Phytochemical Characterization and Evaluation of Bioactive Properties of Tisanes Prepared from Promising Medicinal and Aromatic Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Tisanes Preparations
2.2. Phytochemical Characterization
2.2.1. Phenolic Compounds
2.2.2. Organic Acids and Tocopherols (Vitamin E)
2.3. Evaluation of Bioactive Properties
2.3.1. Antioxidant Activity
2.3.2. Antimicrobial Activity
2.3.3. Anti-Inflammatory Activity
2.3.4. Cytotoxic and Hepatotoxic Activity
2.3.5. Anti-Tyrosinase Activity
2.3.6. Antidiabetic Activity
2.4. Statistical Analysis
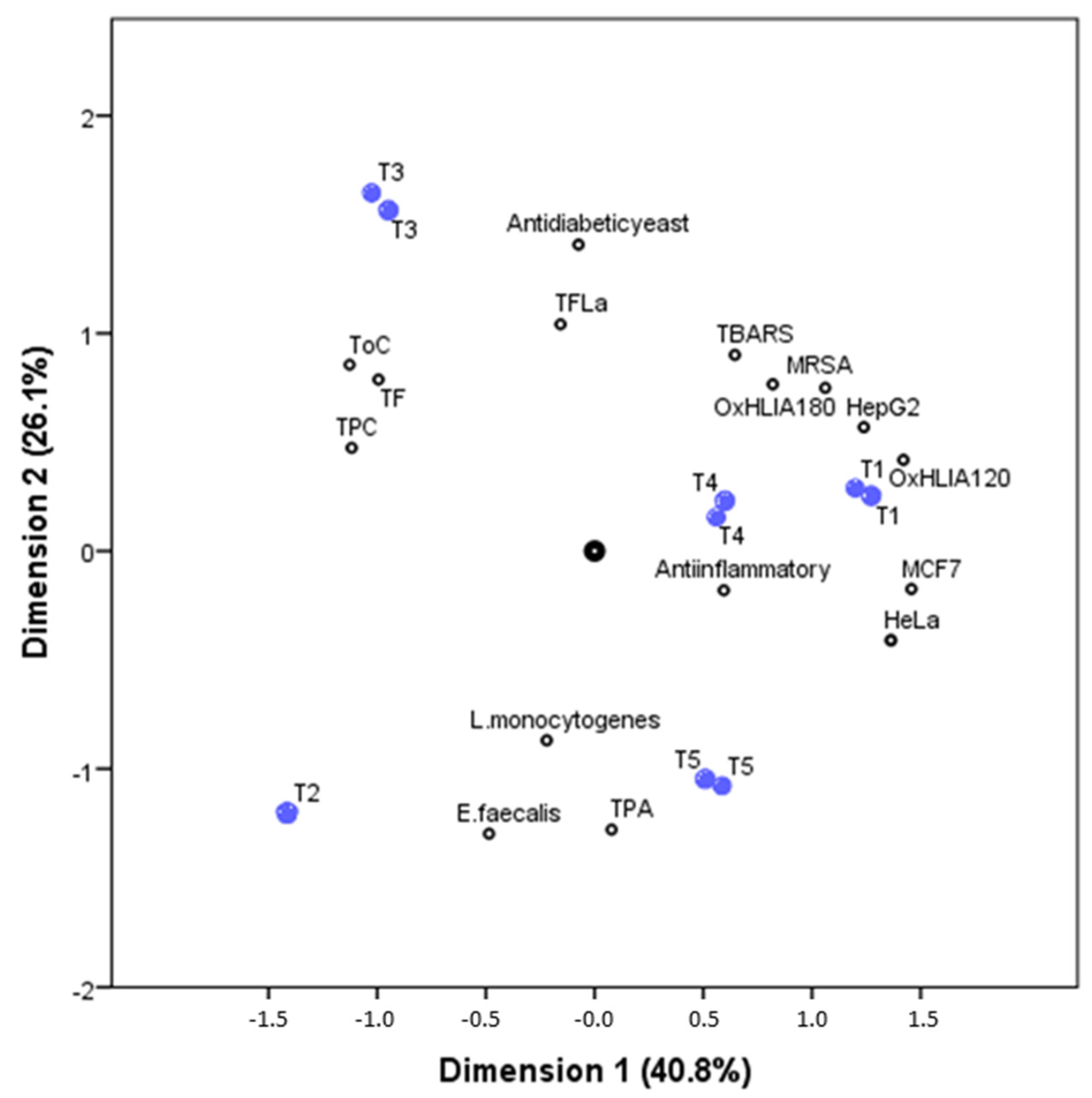
3. Results and Discussion
3.1. Phenolic Compounds
3.2. Organic Acids and Tocopherols
3.3. Evaluation of Bioactive Properties
3.3.1. Antioxidant Activity
3.3.2. Antimicrobial Activity
3.3.3. Anti-Inflammatory Activity
3.3.4. Cytotoxic and Hepatotoxic Activity
3.3.5. Anti-Tyrosinase Activity
3.3.6. Antidiabetic Activity
3.4. Selecting the Most Promisssing Tisane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pereira, E.; Antonio, A.L.; Barreira, J.C.M.; Barros, L.; Bento, A.; Ferreira, I.C.F.R. Gamma irradiation as a practical alternative to preserve the chemical and bioactive wholesomeness of widely used aromatic plants. Food Res. Int. 2015, 67, 338–348. [Google Scholar] [CrossRef]
- Pohl, P.; Dzimitrowicz, A.; Jedryczko, D.; Szymczycha-Madeja, A.; Welna, M.; Jamroz, P. The determination of elements in herbal teas and medicinal plant formulations and their tisanes. J. Pharm. Biomed. Anal. 2016, 130, 326–335. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Pardo-de-Santayana, M.; Tardío, J. Mediterranean non-cultivated vegetables as dietary sources of compounds with antioxidant and biological activity. LWT Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Pawlaczyk, I.; Czerchawski, L.; Pilecki, W.; Lamer-Zarawska, E.; Gancarz, R. Polyphenolic-polysaccharide compounds from selected medicinal plants of Asteraceae and Rosaceae families: Chemical characterization and blood anticoagulant activity. Carbohydr. Polym. 2009, 77, 568–575. [Google Scholar] [CrossRef]
- Dufresne, C.J.; Farnworth, E.R. A review of latest research findings on the health promotion properties of tea. J. Nutr. Biochem. 2001, 12, 404–421. [Google Scholar] [CrossRef]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Scientific validation of synergistic antioxidant effects in commercialised mixtures of Cymbopogon citratus and Pterospartum tridentatum or Gomphrena globosa for infusions preparation. Food Chem. 2015, 185, 16–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Estrada-Reyes, R.; Aguirre Hernández, E.; García-Argáez, A.; Soto Hernández, M.; Linares, E.; Bye, R.; Heinze, G.; Martínez-Vázquez, M. Comparative chemical composition of Agastache mexicana subsp. mexicana and A. mexicana subsp. xolocotziana. Biochem. Syst. Ecol. 2004, 32, 685–694. [Google Scholar] [CrossRef]
- Matić, I.Z.; Juranić, Z.; Šavikin, K.; Zdunić, G.; Nadvinski, N.; Goddevac, D. Chamomile and marigold tea: Chemical characterization and evaluation of anticancer activity. Phyther. Res. 2013, 27, 852–858. [Google Scholar] [CrossRef]
- Husain, I.; Ahmad, R.; Chandra, A.; Raza, S.T.; Shukla, Y.; Mahdi, F. Phytochemical characterization and biological activity evaluation of ethanolic extract of Cinnamomum zeylanicum. J. Ethnopharmacol. 2018, 219, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, M.; Tarnam, A.; Begum, N. Biological potential and phytopharmacological screening of Gomphrena species. Glob. J. Pharmacol. 2014, 3, 58–66. [Google Scholar]
- Liberal, Â.; Calhelha, R.C.; Pereira, C.; Adega, F.; Barros, L.; Dueñas, M.; Santos-Buelga, C.; Abreu, R.M.V.; Ferreira, I.C.F.R. A comparison of the bioactivity and phytochemical profile of three different cultivars of globe amaranth: Red, white, and pink. Food Funct. 2016, 7, 679–688. [Google Scholar] [CrossRef]
- Jabeur, I.; Tobaldini, F.; Martins, N.; Barros, L.; Martins, I.; Calhelha, R.C.; Henriques, M.; Silva, S.; Achour, L.; Santos-Buelga, C.; et al. Bioactive properties and functional constituents of Hypericum androsaemum L.: A focus on the phenolic profile. Food Res. Int. 2016, 89, 422–431. [Google Scholar] [CrossRef]
- Zhou, M.; Xing, H.H.; Ma, H.Y.; Zhou, L.; Yang, Y.; Li, G.P.; Hu, W.Y.; Liu, Q.; Li, X.M.; Hu, Q.F. Three new isobenzofurans from Lavandula angustifolia and their bioactivities. Phytochem. Lett. 2017, 19, 156–159. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikolaidou, E.; Stamatakis, A.; Tzortzakis, N. Vegetative, physiological, nutritional and antioxidant behavior of spearmint (Mentha spicata L.) in response to different nitrogen supply in hydroponics. J. Appl. Res. Med. Aromat. Plants 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Kapp, K.; Hakala, E.; Orav, A.; Pohjala, L.; Vuorela, P.; Püssa, T.; Vuorela, H.; Raal, A. Commercial peppermint (Mentha×piperita L.) teas: Antichlamydial effect and polyphenolic composition. Food Res. Int. 2013, 53, 758–766. [Google Scholar] [CrossRef]
- Flanigan, P.M.; Niemeyer, E.D. Effect of cultivar on phenolic levels, anthocyanin composition, and antioxidant properties in purple basil (Ocimum basilicum L.). Food Chem. 2014, 164, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- De Almeida Gonçalves, G.; De Sá-Nakanishi, A.B.; Comar, J.F.; Bracht, L.; Dias, M.I.; Barros, L.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Water soluble compounds of: Rosmarinus officinalis L. improve the oxidative and inflammatory states of rats with adjuvant-induced arthritis. Food Funct. 2018, 9, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.R.; Peres, A.M.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Simultaneous characterization and quantification of phenolic compounds in Thymus x citriodorus using a validated HPLC-UV and ESI-MS combined method. Food Res. Int. 2013, 54, 1773–1780. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C.F.R. A Comparison of the nutritional contribution of thirty-nine aromatic plants used as condiments and/or herbal infusions. Plant Foods Hum. Nutr. 2015, 70, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized Analysis of Organic Acids in Edible Mushrooms from Portugal by Ultra Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Souilem, F.; Fernandes, Â.; Calhelha, R.C.; Barreira, J.C.M.; Barros, L.; Skhiri, F.; Martins, A.; Ferreira, I.C.F.R. Wild mushrooms and their mycelia as sources of bioactive compounds: Antioxidant, anti-inflammatory and cytotoxic properties. Food Chem. 2017, 230, 40–48. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Malatesta, L.; De Luca, E.; Bellagamba, G.; Uysal, S.; Aktumsek, A.; Locatelli, M. Comparative study of biological activities and multicomponent pattern of two wild Turkish species: Asphodeline anatolica and Potentilla speciosa. J. Enzyme Inhib. Med. Chem. 2016, 31, 203–208. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro). Phytochemistry 2017, 143, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Venditti, A.; Cásedas, G.; Frezza, C.; Guiso, M.; Sciubba, F.; Serafini, M.; Bianco, A.; Valero, M.S.; López, V. Everlasting flower (Helichrysum stoechas Moench) as a potential source of bioactive molecules with antiproliferative, antioxidant, antidiabetic and neuroprotective properties. Ind. Crops Prod. 2017, 108, 295–302. [Google Scholar] [CrossRef]
- Khoza, B.S.; Gbashi, S.; Steenkamp, P.A.; Njobeh, P.B.; Madala, N.E. Identification of hydroxylcinnamoyl tartaric acid esters in Bidens pilosa by UPLC-tandem mass spectrometry. S. Afr. J. Bot. 2016, 103, 95–100. [Google Scholar] [CrossRef]
- Clifford, M.N.; Zheng, W.; Kuhnert, N. Profiling the chlorogenic acids of aster by HPLC–MSn. Phytochem. Anal. 2006, 17, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSnidentification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Phenolic profile and bioactive properties of carissa macrocarpa (Eckl.) A.DC.: An in vitro comparative study between leaves, stems, and flowers. Molecules 2019, 24, 1696. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- Ozarowski, M.; Mikolajczak, P.L.; Bogacz, A.; Gryszczynska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R.; et al. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.P.; Nocera, P.; Lettieri, A.; Bauer, R.; Monaco, P. Winter wild fennel leaves as a source of anti-inflammatory and antioxidant polyphenols. Arab. J. Chem. 2018, 11, 513–524. [Google Scholar] [CrossRef]
- Tahir, N.I.; Shaari, K.; Abas, F.; Parveez, G.K.A.; Ishak, Z.; Ramli, U.S. Characterization of apigenin and luteolin derivatives from oil palm (Elaeis guineensis Jacq.) Leaf using LC-ESI-MS/MS. J. Agric. Food Chem. 2012, 60, 11201–11210. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Fernandes, I.P.; Ruphuy, G.; Oliveira, M.B.P.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. A bioactive formulation based on Fragaria vesca L. vegetative parts: Chemical characterisation and application in κ-carrageenan gelatin. J. Funct. Foods 2015, 16, 243–255. [Google Scholar] [CrossRef]
- Majdi, C.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Frih, B.; Charrouf, Z.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Phytochemical characterization and bioactive properties of cinnamon basil (Ocimum basilicum cv. ‘cinnamon’) and lemon basil (ocimum x citriodorum). Antioxidants 2020, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Ziani, B.E.C.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds characterization by LC-DAD- ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019, 116, 312–319. [Google Scholar] [CrossRef]
- Ribeiro, A.; Caleja, C.; Barros, L.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. Rosemary extracts in functional foods: Extraction, chemical characterization and incorporation of free and microencapsulated forms in cottage cheese. Food Funct. 2016, 7, 2185–2196. [Google Scholar] [CrossRef]
- Rita, I.; Pereira, C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Mentha spicata L. infusions as sources of antioxidant phenolic compounds: Emerging reserve lots with special harvest requirements. Food Funct. 2016, 7, 4188–4192. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kiprotich, J.; Kuhnert, N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae family. Phytochemistry 2011, 72, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Karolak, I.; Kiss, A.K. Determination of the phenolic profile and antioxidant properties of Salvia viridis L. Shoots: A comparison of aqueous and hydroethanolic extracts. Molecules 2018, 23, 1468. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, C.F.; Tian, G.G.; Qian, L.R.; Yu, L.J. Characterization of active constituents in Pyracantha fortuneana fruit extract and their effects on hyperlipidaemia, obesity, and oxidative stress in rodents. J. Funct. Foods 2016, 22, 278–290. [Google Scholar] [CrossRef]
- Miguel, M.G.; Cruz, C.; Faleiro, L.; Simoes, M.T.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Foeniculum vulgare essential oils: Chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Commun. 2010, 5, 319–328. [Google Scholar] [CrossRef]
- Rita, I.; Pereira, C.; Barros, L.; Ferreira, I.C.F.R. Exploring reserve lots of Cymbopogon citratus, Aloysia citrodora and Thymus × citriodorus as improved sources of phenolic compounds. Food Chem. 2018, 257, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: A phytochemical study focused on antioxidant compounds. Food Res. Int. 2014, 62, 684–693. [Google Scholar] [CrossRef]
- Pereira, E.; Pimenta, A.I.; Calhelha, R.C.; Antonio, A.L.; Barros, L.; Santos-Buelga, C.; Verde, S.C.; Ferreira, I.C.F.R. Infusions of gamma irradiated Aloysia citrodora L. and Mentha x piperita L.: Effects on phenolic composition, cytotoxicity, antibacterial and virucidal activities. Ind. Crops Prod. 2017, 97, 582–590. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Barros, L.; Abreu, R. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.K.; Thuong, P.T.; Su, N.D.; Sok, D.E.; Song, K.S.; Seong, Y.H.; Bae, K.H. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Antimicrobial activity of malic acid against Listeria monocytogenes, Salmonella Enteritidis and Escherichia coli O157:H7 in apple, pear and melon juices. Food Control 2009, 20, 105–112. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Antonio, A.L.; Barreira, J.C.M.; Bento, A.; Kaluska, I.; Ferreira, I.C.F.R. Analysis of organic acids in electron beam irradiated chestnuts (Castanea sativa Mill.): Effects of radiation dose and storage time. Food Chem. Toxicol. 2013, 55, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, K.S.; Khalil, O.A.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Hypoglycemic and hepatoprotective activity of Rosmarinus officinalis extract in diabetic rats. J. Physiol. Biochem. 2013, 69, 779–783. [Google Scholar] [CrossRef]
- Taheri Gandomani, V.; Mahdavi, A.H.; Rahmani, H.R.; Riasi, A.; Jahanian, E. Effects of different levels of clove bud (Syzygium aromaticum) on performance, intestinal microbial colonization, jejunal morphology, and immunocompetence of laying hens fed different n-6 to n-3 ratios. Livest. Sci. 2014, 167, 236–248. [Google Scholar] [CrossRef]
- Hosni, K.; Msaâda, K.; Taârit, M.B.; Marzouk, B. Fatty acid composition and tocopherol content in four Tunisian Hypericum species: Hypericum perforatum, Hypericum tomentosum, Hypericum perfoliatum and Hypericum ericoides Ssp. Roberti. Arab. J. Chem. 2017, 10, S2736–S2741. [Google Scholar] [CrossRef]
- Instituto Nacional de Saúde Doutor Ricardo Jorge, IP; Instituto Nacional de Estatística, IP. INSA-Inquérito Nacional de Saúde; Instituto Nacional de Estatística: Lisboa, Portugal, 2005. [Google Scholar]
- Chen, Y.; Deuster, P. Comparison of quercetin and dihydroquercetin: Antioxidant-independent actions on erythrocyte and platelet membrane. Chem. Biol. Interact. 2009, 182, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Silva, L.R.; Valentão, P.; Faria, J.; Ferreres, F.; Sousa, C.; Gil-Izquierdo, A.; Pinho, B.R.; Andrade, P.B. Phytochemical investigations and biological potential screening with cellular and non-cellular models of globe amaranth (Gomphrena globosaL.) inflorescences. Food Chem. 2012, 135, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Tovar, I.; Sponza, S.; Asensio-S-Manzanera, M.C.; Novak, J. Contribution of the main polyphenols of Thymus mastichina subsp: Mastichina to its antioxidant properties. Ind. Crops Prod. 2015, 66, 291–298. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Futur. Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef]
- Ryu, B.; Kim, H.M.; Woo, J.H.; Choi, J.H.; Jang, D.S. A new acetophenone glycoside from the flower buds of Syzygium aromaticum (cloves). Fitoterapia 2016, 115, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lee, J. Anti-inflammatory activity of butein and luteolin through suppression of NFκB activation and induction of heme oxygenase-1. J. Med. Food 2015, 18, 557–564. [Google Scholar] [CrossRef]
- Correa, V.G.; Gonçalves, G.A.; de Sá-Nakanishi, A.B.; Ferreira, I.C.F.R.; Barros, L.; Dias, M.I.; Koehnlein, E.A.; de Souza, C.G.M.; Bracht, A.; Peralta, R.M. Effects of in vitro digestion and in vitro colonic fermentation on stability and functional properties of yerba mate (Ilex paraguariensis A. St. Hil.) beverages. Food Chem. 2017, 237. [Google Scholar] [CrossRef]
- Petiwala, S.M.; Johnson, J.J. Diterpenes from rosemary (Rosmarinus officinalis): Defining their potential for anti-cancer activity. Cancer Lett. 2015, 367, 93–102. [Google Scholar] [CrossRef]
- Seo, S.Y.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Tamura, N. Inhibitory compound of tyrosinase activity from the sprout of Polygonum hydropiper L. (Benitade). Biol. Pharm. Bull. 2007, 30, 595–597. [Google Scholar] [CrossRef]
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ghosh, R.; Pal, B.C. α-Glucosidase inhibitory terpenoids from Potentilla fulgens and their quantitative estimation by validated HPLC method. J. Funct. Foods 2013, 5, 1135–1141. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Jiphimai, P.; Prutanopajai, P.; Chanathong, B.; Sapwarobol, S.; Ariyapitipan, T. Evaluation of α-glucosidase, α-amylase and protein glycation inhibitory activities of edible plants. Int. J. Food Sci. Nutr. 2010, 61, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]


| Plant | Family | Applicability/Common Uses | Bioactive Properties | Ref. |
|---|---|---|---|---|
| Agastache foeniculum (Pursh) Kuntze | Lamiaceae | Medicine | Antimicrobial | [11] |
| Calendula officinalis L. | Asteraceae | Cosmetic, medicinal, food, and colorant | Anti-inflammatory, antitumor, and antimicrobial | [12] |
| Cinnamomum zeylanicum Blume | Lauraceae | Food | Antitumor and antidiabetics | [13] |
| Cymbopogon citratus (D.C.) Stapf | Poaceae | Cosmetic, pharmaceutical, and infusion | Antioxidant, intestinal anti-inflammatory, antitumor, and antidiabetics | [10] |
| Gomphrena globosa L. | Amaranthaceae | Infusion | Antioxidant, anti-inflammatory, and antidiabetics | [14] |
| Gomphrena sp. | Amaranthaceae | Bioactive and infusion | Antioxidant, antimicrobial, and antitumor | [15] |
| Hypericum androsaemum L. | Hypericaceae | Medicine | Anti-inflammatory, antitumor, antimicrobial, antioxidant, and antiviral | [16] |
| Lavandula angustifolia Mill. | Lamiaceae | Perfumery, cosmetics, and medicine | Antimicrobial, antioxidant, antiviral, and cytotoxic | [17] |
| Mentha spicata L. | Lamiaceae | Food, pharmaceutical, perfumery, confectionery | Antioxidant, antimicrobial, and neuroprotective effects | [18] |
| Mentha x piperita L. | Lamiaceae | Food, infusion | Antioxidant, antimicrobial, and anti-inflammatory | [19] |
| Ocimum basilicum L. | Lamiaceae | Food | Anti-inflammatory, antimicrobial, and neuroprotective effects | [20] |
| Ocimum basilicum "Cinnamon” | Labiatae | Flavoring agent and essential oil | Antioxidant and antimicrobial | [21,22] |
| Rosmarinus officinalis L. | Lamiaceae | Culinary and medicine | Antioxidant, antimicrobial, anti-inflammatory, anti-tumor, and anti-diabetic | [23] |
| Syzygium aromaticum L. | Myrtaceae | Medicine | Anti-inflammatory, antioxidant, antimicrobial, and anti-tumor | [23] |
| Thymus mastichina L. | Lamiaceae | Food, cosmetics, and infusion | Antioxidant, anti-inflammatory, antitumor, and antifungal | [23] |
| Thymus x citriodorus (Pers.) Schreb | Lamiaceae | Food, infusion | Antimicrobial, anti-inflammatory, and antioxidant | [24,25] |
| Peak | Rt (min) | λmax (nm) | [M-H]− (m/z) | MS2 (m/z) | Tentative Identification | Reference/Method Used for Identification |
|---|---|---|---|---|---|---|
| 1 | 4.75 | 263/325 | 311 | 179(72),149(100),135(5) | Caftaric acid | [33] |
| 2 | 4.79 | 324 | 353 | 191(100),179(49),173(10),135(12) | 3-O-Caffeoylquinic acid | DAD/MS [34,35] |
| 3 | 5.5 | 280 | 359 | 197(100),153(8),113(5) | Syringic acid hexoside | [36] |
| 4 | 5.81 | 312 | 295 | 163(100),149(5),119(5) | Coutaric acid | [33] |
| 5 | 6.31 | 264 | 325 | 163(100),119(18) | p-Coumaric acid hexoside | [37] |
| 6 | 6.62 | 324 | 353 | 191(80),179(9),173(100),135(5) | cis 4-O-Caffeoylquinic acid | [34,35] |
| 7 | 6.67 | 290/321 | 325 | 193(100),149(5),134(5) | Fertaric acid | [33] |
| 8 | 7.06 | 285 | 611 | 449(100),287(13) | Eriodictyol-O-dihexoside | [33] |
| 9 | 7.11 | 324 | 353 | 191(100),179(5),173(5),135(5) | trans 4-O-Caffeoylquinic acid | [34,35] |
| 10 | 7.87 | 325 | 353 | 191(100),179(9),173(5),135(5) | cis 5-O-Caffeoylquinic acid | [34,35] |
| 11 | 8.41 | 281 | 387 | 369(25),207(100),163(41),119(5) | Medioresinol | [38,39] |
| 12 | 8.54 | 321 | 353 | 191(100),179(12),173(5),135(5) | trans 5-O-Caffeoylquinic acid | [34,35] |
| 13 | 9.95 | 325 | 593 | 473(100),383(13),353(21),325(5) | Apigenin-C-hexoside-C-hexoside | [40] |
| 14 | 10.05 | 322 | 179 | 135(100) | Caffeic acid | DAD/MS |
| 15 | 11.1 | 281 | 865 | 739(83),713(50),695(100),577(77),425(12),407(10),289(5),287(12) | Type B (epi)-catechin trimer | [41] |
| 16 | 11.44 | 279 | 325 | 163(100),145(5),119(8) | p-Coumaric acid hexoside | [37] |
| 17 | 12.12 | 283 | 1153 | 865(37),577(12),561(5),289(5),287(5) | Type B (epi)-catechin tetramer | [41] |
| 18 | 12.22 | 327 | 473 | 311(100),293(98),179(61),149(80),135(5) | cis Chicoric acid | [42] |
| 19 | 12.3 | 327 | 473 | 311(100),293(95),179(50),149(55),135(5) | trans Chicoric acid | [42] |
| 20 | 12.59 | 324 | 637 | 461(100),285(15) | Kaempherol-O-diglucuronide | DAD/MS |
| 21 | 13.46 | 346 | 637 | 285(100) | Luteolin-O-diglucuronide | DAD/MS |
| 22 | 14.47 | 330 | 555 | 537(100),493(12),359(10),179(5),161(5) | Salvianolic acid K isomer | [43] |
| 23 | 14.5 | 285/325 | 537 | 493(100),359(34),313(13),295(4),269(3),197(5),179(5) | Lithospermic acid A | [44] |
| 24 | 15.16 | 284 | 595 | 287(100) | Eriodictyol-O-deoxyhexosyl-hexoside | [45] |
| 25 | 15.18 | 341 | 477 | 301(100) | Quercetin-O-glucuronide | [46] |
| 26 | 15.8 | 344 | 463 | 301(100) | Quercetin-3-O-glucoside | DAD/MS |
| 27 | 15.99 | 282 | 463 | 287(100) | Eriodictyol-O-glucuronide | DAD/MS |
| 28 | 16.96 | 342 | 769 | 315(100) | Isorhamnetin-O-di-deoxyhexosyl-hexoside | [46] |
| 29 | 17.37 | 322 | 521 | 359(100),197(45),179(40),161(15),135(5) | Rosmarinic acid hexoside | [44] |
| 30 | 17.69 | 343 | 609 | 301(100) | Quercetin-3-O-rutinoside | DAD/MS |
| 31 | 17.98 | 345 | 593 | 285(100) | Luteolin-O-deoxyhexosyl-hexoside | [45] |
| 32 | 18.02 | 346 | 593 | 285(100) | Luteolin-7-O-rutinoside | DAD/MS |
| 33 | 18.07 | 348 | 477 | 301(100) | Quercetin-O-glucuronide | [46] |
| 34 | 18.38 | 347 | 461 | 285(100) | Luteolin-7-O-glucoside | DAD/MS |
| 35 | 18.93 | 347 | 461 | 285(100) | Luteolin-O-glucuronide | [46] |
| 36 | 18.57 | 280 | 717 | 519(100),475(20),339(7),321(5) | Salvianolic acid B | [44] |
| 37 | 18.96 | 281/327 | 487 | 325(90),307(51),293(100),193(5),179(12) | Feruloylcaffeoyl tartaric acid | [38] |
| 38 | 19.2 | 347 | 521 | 359(100),197(44),179(38),161(14),135(5) | Rosmarinic acid hexoside | [44] |
| 39 | 19.29 | 282/323 | 719 | 539(24),521(17),339(100),197(10),179(5),161(5),135(5) | Sagerinic acid | [44] |
| 40 | 19.85 | 337 | 717 | 537(40),519(100),493(5),359(10),339(5),321(5),295(5),197(5),179(5) | Salvianolic acid B isomer | [44] |
| 41 | 20.17 | 340 | 549 | 505(100),301(10) | Quercetin-O-malonylhexoside | DAD/MS |
| 42 | 21.92 | 330 | 359 | 197(29),179(36),161(100),135(5) | Rosmarinic acid | DAD/MS |
| 43 | 22.25 | 325 | 555 | 537(5),511(8),493(45),311(100),197(5),179(5),161(5),135(5) | Salvianolic acid K | [43] |
| 44 | 23.92 | 340 | 533 | 489(100),285(10) | Luteolin-O-malonylhexoside | DAD/MS |
| 45 | 24.05 | 341 | 549 | 387(100),369(21),207(50),163(41),119(5) | Medioresinol caffeate | [38,39] |
| 46 | 24.71 | 333 | 461 | 285(100) | Luteolin-O-glucuronide | [46] |
| 47 | 25.51 | 335 | 475 | 299(100),285(5) | methylluteolin-O-hexuronide (chrysoeriol hexuronide) | [47] |
| 48 | 25.85 | 331 | 717 | 537(10),519(100),493(5),359(5),339(21),321(50),295(9),197(5),179(5) | Salvianolic acid B isomer | [44] |
| 49 | 25.97 | 324 | 537 | 493(100),359(10),313(6),295(6),269(5),197(5),179(5) | Lithospermic acid A | [44] |
| 50 | 28.17 | 330 | 517 | 473(20),269(100) | Apigenin-O-malonylhexoside | DAD/MS |
| 51 | 29.02 | 331 | 503 | 285(100) | Luteolin-O-malonylpentoside | DAD/MS |
| 52 | 29.71 | 341 | 547 | 503(50),299(100) | Diosmetin-O-malonylhexoside | DAD/MS |
| 53 | 30.17 | 284/329 | 537 | 493(100),439(51),197(24),179(6),359(20) | Lithospermic acid A isomer 2 | [44] |
| 54 | 30.48 | 280/339 | 493 | 359(100),313(15),295(9),269(7),197(5),179(5) | Salvianolic acid A | [45] |
| 55 | 30.84 | 330 | 503 | 285(100) | Luteolin-O-malonylpentoside | DAD/MS |
| 56 | 33 | 268/331 | 677 | 659(21),451(100),367(10),225(20) | Unkwown | - |
| 57 | 34.19 | 290 | 791 | 773(100),678(11),546(2),451(35),337(2),225(11) | Oleanolic acid derivative | [48] |
| 58 | 35.77 | 268/333 | 1063 | 771(100),283(50),269(10) | Unkwown | - |
| 59 | 36.87 | 338 | 589 | 545(100),299(5) | Diosmetin-malonyl methyl hexoside | DAD/MS |
| 60 | 38.08 | 269/329 | 283 | 269(100) | Calycosin | [49] |
| 61 | 40.01 | 269/329 | 573 | 283(100) | Calycosin derivative | DAD/MS |
| 62 | 40.36 | 268/329 | 533 | 283(100) | Calycosin derivative | DAD/MS |
| 63 | 41.83 | 268/330 | 573 | 283(100) | Calycosin derivative | DAD/MS |
| Peak | T1 | T2 | T3 | T4 | T5 | t-Students Test p-Value |
|---|---|---|---|---|---|---|
| 1 A | 0.41 ± 0.01 | nd | nd | nd | nd | - |
| 2 B | nd | nd | nd | nd | 3.13 ± 0.14 | - |
| 3 C | nd | 0.163 ± 0.002 | nd | nd | nd | - |
| 4 D | 0.143 ± 0.001 | nd | nd | 0.151 ± 0.002 | nd | 0.001 |
| 5 E | nd | 0.863 ± 0.01 | nd | 0.29 ± 0.01 | nd | <0.001 |
| 6 B | nd | nd | 0.69 ± 0.02 | nd | 0.63 ± 0.03 | <0.001 |
| 7 F | 0.172 ± 0.002 | nd | nd | nd | nd | - |
| 8 G | nd | 0.086 ± 0.004 | nd | 0.115 ± 0.004 | nd | - |
| 9 B | nd | nd | 1.19 ± 0.02 | nd | 30.88 ± 1.17 | <0.001 |
| 10 B | nd | nd | nd | nd | 3.66 ± 0.01 | - |
| 11 D | nd | 0.215 ± 0.004 | nd | nd | nd | - |
| 12 B | nd | nd | nd | nd | 2.07 ± 0.06 | - |
| 13 H | nd | 1.016 ± 0.003 a | nd | 0.606 ± 0.002 b | 0.442 ± 0.001 c | - |
| 14 A | 0.1303 ± 0.002 | nd | 0.15 ± 0.01 | nd | nd | 0.001 |
| 15 I | nd | nd | nd | nd | 1.65 ± 0.03 | - |
| 16 E | nd | 1.07 ± 0.03 | nd | nd | nd | - |
| 17 I | nd | nd | nd | nd | 1.98 ± 0.76 | - |
| 18 A | 2.69 ± 0.05 | nd | nd | 0.263 ± 0.004 | nd | <0.001 |
| 19 A | 0.82 ± 0.01 | nd | nd | 0.49 ± 0.02 | nd | <0.001 |
| 20 J | nd | tr | nd | nd | nd | - |
| 21 J | nd | nd | 2.47 ± 0.04 | 2.27 ± 0.08 | nd | <0.001 |
| 22 K | 2.33 ± 0.01 | nd | nd | 1.29 ± 0.01 | nd | <0.001 |
| 23 K | nd | nd | 0.747 ± 0.004 | nd | nd | - |
| 24 G | nd | nd | 6.49 ± 0.23 | 5.77 ± 0.12 | nd | <0.001 |
| 25 J | nd | 1.52 ± 0.03 | nd | nd | 0.90 ± 0.01 | <0.001 |
| 26 L | nd | 4.58 ± 0.18 | nd | nd | nd | - |
| 27 G | nd | nd | nd | 1.996 ± 0.003 | 3.17 ± 0.07 | - |
| 28 J | nd | nd | nd | 1.19 ± 0.01 | nd | - |
| 29 K | nd | 1.99 ± 0.01 | nd | nd | nd | - |
| 30 J | 0.23 ± 0.003 | nd | nd | 1.59 ± 0.01 | nd | - |
| 31 J | nd | nd | nd | 3.533 ± 0.003 | nd | - |
| 32 J | nd | nd | 5.91 ± 0.02 | nd | nd | - |
| 33 J | nd | nd | nd | nd | 2.29 ± 0.01 | - |
| 34 L | nd | 8.73 ± 0.11 d | 7.05 ± 0.13 c | 10.08 ± 0.22 b | 15.92 ± 0.34 a | - |
| 35 L | nd | 3.17 ± 0.04 | nd | nd | 8.09 ± 0.25 | <0.001 |
| 36 K | 0.61 ± 0.01 | nd | nd | nd | nd | - |
| 37 F | 0.107 ± 0.004 | nd | nd | nd | nd | - |
| 38 K | nd | 6.12 ± 0.12 | 1.23 ± 0.02 | nd | nd | <0.001 |
| 39 K | 0.828 ± 0.013 c | nd | nd | 1.84 ± 0.05 a | 1.55 ± 0.07 b | - |
| 40 K | nd | nd | 1.71 ± 0.07 | 1.79 ± 0.01 | nd | <0.001 |
| 41 J | 1.19 ± 0.05 | nd | nd | nd | nd | - |
| 42 K | 10.84 ± 0.14 d | 20.78 ± 0.34 b | 22.65 ± 0.33 a | 20.66 ± 0.26 b | 19.9 ± 0.13 c | - |
| 43 K | nd | 9.60 ± 0.39 | nd | nd | nd | - |
| 44 J | nd | nd | 0.278 ± 0.005 | nd | nd | - |
| 45 D | nd | nd | 3.16 ± 0.02 | nd | nd | - |
| 46 J | nd | nd | 1.19 ± 0.04 | 0.88 ± 0.03 | nd | <0.001 |
| 47 J | nd | 0.68 ± 0.01 | nd | nd | nd | - |
| 48 K | nd | nd | 3.34 ± 0.02 | 5.46 ± 0.19 | nd | - |
| 49 K | 6.84 ± 0.09 a | nd | nd | 1.14 ± 0.03 c | 5.61 ± 0.09 b | - |
| 50 M | nd | nd | 1.03 ± 0.01 | nd | nd | - |
| 51 J | nd | nd | nd | 0.40 ± 0.02 | nd | - |
| 52 G | nd | nd | 36.04 ± 0.05 | nd | nd | - |
| 53 K | nd | nd | nd | 0.95 ± 0.01 | nd | - |
| 54 K | 2.59 ± 0.03 | nd | nd | nd | 0.87 ± 0.01 | <0.001 |
| 55 J | nd | nd | nd | 0.080 ± 0.001 | nd | - |
| 56 | nd | nd | nq | nd | nd | - |
| 57 | nd | nd | nd | nd | nq | - |
| 58 | nd | nd | nq | nd | nd | - |
| 59 G | nd | nd | 0.026 ± 0.001 | nd | nd | - |
| 60 K | nd | nd | 1.07 ± 0.06 | nd | nd | - |
| 61 K | nd | nd | 0.25 ± 0.01 | nd | nd | - |
| 62 K | nd | nd | 7.28 ± 0.13 | nd | nd | - |
| 63 K | nd | nd | 9.22 ± 0.21 | nd | nd | - |
| TPA | 28.51 ± 0.28 e | 40.59 ± 0.13 b | 31.73 ± 0.23 d | 34.34 ± 0.10 c | 68.30 ± 0.78 a | - |
| TiF | nd | nd | 17.81 ± 0.14 | nd | nd | - |
| TFa | nd | 0.086 ± 0.004 d | 6.495 ± 0.23 b | 7.88 ± 0.12 a | 3.17 ± 0.07 c | - |
| TFo | 1.42 ± 0.05 e | 19.7 ± 0.3 d | 53.98 ± 0.08 a | 20.58 ± 0.19 c | 27.66 ± 0.08 b | - |
| TF3O | nd | nd | nd | nd | 3.64 ± 0.04 | - |
| TOC | nd | 0.215 ± 0.004 | 3.16 ± 0.02 | nd | nd | <0.001 |
| TPC | 29.93 ± 0.34 e | 60.58 ± 0.45 d | 113.19 ± 0.68 a | 62.79 ± 0.41 c | 102.78 ± 0.84 b | - |
| Extracts | Infusion Preparations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T1 | T2 | T3 | T4 | T5 | |
| Organic acids | (g/100 g·dw) | (mg/100 mL) | ||||||||
| Oxalic acid | 2.47 ± 0.01 a | 0.38 ± 0.002 d | 1.30 ± 0.02 b | 0.79 ± 0.01 c | tr | 98 ± 1 a | 6.9 ± 0.1 d | 16.8 ± 0.3 c | 21.1 ± 0.5 b | tr |
| Quinic acid | 1.65 ± 0.07 a | 0.10 ± 0.04 d | 1.00 ± 0.08 c | 1.33 ± 0.09 b | nd | 69 ± 1 a | 2.8 ± 0.1 d | 28 ± 1 c | 33 ± 1 b | nd |
| Malic acid | 0.48 ± 0.03 a | 0.36 ± 0.02 d | 0.41 ± 0.01 c | 0.43 ± 0.01 b | tr | 68 ± 1 a | 11.5 ± 0.3 b | 11 ± 1 b | 10.5 ± 0.1 c | tr |
| Fumaric acid | nd | nd | nd | nd | tr | nd | nd | nd | nd | tr |
| Sum | 4.59 ± 0.09 a | 0.83 ± 0.07 d | 2.71 ± 0.08 b | 2.54 ± 0.09 c | tr | 234 ± 3 a | 21.2 ± 0.4 d | 56 ± 2 c | 65 ± 1 b | tr |
| Tocopherols | (mg/100 g·dw) | (mg/100 mL) | ||||||||
| α-Tocopherol | 12.2 ± 0.9 a | 3.3 ± 0.3 c | 0.87 ± 0.01 e | 3.5 ± 0.2 b | 2.3 ± 0.1 d | nd | nd | nd | nd | nd |
| γ-Tocopherol | 35.8 ± 0.4 b | nd | 4.71 ± 0.01 d | 11.2 ± 0.3 c | 109 ± 5 a | nd | nd | nd | nd | nd |
| Sum | 48.0 ± 0.6 b | 3.3 ± 0.3 e | 5.58 ± 0.01 d | 14.7 ± 0.3 c | 112 ± 5 a | nd | nd | nd | nd | nd |
| T1 | T2 | T3 | T4 | T5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antioxidant Activity (IC50 values, μg mL) A | ||||||||||
| OxHLIA Δt = 120 | 27.9 ± 0.2 a | 17.8 ± 0.2 e | 22.0 ± 0.2 d | 25.2 ± 0.3 b | 23.4 ± 0.3 c | |||||
| Δt = 180 | 45.3 ± 0.3 a | 30.3 ± 0.3 e | 36.5 ± 0.2 c | 37.3 ± 0.2 b | 34.4 ± 0.7 d | |||||
| TBARS inhibition | 13.2 ± 0.3 a | 7.4 ± 0.1 c | 9.7 ± 0.3 b | 9.8 ± 0.1 b | 5.07 ± 0.04 d | |||||
| Antimicrobial activity (mg/mL) B | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Gram-negative bacteria | ||||||||||
| Escherichia coli | 20 | >20 | 20 | >20 | >20 | >20 | >20 | >20 | 20 | >20 |
| Klebsiella pneumoniae | 20 | >20 | 20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 |
| Morganella morganii | >20 | >20 | 20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 |
| Proteus mirabilis | >20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 |
| Pseudomonas aeruginosa | >20 | >20 | 20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 |
| Gram-positive bacteria | ||||||||||
| Enterococcus faecalis | 10 | >20 | 20 | >20 | 5 | >20 | 10 | >20 | >20 | >20 |
| Listeria monocytogenes | 5 | >20 | >20 | >20 | 5 | >20 | 20 | >20 | >20 | >20 |
| MRSA | 10 | >20 | 2.5 | >20 | 10 | >20 | 5 | >20 | 10 | >20 |
| Anti-inflammatory activity (IC50 values µg/mL) C | ||||||||||
| RAW 264.7 | >400 | >400 | >400 | 354 ± 6 * | 345 ± 9 * | |||||
| Cytotoxicity Activity (GI50 values µg/mL) D | ||||||||||
| HepG2 | 311 ± 9 a | >400 | 299 ± 9 b | 264 ± 15 c | 316 ± 13 a | |||||
| NCI H460 | 257 ± 9 b | >400 | >400 | 248 ± 10 c | 317 ± 8 a | |||||
| HeLa | 294 ± 14 b | >400 | >400 | 253 ± 8 c | 313 ± 5 a | |||||
| MCF7 | 320 ± 3 a | >400 | >400 | 308 ± 13 b | 285 ± 10 c | |||||
| Hepatotoxicity (GI50 values µg/mL) D | ||||||||||
| PLP2 | >400 | >400 | >400 | >400 | >400 | |||||
| Anti-tyrosinase (IC50 values mg/mL or PI at 8 mg/mL) E | ||||||||||
| Mushroom tyrosinase inhibition | >8 | >8 | >8 | >8 | >8 | |||||
| Antidiabetic activity (IC50 values µg/mL or PI at 8 mg/mL) F | ||||||||||
| Mammalian α-glucosidase inhibition | >8 | >8 | >8 | >8 | 37 ± 6 | |||||
| Yeast α-glucosidase inhibition | 41% ± 4% | 1.19 ± 0.02 | 43% ± 3% | 6.9 ± 0.4 | 0.054 ± 0.003 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschoalinotto, B.H.; Dias, M.I.; Pinela, J.; Pires, T.C.S.P.; Alves, M.J.; Mocan, A.; Calhelha, R.C.; Barros, L.; Ineu, R.P.; Ferreira, I.C.F.R. Phytochemical Characterization and Evaluation of Bioactive Properties of Tisanes Prepared from Promising Medicinal and Aromatic Plants. Foods 2021, 10, 475. https://doi.org/10.3390/foods10020475
Paschoalinotto BH, Dias MI, Pinela J, Pires TCSP, Alves MJ, Mocan A, Calhelha RC, Barros L, Ineu RP, Ferreira ICFR. Phytochemical Characterization and Evaluation of Bioactive Properties of Tisanes Prepared from Promising Medicinal and Aromatic Plants. Foods. 2021; 10(2):475. https://doi.org/10.3390/foods10020475
Chicago/Turabian StylePaschoalinotto, Beatriz H., Maria Inês Dias, José Pinela, Tânia C.S.P. Pires, Maria José Alves, Andrei Mocan, Ricardo C. Calhelha, Lillian Barros, Rafael P. Ineu, and Isabel C.F.R. Ferreira. 2021. "Phytochemical Characterization and Evaluation of Bioactive Properties of Tisanes Prepared from Promising Medicinal and Aromatic Plants" Foods 10, no. 2: 475. https://doi.org/10.3390/foods10020475
APA StylePaschoalinotto, B. H., Dias, M. I., Pinela, J., Pires, T. C. S. P., Alves, M. J., Mocan, A., Calhelha, R. C., Barros, L., Ineu, R. P., & Ferreira, I. C. F. R. (2021). Phytochemical Characterization and Evaluation of Bioactive Properties of Tisanes Prepared from Promising Medicinal and Aromatic Plants. Foods, 10(2), 475. https://doi.org/10.3390/foods10020475