | 1000 | 500 | 250 | 500 | ||
| Water | 5% | Diameter of ZOI in mm | 9 ± 0.1 a | 10 ± 0.6 b | 12 ± 0.2 c | 8 ± 0.5 a | 10 ± 0.1 b | 13 ± 0.6 d |
| Extract Concentration (mg/mL) | 125 | 62.5 | 500 | 250 | 62.5 | 62.5 | ||
| 10% | Diameter of ZOI in mm | 10 ± 0.6 c | 9 ± 0.3 b | 10 ± 0.4 c | 8 ± 0.3 a | 9 ± 0.2 b | 11 ± 0.6 d | |
| Extract Concentration (mg/mL) | 125 | 125 | 125 | 500 | 250 | 500 | ||
| 20% | Diameter of ZOI in mm | 12 ± 0.3 d | 9 ± 0.5 b | 14 ± 0.3 c | 8 ± 0.1 a | 11 ± 0.6 c | 12 ± 0.3 d | |
| Extract Concentration (mg/mL) | 250 | 250 | 1000 | 500 | 500 | 1000 | ||
| Penicillin | - | - | 12 ± 1.7 | 10.0 ± 0.0 | - | - | ||
| Levofloxacin | 32.3 ± 0.6 | 31.7 ± 2.9 | - | - | - | - | ||
| Vancomycin | - | - | - | - | 10.7 ± 0.6 | 14 ± 1.7 | ||
| Extract | Strain | EtOH | Water | ANOVA (F,p) |
|---|---|---|---|---|
| Cranberry | E. coli 7 | 11.3 ± 0.6 a,2 | 10.3 ± 1.3 a,2 | 4.14, p > 0.05 |
| E. coli 1303 | 9.3 ± 0.6 a,1 | 11.3 ± 1.3 b,3,4 | 16.51, p < 0.05 | |
| S. aureus 22 | 8.7 ± 0.6 a,1 | 10.7 ± 0.6 b,2,3 | 46.13, p < 0.05 | |
| S. aureus 8 | 8.3 ± 0.6 a,1 | 9.3 ± 1.0 b,1 | 6.20, p < 0.05 | |
| S. pyogenes 29 | 10.7 ± 2.8 a,2 | 12.0 ± 0.3 a,4 | 2.00, p > 0.05 | |
| S. pyogenes 1143 | 13.0 ± 0.9 a,3 | 13.0 ± 0.9 a,5 | 0.01, p > 0.05 | |
| ANOVA (F,p) | 17.15, p < 0.05 | 14.83, p < 0.05 | ||
| Black chokeberry | E. coli 7 | 8.0 ± 0.4 a,1 | 9.0 ± 0.9 d,2 | 9.45, p < 0.05 |
| E. coli 1303 | 10.3 ± 0.7 a,2 | 11.3 ± 1.8 a,3,4 | 2.47, p > 0.05 | |
| S. aureus 22 | 8.6 ± 0.6 a,1 | 10.6 ± 0.6 b,2,3 | 50.17, p < 0.05 | |
| S. aureus 8 | 8.3 ± 0.6 a,1 | 9.3 ± 1.06 b,1 | 5.99, p < 0.05 | |
| S. pyogenes 29 | 10.7 ± 2.8 a,2 | 12.0 ± 0.3 a,4 | 2.04, p > 0.05 | |
| S. pyogenes 1143 | 11.0 ± 1.0 a,2 | 12.6 ± 0.6 b,5 | 18.81, p < 0.05 | |
| ANOVA (F,p) | 9.19, p < 0.05 | 18.88, p < 0.05 | ||
| Pomegranate | E. coli 7 | 9.98 ± 0.4 a,1 | 10.3 ± 1.4 a,2 | 0.54, p > 0.05 |
| E. coli 1303 | 10.7 ± 0.5 a,1 | 9.3 ± 0.7 b,2 | 22.38, p < 0.05 | |
| S. aureus 22 | 13.0 ± 2.3 a,2 | 12.0 ± 1.7 a,3 | 1.08, p > 0.05 | |
| S. aureus 8 | 9.9 ± 1.7 a,1 | 7.9 ± 0.3 b,1 | 11.64, p < 0.05 | |
| S. pyogenes 29 | 10.7 ± 0.5 a,1 | 10.0 ± 0.9 a,2 | 3.27, p > 0.05 | |
| S. pyogenes 1143 | 12.7 ± 1.0 a,2 | 12.0 ± 0.9 a,3 | 1.92, p > 0.05 | |
| (ANOVA F, p) | 9.40, p < 0.05 | 18.28, p < 0.05 |
| Extract | Strain | EtOH | Water |
|---|---|---|---|
| Cranberry | E. coli 7 | ns | 0.98 ** |
| E. coli 1303 | ns | 0.98 ** | |
| S. aureus 22 | −0.72 * | ns | |
| S. aureus 8 | ns | 0.72 * | |
| S. pyogenes 29 | ns | ns | |
| S. pyogeness 1143 | ns | −0.90 ** | |
| Black chokeberry | E. coli 7 | ns | ns |
| E. coli 1303 | ns | ns | |
| S. aureus 22 | −0.83 ** | ns | |
| S. aureus 8 | 0.69 * | ||
| S. pyogenes 29 | ns | ns | |
| S. pyogeness 1143 | ns | −0.84 ** | |
| Pomegranate | E. coli 7 | ns | 0.97 ** |
| E. coli 1303 | ns | ns | |
| S. aureus 22 | ns | ns | |
| S. aureus 8 | ns | ns | |
| S. pyogenes 29 | −0.69 * | ns | |
| S. pyogeness 1143 | ns | ns |
| Species | Sample | First Day | Third Day | Fifth Day | Eighth Day |
|---|---|---|---|---|---|
| Enterobacteriaceae | Control | 3.1 ± 0.8 a1 | 5.1 ± 1.1 ab1 | 6.5 ± 1.4 ab1 | 7.5 ± 1.8 b1 |
| WP | 2.0 ± 0.8 a12 | 4.0 ± 0.7 ab1 | 5.2 ± 1.1 b1 | 6.3 ± 1.1 b1 | |
| WC | 1.7 ± 0.4 a12 | 4.8 ± 1.1 b1 | 5.8 ± 1.4 b1 | 6.8 ± 1.1 b1 | |
| WA | 1.3 ± 0.2 a2 | 3.9 ± 1.4 ab1 | 4.2 ± 1.2 ab1 | 6.4 ± 1.7 b1 | |
| LABs | Control | 2.8 ± 0.3 a1 | 3.4 ± 0.8 ab1 | 4.7 ± 0.8 ab1 | 5.2 ± 1.1 b1 |
| WP | 3.6 ± 0.3 a2 | 5.3 ± 0.9 ab1 | 5.5 ± 1 ab1 | 6.9 ± 1.4 b1 | |
| WC | 3.6 ± 0.1 a2 | 5.0 ± 1.3 a1 | 5.6 ± 2 a1 | 6.5 ± 2.3 a1 | |
| WA | 3.6 ± 0.4 a2 | 5.0 ± 1.4 a1 | 5.6 ± 2.1 a1 | 6.5 ± 2.4 a1 | |
| TMC | Control | 3.5 ± 0.3 a1 | 7.9 ± 1.1 b1 | 6.9 ± 1.5 b1 | 9.0 ± 1.5 b1 |
| WP | 3.2 ± 0.5 a1 | 4.2 ± 1.4 a2 | 5.4 ± 1.2 a1 | 6.1 ± 2.9 a1 | |
| WC | 3.2 ± 0.3 a1 | 4.3 ± 1.3 a12 | 5.3 ± 2.5 a1 | 6.2 ± 1.5 a1 | |
| WA | 3.4 ± 0.4 a1 | 4.8 ± 1.8 a12 | 5.4 ± 1.5 a1 | 6.2 ± 2.4 a1 | |
| Yeast/Molds | Control | 3.0 ± 0.6 a1 | 3.7 ± 1.7 a1 | 4.7 ± 1.6 a1 | 6.0 ± 2.5 a1 |
| WP | 2.4 ± 0.3 a1 | 3.3 ± 1.5 a1 | 4.0 ± 1.3 a1 | 4.8 ± 2.4 a1 | |
| WC | 2.5 ± 0.7 a1 | 3.4 ± 1.6 a1 | 4.4 ± 1.8 a1 | 5.6 ± 2.2 a1 | |
| WA | 2.8 ± 0.4 a1 | 3.5 ± 1.6 a1 | 4.5 ± 2.3 a1 | 5.4 ± 1.8 a1 | |
| Staphylococcus spp | Control | 3.9 ± 0.7 a1 | 5.8 ± 1.8 a1 | 6.7 ± 1.5 a1 | 7.3 ± 1.5 a1 |
| WP | 2.9 ± 0.3 a1 | 4.7 ± 1.2 a1 | 4.6 ± 1.7 a1 | 4.3 ± 2.2 a1 | |
| WC | 3.4 ± 0.3 a1 | 5 ± 1.6 a1 | 4.8 ± 1.8 a1 | 5.0 ± 1.7 a1 | |
| WA | 3.0 ± 0.4 a1 | 5.1 ± 1.6 a1 | 5.5 ± 1.4 a1 | 5.9 ± 1.5 a1 | |
| Pseudomonas spp | Control | 3.5 ± 0.5 a1 | 6.2 ± 1.1 ab1 | 8.1 ± 1.7 b1 | 9.5 ± 1.6 b1 |
| WP | 2.0 ± 0.3 a2 | 5.8 ± 1.4 b1 | 6.9 ± 0.2 b1 | 7.0 ± 0.9 b1 | |
| WC | 2.8 ± 0.5 a12 | 5.2 ± 1.4 ab1 | 7.8 ± 2.4 b1 | 8.4 ± 1.5 b1 | |
| WA | 2.9 ± 0.5 a12 | 4.8 ± 1.8 ab1 | 8.0 ± 1.1 bc1 | 8.6 ± 1.3 c1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daoutidou, M.; Plessas, S.; Alexopoulos, A.; Mantzourani, I. Assessment of Antimicrobial Activity of Pomegranate, Cranberry, and Black Chokeberry Extracts against Foodborne Pathogens. Foods 2021, 10, 486. https://doi.org/10.3390/foods10030486
Daoutidou M, Plessas S, Alexopoulos A, Mantzourani I. Assessment of Antimicrobial Activity of Pomegranate, Cranberry, and Black Chokeberry Extracts against Foodborne Pathogens. Foods. 2021; 10(3):486. https://doi.org/10.3390/foods10030486
Chicago/Turabian StyleDaoutidou, Maria, Stavros Plessas, Athanasios Alexopoulos, and Ioanna Mantzourani. 2021. "Assessment of Antimicrobial Activity of Pomegranate, Cranberry, and Black Chokeberry Extracts against Foodborne Pathogens" Foods 10, no. 3: 486. https://doi.org/10.3390/foods10030486
APA StyleDaoutidou, M., Plessas, S., Alexopoulos, A., & Mantzourani, I. (2021). Assessment of Antimicrobial Activity of Pomegranate, Cranberry, and Black Chokeberry Extracts against Foodborne Pathogens. Foods, 10(3), 486. https://doi.org/10.3390/foods10030486








