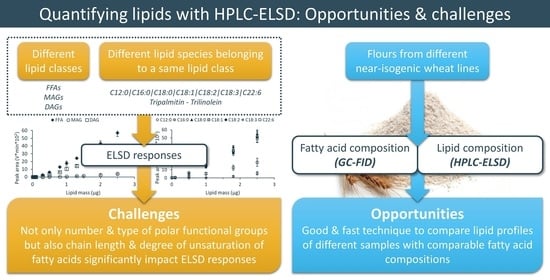
Normal-Phase HPLC-ELSD to Compare Lipid Profiles of Different Wheat Flours
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ELSD Responses of Different Lipids
2.3. Lipid Extraction
2.4. Fatty Acid Composition
2.5. Lipid Composition
2.6. Statistical Analyses
3. Results
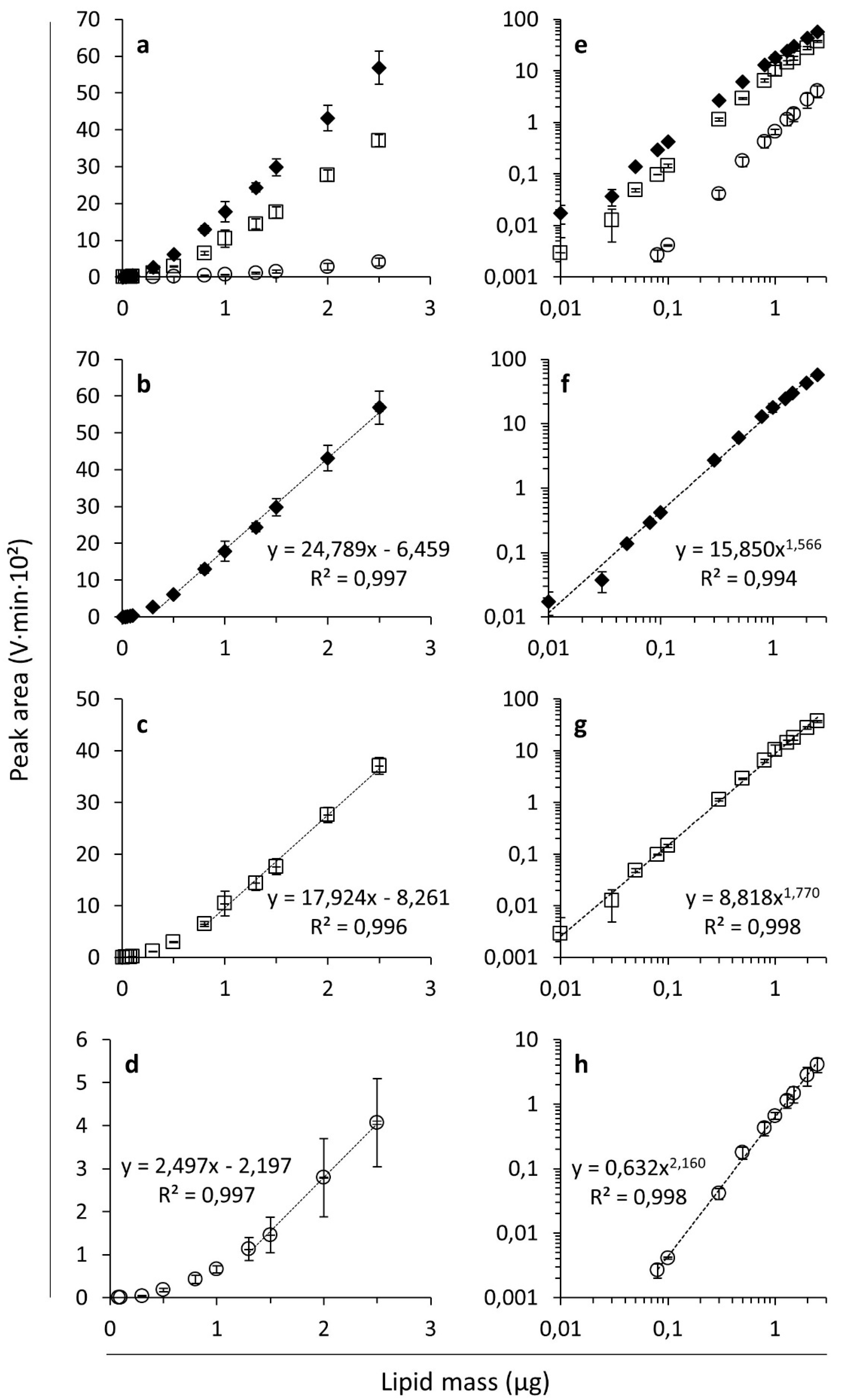
3.1. ELSD Responses of Different Simple Lipid Classes
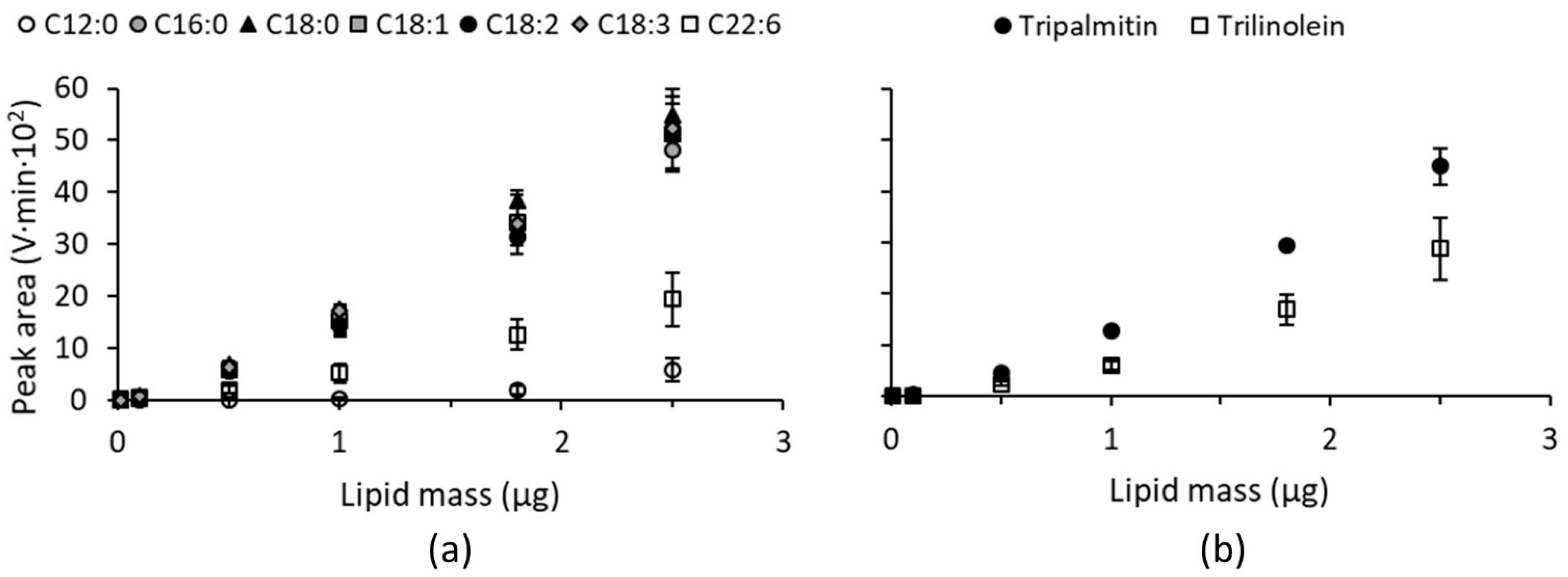
3.2. ELSD Responses of Different Simple Lipid Species Belonging to the Same Lipid Class
3.3. Fatty Acid Composition of Flour from Near-Isogenic Wheat Lines
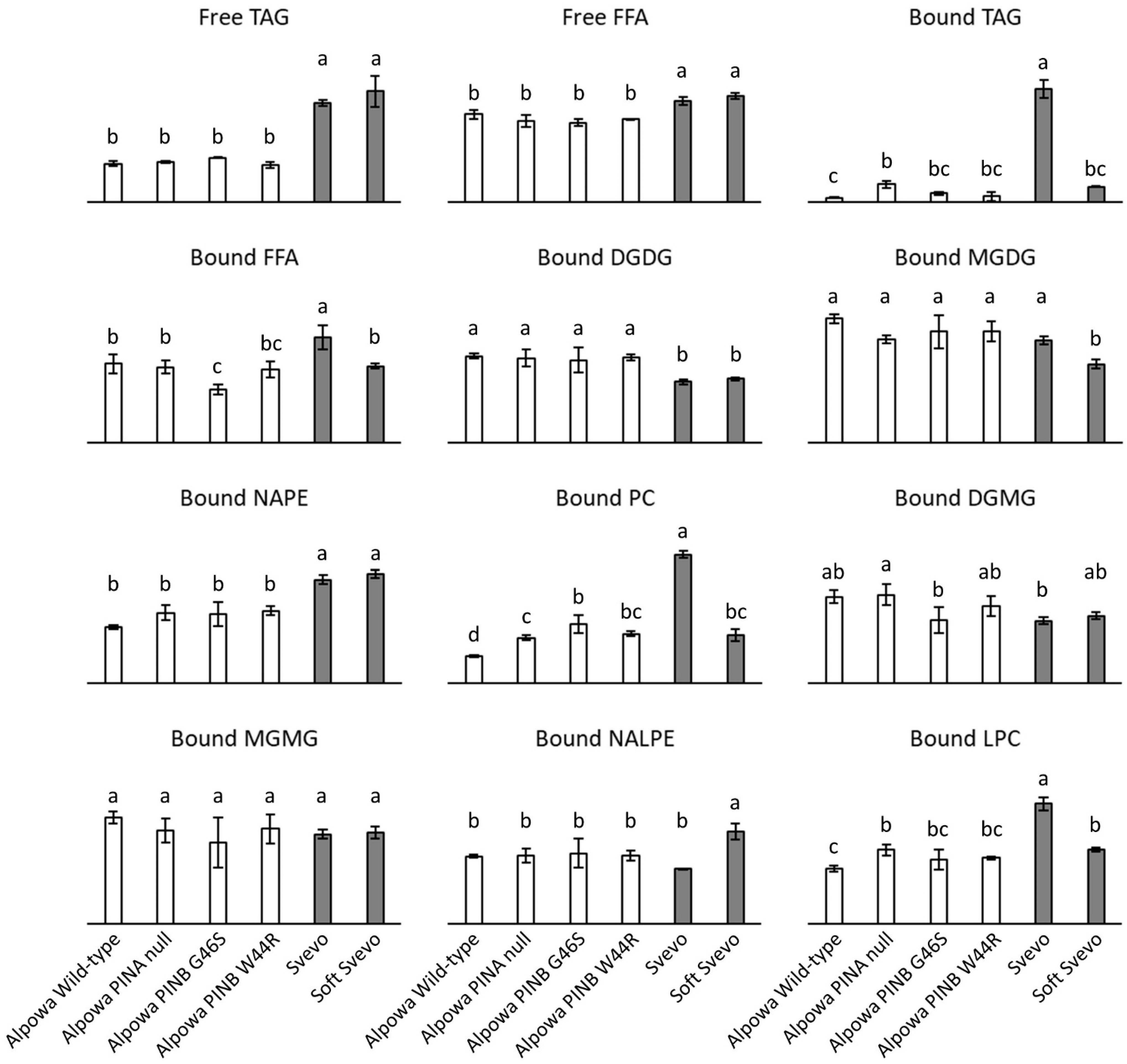
3.4. Lipid Composition of Flour from Near-Isogenic Wheat Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christie, W.W. AOCS Lipid Library. Available online: http://lipidlibrary.aocs.org/ (accessed on 10 January 2021).
- Christie, W.W.; Han, X. Lipid Analysis—Isolation, Separation, Identification and Lipidomic Analysis, 4th ed.; The Oily Press: Bridgewater, UK, 2010; Volume 24, ISBN 978-0-9552512-4-5. [Google Scholar]
- Melis, S.; Delcour, J.A. Impact of wheat endogenous lipids on the quality of fresh bread: Key terms, concepts, and underlying mechanisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3715–3754. [Google Scholar] [CrossRef]
- Chung, O.K.; Ohm, J.-B.; Ram, M.S.; Park, S.-H.; Howitt, C.A. Wheat lipids. In Wheat Chemistry and Technology; Khan, K., Shewry, P.R., Eds.; AACC International: St. Paul, MN, USA, 2009; pp. 363–399. ISBN 1891127551. [Google Scholar]
- Holčapek, M.; Liebisch, G.; Ekroos, K. Lipidomic analysis. Anal. Chem. 2018, 90, 4249–4257. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gómez, P.; Montero, O.; Fontecha, J. In-depth lipidomic analysis of molecular species of triacylglycerides, diacylglycerides, glycerophospholipids, and sphingolipids of buttermilk by GC-MS/FID, HPLC-ELSD, and UPLC-QToF-MS. Int. J. Mol. Sci. 2017, 18, 605. [Google Scholar] [CrossRef]
- González-Thuillier, I.; Salt, L.; Chope, G.; Penson, S.; Skeggs, P.; Tosi, P.; Powers, S.J.; Ward, J.L.; Wilde, P.; Shewry, P.R.; et al. Distribution of lipids in the grain of wheat (cv. Hereward) determined by lipidomic analysis of milling and pearling fractions. J. Agric. Food Chem. 2015, 63, 10705–10716. [Google Scholar] [CrossRef]
- Righetti, L.; Rubert, J.; Galaverna, G.; Hurkova, K.; Dall’Asta, C.; Hajslova, J.; Stranska-Zachariasova, M. A novel approach based on untargeted lipidomics reveals differences in the lipid pattern among durum and common wheat. Food Chem. 2018, 240, 775–783. [Google Scholar] [CrossRef]
- Sun, F.; Chen, H.; Chen, D.; Tan, H.; Huang, Y.; Cozzolino, D. Lipidomic changes in banana (Musa cavendish) during ripening and comparison of extraction by Folch and Bligh–Dyer methods. J. Agric. Food Chem. 2020, 68, 11309–11316. [Google Scholar] [CrossRef]
- Janssen, F.; Wouters, A.G.B.; Linclau, L.; Waelkens, E.; Derua, R.; Dehairs, J.; Moldenaers, P.; Vermant, J.; Delcour, J.A. The role of lipids in determining the air-water interfacial properties of wheat, rye, and oat dough liquor constituents. Food Chem. 2020, 319, 126565. [Google Scholar] [CrossRef]
- Hashemi, F.S.; Farzadnia, F.; Aghajani, A.; Ahmadzadeh NobariAzar, F.; Pezeshki, A. Conjugated linoleic acid loaded nanostructured lipid carrier as a potential antioxidant nanocarrier for food applications. Food Sci. Nutr. 2020, 8, 4185–4195. [Google Scholar] [CrossRef]
- Rohmah, M.; Choiri, S.; Raharjo, S.; Hidayat, C.; Martien, R. Palm stearin and olein binary mixture incorporated into nanostructured lipids carrier: Improvement food functionality for micronutrient delivery. J. Food Process. Preserv. 2020, 44, e14761. [Google Scholar] [CrossRef]
- Schjoerring-Thyssen, J.; Olsen, K.; Koehler, K.; Jouenne, E.; Rousseau, D.; Andersen, M.L. Morphology and structure of solid lipid nanoparticles loaded with high concentrations of β-carotene. J. Agric. Food Chem. 2019, 67, 12273–12282. [Google Scholar] [CrossRef]
- Martini, W.S.; Porto, B.L.S.; Oliveira, M.A.L.; de Sant’Ana, A.C. Comparative study of the lipid profiles of oils from kernels of peanut, babassu, coconut, castor and grape by GC-FID and Raman spectroscopy. J. Braz. Chem. Soc. 2018, 29, 390–397. [Google Scholar] [CrossRef]
- Velasco, J.; Morales-Barroso, A.; Ruiz-Méndez, M.V.; Márquez-Ruiz, G. Quantitative determination of major oxidation products in edible oils by direct NP-HPLC-DAD analysis. J. Chromatogr. A 2018, 1547, 62–70. [Google Scholar] [CrossRef]
- Yurchenko, S.; Sats, A.; Tatar, V.; Kaart, T.; Mootse, H.; Jõudu, I. Fatty acid profile of milk from Saanen and Swedish Landrace goats. Food Chem. 2018, 254, 326–332. [Google Scholar] [CrossRef]
- Panayotova, V.; Merzdhanova, A.; Dobreva, D.A.; Zlatanov, M.; Makedonski, L. Lipids of Black Sea algae: Unveiling their potential for pharmaceutical and cosmetic applications. J. IMAB 2017, 23, 1747–1751. [Google Scholar] [CrossRef]
- Yang, Z.; Piironen, V.; Lampi, A.-M. Epoxy and hydroxy fatty acids as non-volatile lipid oxidation products in oat. Food Chem. 2019, 295, 82–93. [Google Scholar] [CrossRef]
- Señoráns, M.; Castejón, N.; Señoráns, F.J. Advanced extraction of lipids with DHA from Isochrysis galbana with enzymatic pre-treatment combined with pressurized liquids and ultrasound assisted extractions. Molecules 2020, 25, 3310. [Google Scholar] [CrossRef]
- Rosa, A.; Nieddu, M.; Masala, C.; Marincola, F.C.; Porcedda, S.; Piras, A. Waste salt from the manufacturing process of mullet bottarga as source of oil with nutritional and nutraceutical properties. J. Sci. Food Agric. 2020, 100, 5363–5372. [Google Scholar] [CrossRef]
- Donot, F.; Strub, C.; Fontana, A.; Jouy, N.; Delbes, C.; Gunata, Z.; Schorr-Galindo, S. Rapid analysis and quantification of major neutral lipid species and free fatty acids by HPLC-ELSD from microalgae. Eur. J. Lipid Sci. Technol. 2016, 118, 1550–1556. [Google Scholar] [CrossRef]
- Jones, J.; Manning, S.; Montoya, M.; Keller, K.; Poenie, M. Extraction of algal lipids and their analysis by HPLC and mass spectrometry. J. Am. Oil Chem. Soc. 2012, 89, 1371–1381. [Google Scholar] [CrossRef]
- Rombaut, R.; Dewettinck, K.; Van Camp, J. Phospho- and sphingolipid content of selected dairy products as determined by HPLC coupled to an evaporative light scattering detector (HPLC–ELSD). J. Food Compos. Anal. 2007, 20, 308–312. [Google Scholar] [CrossRef]
- Rodríguez-Alcalá, L.M.; Fontecha, J. Major lipid classes separation of buttermilk, and cows, goats and ewes milk by high performance liquid chromatography with an evaporative light scattering detector focused on the phospholipid fraction. J. Chromatogr. A 2010, 1217, 3063–3066. [Google Scholar] [CrossRef] [PubMed]
- Contarini, G.; Pelizzola, V.; Scurati, S.; Povolo, M. Polar lipid of donkey milk fat: Phospholipid, ceramide and cholesterol composition. J. Food Compos. Anal. 2017, 57, 16–23. [Google Scholar] [CrossRef]
- Mourey, T.H.; Oppenheimer, L.E. Principles of operation of an evaporative light-scattering detector for liquid chromatography. Anal. Chem. 1984, 56, 2427–2434. [Google Scholar] [CrossRef]
- Stolyhwo, A.; Colin, H.; Martin, M.; Guiochon, G. Study of the qualitative and quantitative properties of the light-scattering detector. J. Chromatogr. A 1984, 288, 253–275. [Google Scholar] [CrossRef]
- Oppenheimer, L.E.; Mourey, T.H. Examination of the concentration response of evaporative light-scattering mass detectors. J. Chromatogr. A 1985, 323, 297–304. [Google Scholar] [CrossRef]
- Christie, W.W. Rapid separation and quantification of lipid classes by high performance liquid chromatography and mass (light-scattering) detection. J. Lipid Res. 1985, 26, 507–512. [Google Scholar] [CrossRef]
- Gerits, L.R.; Pareyt, B.; Masure, H.G.; Delcour, J.A. A lipase based approach to understand the role of wheat endogenous lipids in bread crumb firmness evolution during storage. LWT Food Sci. Technol. 2015, 64, 874–880. [Google Scholar] [CrossRef]
- Graeve, M.; Janssen, D. Improved separation and quantification of neutral and polar lipid classes by HPLC–ELSD using a monolithic silica phase: Application to exceptional marine lipids. J. Chromatogr. B 2009, 877, 1815–1819. [Google Scholar] [CrossRef]
- Kobayashi, N.; Noel, E.A.; Barnes, A.; Rosenberg, J.; DiRusso, C.; Black, P.; Oyler, G.A. Rapid detection and quantification of triacylglycerol by HPLC–ELSD in Chlamydomonas reinhardtii and Chlorella strains. Lipids 2013, 48, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Gerits, L.R.; Pareyt, B.; Delcour, J.A. Single run HPLC separation coupled to evaporative light scattering detection unravels wheat flour endogenous lipid redistribution during bread dough making. LWT Food Sci. Technol. 2013, 53, 426–433. [Google Scholar] [CrossRef]
- Gerits, L.R.; Pareyt, B.; Delcour, J.A. A lipase based approach for studying the role of wheat lipids in bread making. Food Chem. 2014, 156, 190–196. [Google Scholar] [CrossRef]
- De Brier, N.; Delcour, J.A. Pearling affects the lipid content and composition and lipase activity levels of wheat (Triticum aestivum L.) roller milling fractions. Cereal Chem. 2017, 94, 588–593. [Google Scholar] [CrossRef]
- Bosmans, G.M.; Peene, L.J.; Van Haesendonck, I.; Brijs, K.; Delcour, J.A. Impact of chlorine treatment on properties of wheat flour and its components in the presence of sucrose. Food Chem. 2019, 274, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Pycarelle, S.C.; Winnen, K.L.J.; Bosmans, G.M.; Van Haesendonck, I.; Pareyt, B.; Brijs, K.; Delcour, J.A. Wheat (Triticum aestivum L.) flour free lipid fractions negatively impact the quality of sponge cake. Food Chem. 2019, 271, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Van Wayenbergh, E.; Struyf, N.; Rezaei, M.N.; Sagalowicz, L.; Bel-Rhlid, R.; Moccand, C.; Courtin, C.M. Cereal bran protects vitamin A from degradation during simmering and storage. Food Chem. 2020, 331, 127292. [Google Scholar] [CrossRef]
- Pauly, A.; Pareyt, B.; De Brier, N.; Delcour, J.A. Incubation of isolated wheat starch with proteolytic or lipolytic enzymes and different extraction media reveals a tight interaction between puroindolines and lipids at its granule surface. Cereal Chem. 2014, 91, 240–246. [Google Scholar] [CrossRef]
- Melis, S.; Pauly, A.; Gerits, L.R.; Pareyt, B.; Delcour, J.A. Lipases as processing aids in the separation of wheat flour into gluten and starch: Impact on the lipid population, gluten agglomeration, and yield. J. Agric. Food Chem. 2017, 65, 1932–1940. [Google Scholar] [CrossRef]
- Melis, S.; Meza Morales, W.R.; Delcour, J.A. Lipases in wheat flour bread making: Importance of an appropriate balance between wheat endogenous lipids and their enzymatically released hydrolysis products. Food Chem. 2019, 298, 125002. [Google Scholar] [CrossRef]
- Melis, S.; Verbauwhede, B.C.; Van de Vondel, J.; Meza Morales, W.R.; Delcour, J.A. Do puroindolines affect the impact of enzymatic lipid hydrolysis on loaf volume in bread making? Food Chem. 2019, 301, 125273. [Google Scholar] [CrossRef]
- Pycarelle, S.C.; Bosmans, G.M.; Nys, H.; Brijs, K.; Delcour, J.A. Stabilization of the air-liquid interface in sponge cake batter by surface-active proteins and lipids: A foaming protocol based approach. Food Hydrocoll. 2020, 101, 105548. [Google Scholar] [CrossRef]
- Janssen, F.; Wouters, A.G.B.; Pauly, A.; Delcour, J.A. Relating the composition and air/water interfacial properties of wheat, rye, barley, and oat dough liquor. Food Chem. 2018, 264, 126–134. [Google Scholar] [CrossRef]
- Gerits, L.R.; Pareyt, B.; Masure, H.G.; Delcour, J.A. Native and enzymatically modified wheat (Triticum aestivum L.) endogenous lipids in bread making: A focus on gas cell stabilization mechanisms. Food Chem. 2015, 172, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.; Solgadi, A.; Chaminade, P. Optimization of normal phase chromatographic conditions for lipid analysis and comparison of associated detection techniques. J. Chromatogr. A 2017, 1514, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Schaffarczyk, M.; Østdal, H.; Koehler, P. Lipases in wheat breadmaking: Analysis and functional effects of lipid reaction products. J. Agric. Food Chem. 2014, 62, 8229–8237. [Google Scholar] [CrossRef] [PubMed]
- Gerits, L.R.; Pareyt, B.; Decamps, K.; Delcour, J.A. Lipases and their functionality in the production of wheat-based food systems. Compr. Rev. Food Sci. Food Saf. 2014, 13, 978–989. [Google Scholar] [CrossRef]
- Morris, C.F.; Simeone, M.C.; King, G.E.; Lafiandra, D. Transfer of soft kernel texture from Triticum aestivum to durum wheat, Triticum turgidum ssp. durum. Crop Sci. 2011, 51, 114–122. [Google Scholar] [CrossRef]
- Morris, C.F.; King, G.E. Registration of hard kernel puroindoline allele near-isogenic line hexaploid wheat genetic stocks. J. Plant Regist. 2008, 2, 67–68. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an analytical procedure for extraction of lipids from microalgae. J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Gheysen, L.; Bernaerts, T.; Bruneel, C.; Goiris, K.; Van Durme, J.; Van Loey, A.; De Cooman, L.; Foubert, I. Impact of processing on n-3 LC-PUFA in model systems enriched with microalgae. Food Chem. 2018, 268, 441–450. [Google Scholar] [CrossRef]
- Piironen, V.; Toivo, J.; Lampi, A.-M. Plant sterols in cereals and cereal products. Cereal Chem. 2002, 79, 148–154. [Google Scholar] [CrossRef]
- Ramos, R.G.; Libong, D.; Rakotomanga, M.; Gaudin, K.; Loiseau, P.M.; Chaminade, P. Comparison between charged aerosol detection and light scattering detection for the analysis of Leishmania membrane phospholipids. J. Chromatogr. A 2008, 1209, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Megoulas, N.C.; Koupparis, M.A. Twenty Years of Evaporative Light Scattering Detection. Crit. Rev. Anal. Chem. 2005, 35, 301–316. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J. The Lipid Handbook, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Krog, N. Theoretical aspects of surfactants in relation to their use in breadmaking. Cereal Chem. 1981, 58, 158–164. [Google Scholar]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9928021571035961-4987-5428-71-4987-5429-5. [Google Scholar]
- ChemSpider ChemSpider ID 3756, 960, 5091, 393217, 4444105, 4444437, 7923, 4444561, 393183, 10674, 10673, 4593733 and 4479674. Available online: http://www.chemspider.com/ (accessed on 10 January 2021).
- Pauly, A.; Pareyt, B.; Fierens, E.; Delcour, J.A. Wheat (Triticum aestivum L. and T. turgidum L. ssp. durum) kernel hardness: I. Current view on the role of puroindolines and polar lipids. Compr. Rev. Food Sci. Food Saf. 2013, 12, 412–426. [Google Scholar]
- Finnie, S.M.; Jeannotte, R.; Morris, C.F.; Faubion, J.M. Variation in polar lipid composition among near-isogenic wheat lines possessing different puroindoline haplotypes. J. Cereal Sci. 2010, 51, 66–72. [Google Scholar] [CrossRef]
- Heinze, K.; Kiszonas, A.M.; Murray, J.C.; Morris, C.F.; Lullien-Pellerin, V. Puroindoline genes introduced into durum wheat reduce milling energy and change milling behavior similar to soft common wheats. J. Cereal Sci. 2016, 71, 183–189. [Google Scholar] [CrossRef]
- Pauly, A.; Pareyt, B.; Fierens, E.; Delcour, J.A. Wheat (Triticum aestivum L. and T. turgidum L. ssp. durum) kernel hardness: II. Implications for end-product quality and role of puroindolines therein. Compr. Rev. Food Sci. Food Saf. 2013, 12, 427–438. [Google Scholar] [CrossRef]
- Hargin, K.D.; Morrison, W.R. The distribution of acyl lipids in the germ, aleurone, starch and non-starch endosperm of four wheat varieties. J. Sci. Food Agric. 1980, 31, 877–888. [Google Scholar] [CrossRef]
- Huang, A.H.C. Oil bodies and oleosins in seeds. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 177–200. [Google Scholar] [CrossRef]
- Tzen, J.T.C.; Huang, A.H.C. Surface structure and properties of plant seed oil bodies. J. Cell Biol. 1992, 117, 327–335. [Google Scholar] [CrossRef]
- Blaszczak, W.; Fornal, J.; Amarowicz, R.; Pegg, R.B. Lipids of wheat, corn and potato starch. J. Food Lipids 2003, 10, 301–312. [Google Scholar] [CrossRef]
- Morrison, W.R.; Mann, D.L.; Soon, W.; Coventry, A.M. Selective extraction and quantitative analysis of non-starch and starch lipids from wheat flour. J. Sci. Food Agric. 1975, 26, 507–521. [Google Scholar] [CrossRef]
- Morrison, W.R.; Law, R.V.; Snape, C.E. Evidence for inclusion complexes of lipids with V-amylose in maize, rice and oat starch. J. Cereal Sci. 1993, 18, 107–109. [Google Scholar] [CrossRef]
- Lin, J.; Turner, C.; Liao, L.P.; McKeon, T.A. Identification and quantification of the molecular species of acylglycerols in castor oil by HPLC using ELSD. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 773–780. [Google Scholar] [CrossRef]
- Lin, J. HPLC separation of acyl lipid classes. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 2005–2020. [Google Scholar] [CrossRef]
- Morrison, W.R. Wheat lipid composition. Cereal Chem. 1978, 55, 548–558. [Google Scholar]
- Min, B.; González-Thuillier, I.; Powers, S.J.; Wilde, P.; Shewry, P.R.; Haslam, R.P. Effects of cultivar and nitrogen nutrition on the lipid composition of wheat flour. J. Agric. Food Chem. 2017, 65, 5427–5434. [Google Scholar] [CrossRef] [PubMed]
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Development and validation of a novel free fatty acid butyl ester gas chromatography method for the determination of free fatty acids in dairy products. J. Agric. Food Chem. 2019, 67, 499–506. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, F.; Xu, S.; Wu, B.; Zheng, C.; Lv, X.; Wu, Z.; Chen, H.; Huang, F. Profiling and quantification of lipids in cold-pressed rapeseed oils based on direct infusion electrospray ionization tandem mass spectrometry. Food Chem. 2019, 285, 194–203. [Google Scholar] [CrossRef]
- Serafim, V.; Tiugan, D.-A.; Andreescu, N.; Mihailescu, A.; Paul, C.; Velea, I.; Puiu, M.; Niculescu, M.D. Development and validation of a LC−MS/MS-based assay for quantification of free and total omega 3 and 6 fatty acids from human plasma. Molecules 2019, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Kokotou, M.G.; Mantzourani, C.; Babaiti, R.; Kokotos, G. Study of the royal jelly free fatty acids by liquid chromatography-high resolution mass spectrometry (LC-HRMS). Metabolites 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
| Lipid | Molecular Weight (g/mol) | Boiling Point * (°C) | Refractive Index | Density (g/cm³) | |
|---|---|---|---|---|---|
| FFAs | Lauric acid | 200.32 | 319 e; 331 a | 1.4183 ° | 0.868 °; 0.883 a,e |
| Palmitic acid | 256.42 | 351 °; 373 c; 391 a | 1.4273 a 1.4335 ° | 0.853 °,a | |
| Stearic acid | 284.48 | 371 °; 413 a | 1.4299 ° | 0.941 °,a | |
| Oleic acid | 282.46 | 360 °; 390 a; 468–469 b | 1.4582 °,a | 0.887 a; 0.894 ° | |
| Linoleic acid | 280.45 | 408 a | 1.4699 °,a | 0.902 °,a,e | |
| Linolenic acid | 278.43 | / | 1.4800 ° | 0.916 °,d | |
| Behenic acid | 340.58 | 440 a | 1.4270 ° | 0.822 ° | |
| Erucic acid | 338.57 | 457 a | 1.4758 ° | 0.860 ° | |
| Cervonic acid | 328.49 | / | / | / | |
| TAGs | Tripalmitin | 807.32 | 624 ° | 1.4381 ° | 0.875 ° |
| Tristearin | 891.48 | / | 1.4395 ° | 0.856 ° | |
| Triolein | 885.43 | 409–416 c | 1.4676 ° | 0.915 ° | |
| Trilinolein | 879.38 | / | / | 0.925 d | |
| Lipid | Linear Trend Line a | Power Trend Line | Polynomial Trend Line | Retention Time b (min) | ||||
|---|---|---|---|---|---|---|---|---|
| Equation | R² | Equation | R² | Equation | R² | |||
| FFAs | Lauric acid | y = 2.787x – 2.020 | 0.888 | y = 0.585x0.855 | 0.647 | y = 1.419x² - 1.386x + 0.182 | 0.992 | 9.38 ± 0.02 A |
| Palmitic acid | y = 21.517x – 5.520 | 0.999 | y = 14.342x1.185 | 0.983 | y = 2.215x² + 14.363x − 0.894 | 0.998 | 9.40 ± 0.05 A | |
| Stearic acid | y = 24.384x – 5.961 | 0.999 | y = 16.889x1.150 | 0.986 | y = 2.447x² + 16.513x − 0.913 | 0.998 | 9.39 ± 0.02 A | |
| Oleic acid | y = 22.912x – 6.591 | 0.998 | y = 14.974x1.177 | 0.987 | y = 3.126x² + 13.148x − 0.679 | 0.999 | 9.39 ± 0.02 A | |
| Linoleic acid | y = 22.747x – 7.277 | 0.992 | y = 14.337x1.157 | 0.984 | y = 4.076x² + 10.411x − 0.305 | 1.000 | 9.39 ± 0.01 A | |
| Linolenic acid | y = 22.698x – 5.479 | 0.997 | y = 15.859x1.193 | 0.992 | y = 2.703x² + 14.366x − 0.576 | 0.999 | 9.40 ± 0.04 A | |
| Cervonic acid | y = 8.810x – 2.972 | 0.995 | y = 5.121x1.166 | 0.958 | y = 1.500x² + 4.179x − 0.241 | 0.999 | 9.39 ± 0.01 A | |
| TAGs | Tripalmitin | y = 20.345x – 6.634 | 0.997 | y = 12.091x1.359 | 0.995 | y = 3.177x² + 10.445x − 0.671 | 0.999 | 5.47 ± 0.02 C |
| Trilinolein | y = 13.429x – 5.975 | 0.981 | y = 6.424x1.396 | 0.986 | y = 3.445x² + 3.030x − 0.134 | 0.999 | 5.52 ± 0.01 B | |
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:1 | |
|---|---|---|---|---|---|---|
| Alpowa Wild-type | 20.4 ± 0.1 A | 0.9 ± 0.1 B | 10.7 ± 0.2 B | 64.8 ± 0.3 A | 2.8 ± 0.2 B | 0.3 ± 0.1 A |
| Alpowa PINA null | 19.5 ± 0.1 C | 1.1 ± 0.1 B | 11.0 ± 0.2 B | 65.3 ± 0.3 A | 3.0 ± 0.1 B | 0.3 ± 0.1 A |
| Alpowa PINB G46S | 19.5 ± 0.1 C | 1.0 ± 0.0 B | 11.3 ± 0.5 B | 64.8 ± 0.5 A | 3.0 ± 0.0 B | 0.3 ± 0.1 A |
| Alpowa PINB W44R | 20.3 ± 0.2 AB | 1.1 ± 0.1 B | 10.8 ± 0.1 B | 64.6 ± 0.1 A | 2.9 ± 0.1 B | 0.3 ± 0.1 A |
| Svevo | 19.5 ± 0.2 C | 1.6 ± 0.1 A | 14.1 ± 0.2 A | 60.9 ± 0.1 B | 3.5 ± 0.2 A | 0.4 ± 0.1 A |
| Soft Svevo | 19.9 ± 0.1 B | 1.6 ± 0.1 A | 13.4 ± 0.2 A | 61.1 ± 0.1 B | 3.5 ± 0.1 A | 0.4 ± 0.1 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melis, S.; Foubert, I.; Delcour, J.A. Normal-Phase HPLC-ELSD to Compare Lipid Profiles of Different Wheat Flours. Foods 2021, 10, 428. https://doi.org/10.3390/foods10020428
Melis S, Foubert I, Delcour JA. Normal-Phase HPLC-ELSD to Compare Lipid Profiles of Different Wheat Flours. Foods. 2021; 10(2):428. https://doi.org/10.3390/foods10020428
Chicago/Turabian StyleMelis, Sara, Imogen Foubert, and Jan A. Delcour. 2021. "Normal-Phase HPLC-ELSD to Compare Lipid Profiles of Different Wheat Flours" Foods 10, no. 2: 428. https://doi.org/10.3390/foods10020428
APA StyleMelis, S., Foubert, I., & Delcour, J. A. (2021). Normal-Phase HPLC-ELSD to Compare Lipid Profiles of Different Wheat Flours. Foods, 10(2), 428. https://doi.org/10.3390/foods10020428