The Cholesterol-Lowering Effect of Capsella Bursa-Pastoris Is Mediated via SREBP2 and HNF-1α-Regulated PCSK9 Inhibition in Obese Mice and HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Diet
2.3. Total and Low-Density Lipoprotein Cholesterol
2.4. HepG2 Cell Culture and Treatment
2.5. Cell Viability
2.6. ELISA
2.7. Western Blot
2.8. Quantitative Real-Time PCR
2.9. HPLC Analysis
2.10. Statistical Analysis
3. Results
3.1. Hypocholesterolemic Activity of CBE Was Attributed to Decreased PCSK9 Gene Expression in WD-Fed Obese Mice
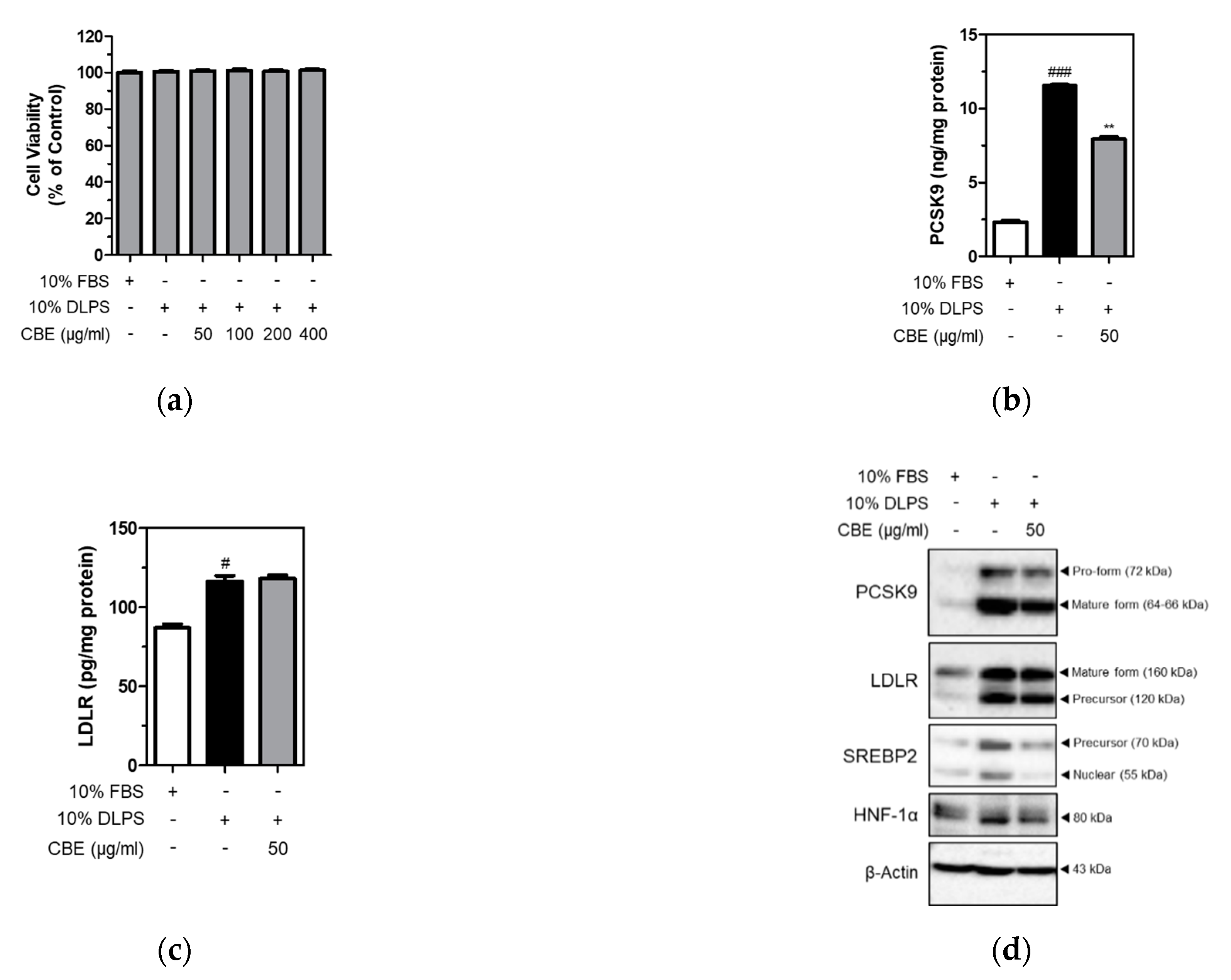
3.2. CBE Significantly and Markedly Suppressed the Levels of Intracellular PCSK9, but Not LDLR, in HepG2 Cells under Lipid Depletion Conditions
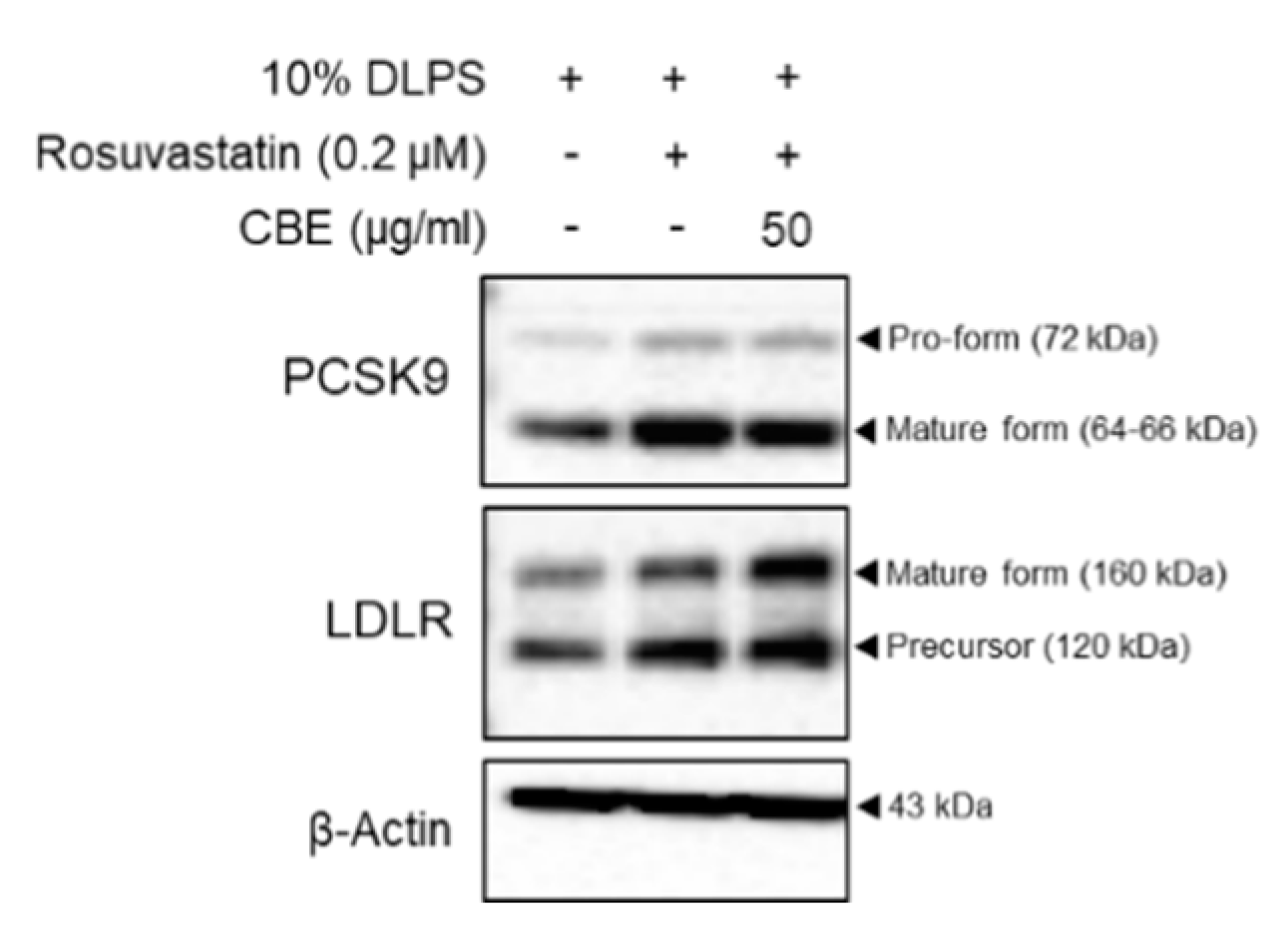
3.3. CBE Synergistically Enhanced LDLR by Inhibiting PCSK9 Protein Expression in HepG2 Cells with Statin Treatment
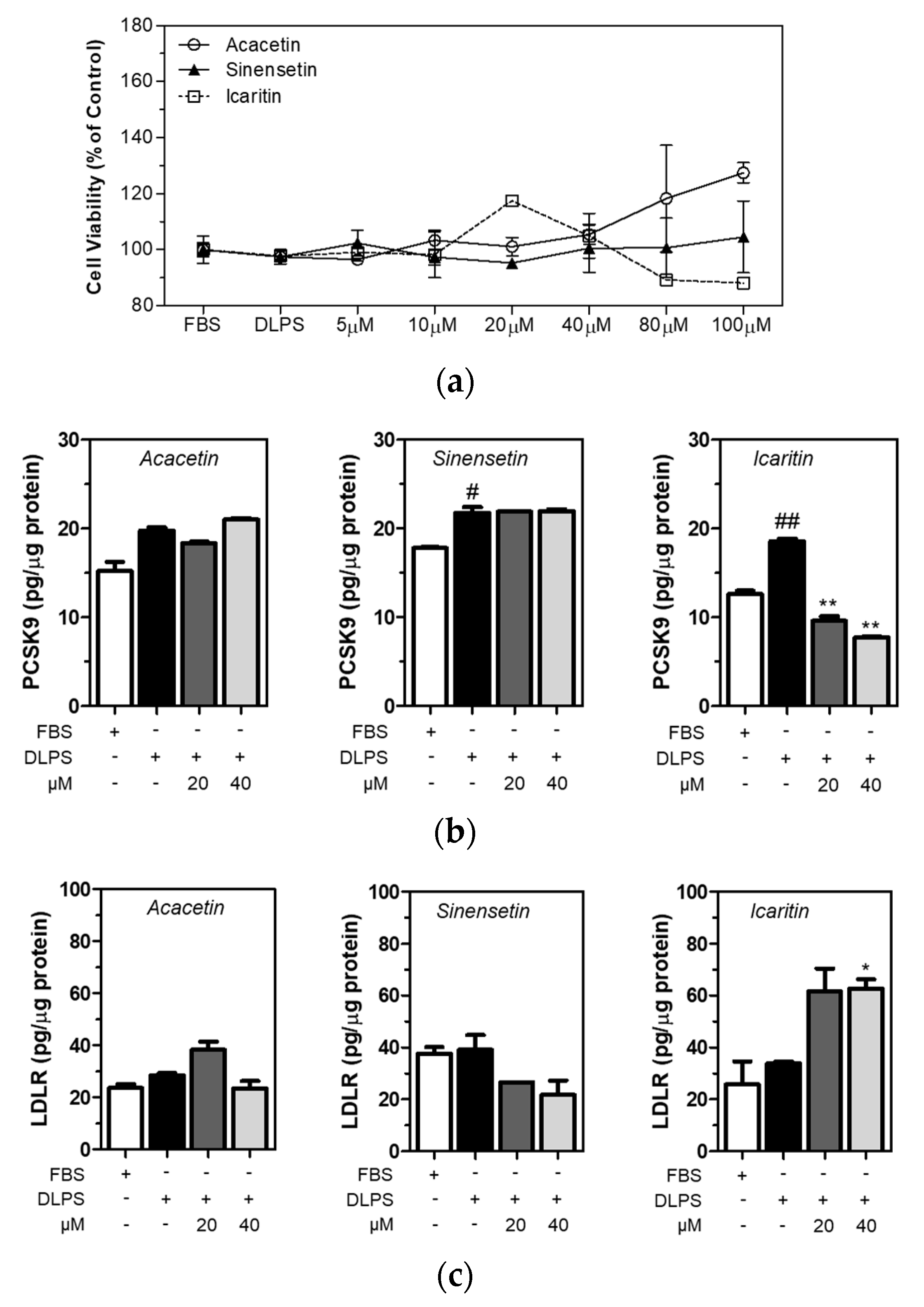
3.4. Icaritin Is the Most Active Compound in CBE in the Regulation of PCSK9 and LDLR in HepG2 Cells
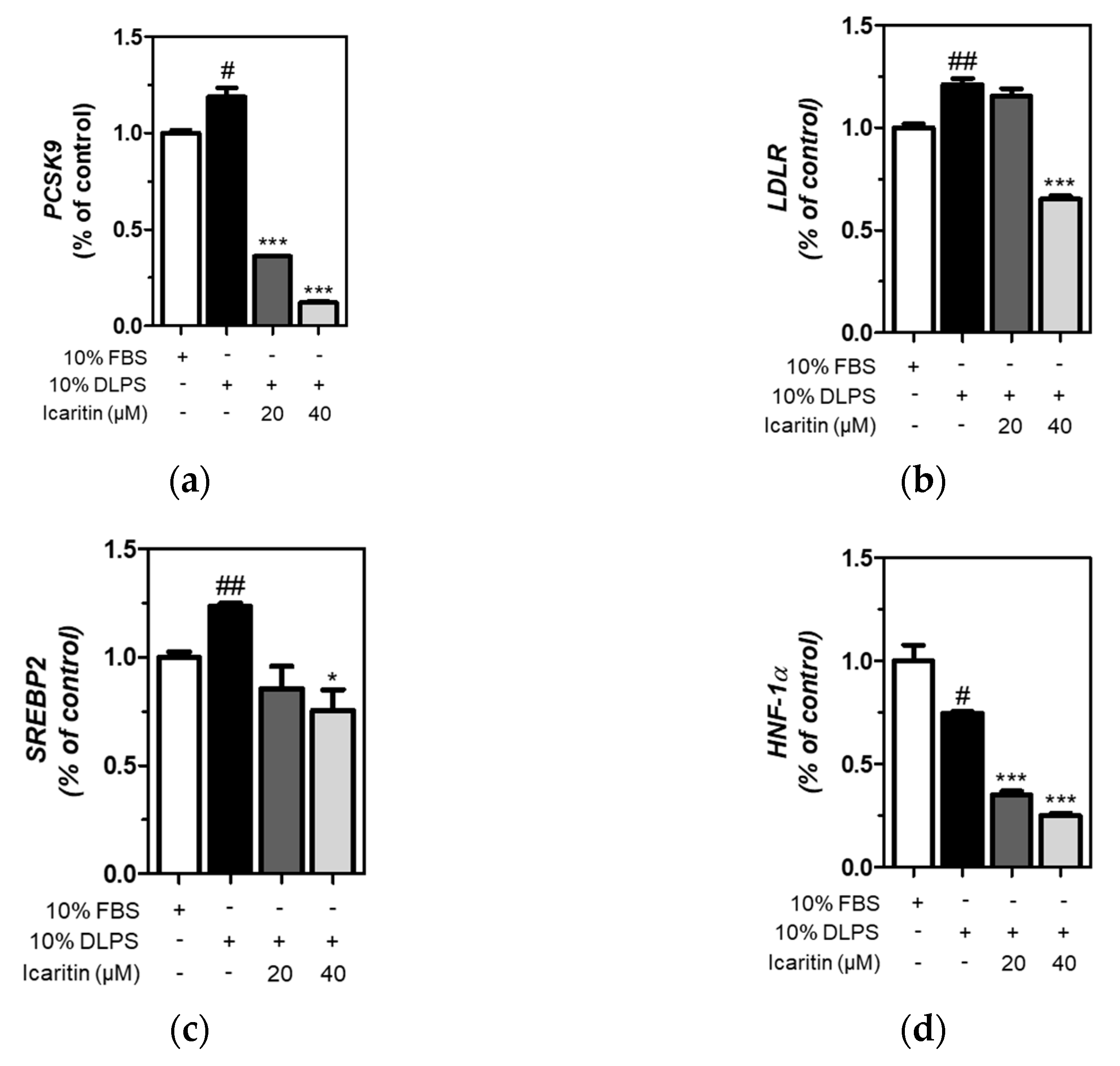
3.5. Icaritin Significantly Attenuated Gene Expression of PCSK9 and LDLR via SREBP2 and HNF-1α Downregulation
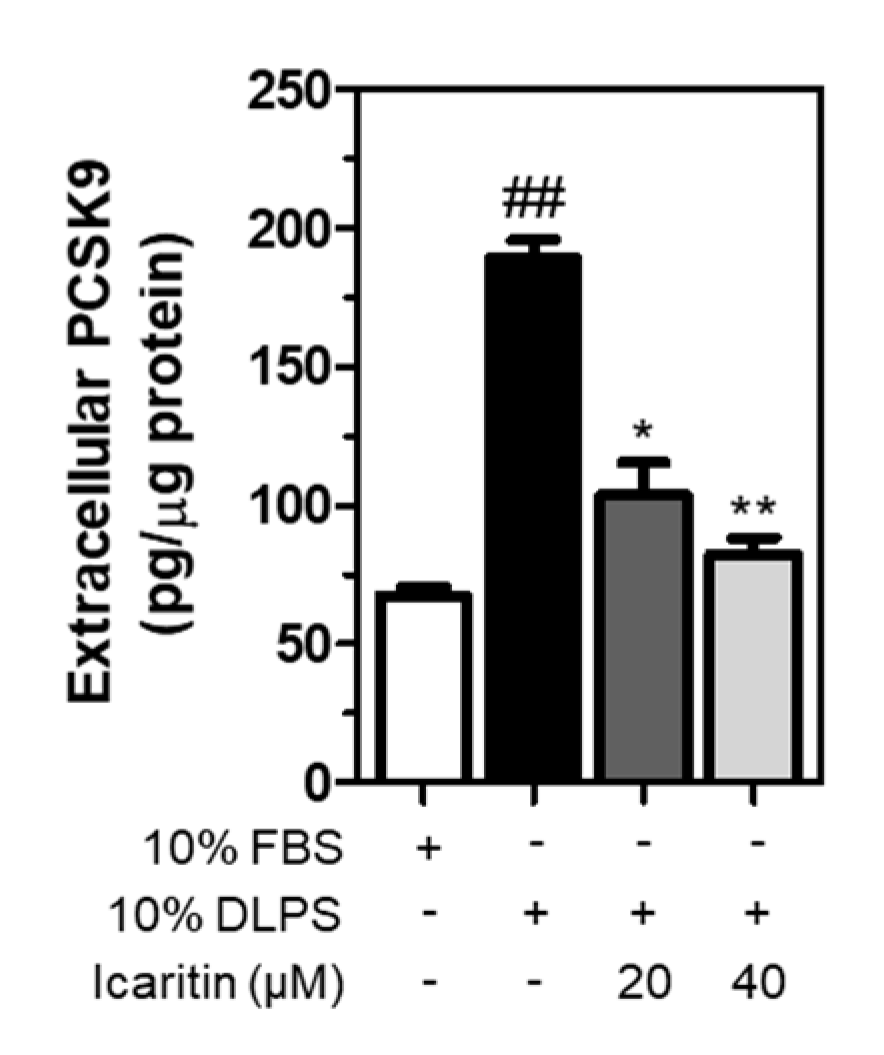
3.6. Icaritin Significantly Suppressed the Extracellular PCSK9 Level Increased by DLPS in HepG2 Cells
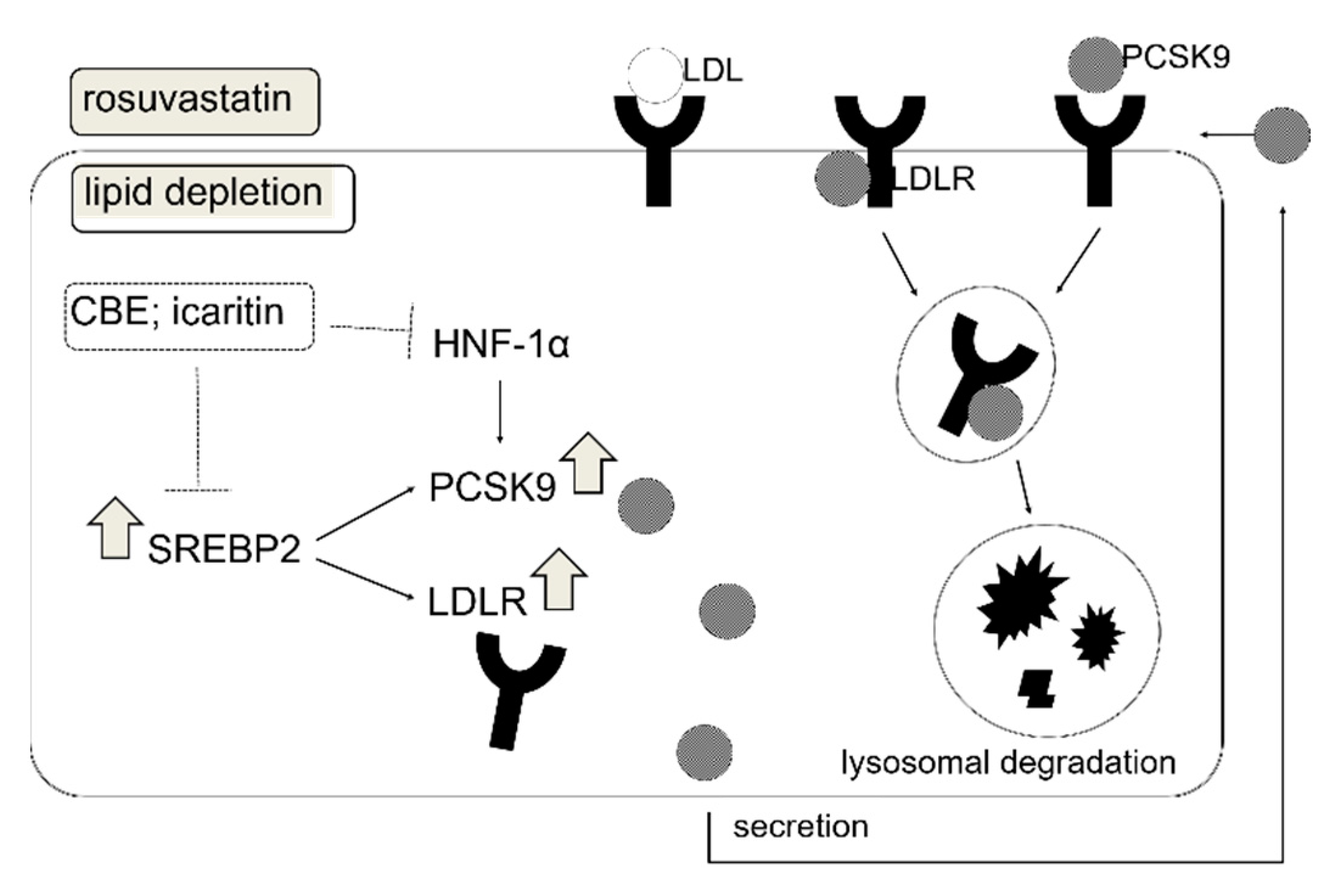
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart. J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Abifadel, M.; Varret, M.; Rabes, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Rashid, S.; Curtis, D.E.; Garuti, R.; Anderson, N.N.; Bashmakov, Y.; Ho, Y.K.; Hammer, R.E.; Moon, Y.A.; Horton, J.D. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA 2005, 102, 5374–5379. [Google Scholar] [CrossRef] [PubMed]
- Beglova, N.; Blacklow, S.C. The LDL receptor: How acid pulls the trigger. Trends Biochem. Sci. 2005, 30, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D. Inhibition of PCSK9 as a novel strategy for the treatment of hypercholesterolemia. Drug. News Perspect. 2008, 21, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G. PCSK9 as a therapeutic target of dyslipidemia. Expert. Opin. Ther. Targets 2009, 13, 19–28. [Google Scholar] [CrossRef]
- Cicero, A.F.; Tartagni, E.; Ertek, S. Efficacy and safety profile of evolocumab (AMG145), an injectable inhibitor of the proprotein convertase subtilisin/kexin type 9: The available clinical evidence. Expert. Opin. Biol. Ther. 2014, 14, 863–868. [Google Scholar] [CrossRef]
- Desai, N.R.; Kohli, P.; Giugliano, R.P.; O’Donoghue, M.L.; Somaratne, R.; Zhou, J.; Hoffman, E.B.; Huang, F.; Rogers, W.J.; Wasserman, S.M.; et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: An analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation 2013, 128, 962–969. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Roth, E.M.; Taskinen, M.R.; Ginsberg, H.N.; Kastelein, J.J.; Colhoun, H.M.; Robinson, J.G.; Merlet, L.; Pordy, R.; Baccara-Dinet, M.T. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: Results of a 24 week, double-blind, randomized Phase 3 trial. Int. J. Cardiol. 2014, 176, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Desai, N.R.; Kohli, P.; Rogers, W.J.; Somaratne, R.; Huang, F.; Liu, T.; Mohanavelu, S.; Hoffman, E.B.; McDonald, S.T.; et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): A randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet 2012, 380, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Wicinski, M.; Zak, J.; Malinowski, B.; Popek, G.; Grzesk, G. PCSK9 signaling pathways and their potential importance in clinical practice. EPMA J. 2017, 8, 391–402. [Google Scholar] [CrossRef]
- Peng, J.; Hu, T.; Li, J.; Du, J.; Zhu, K.; Cheng, B.; Li, K. Shepherd’s Purse Polyphenols Exert Its Anti-Inflammatory and Antioxidative Effects Associated with Suppressing MAPK and NF-kappaB Pathways and Heme Oxygenase-1 Activation. Oxid. Med. Cell Longev. 2019, 2019, 7202695. [Google Scholar] [CrossRef]
- Choi, H.K.; Shin, E.J.; Park, S.J.; Hur, H.J.; Park, J.H.; Chung, M.Y.; Kim, M.S.; Hwang, J.T. Ethanol Extract of Capsella bursa-pastoris Improves Hepatic Steatosis Through Inhibition of Histone Acetyltransferase Activity. J. Med. Food. 2017, 20, 251–257. [Google Scholar] [CrossRef]
- Chung, M.Y.; Shin, E.J.; Choi, H.K.; Kim, S.H.; Sung, M.J.; Park, J.H.; Hwang, J.T. Schisandra chinensis berry extract protects against steatosis by inhibiting histone acetylation in oleic acid-treated HepG2 cells and in the livers of diet-induced obese mice. Nutr. Res. 2017, 46, 1–10. [Google Scholar] [CrossRef]
- Hannah, V.C.; Ou, J.; Luong, A.; Goldstein, J.L.; Brown, M.S. Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. J. Biol. Chem. 2001, 276, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Hwang, J.T.; Nam, T.G.; Kim, S.H.; Min, D.K.; Park, S.W.; Chung, M.Y. Welsh onion extract inhibits PCSK9 expression contributing to the maintenance of the LDLR level under lipid depletion conditions of HepG2 cells. Food. Funct. 2017, 8, 4582–4591. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, J.Y.; Chung, M.Y.; Park, Y.K.; Bower, A.M.; Koo, S.I.; Giardina, C.; Bruno, R.S. Green tea extract suppresses NFkappaB activation and inflammatory responses in diet-induced obese rats with nonalcoholic steatohepatitis. J. Nutr. 2012, 142, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Guo, Y.; Wei, R.; Sang, Z.; Liu, W.; Gao, L.; Liu, T. Flavonoids from Capsella bursa-pastoris and their hepatoprotective activities in vitro. Revista Brasileira de Farmacognosia. 2016, 26, 710–713. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Hobbs, H.H.; Cohen, J.C. Molecular characterization of proprotein convertase subtilisin/kexin type 9-mediated degradation of the LDLR. J. Lipid Res. 2012, 53, 1932–1943. [Google Scholar] [CrossRef]
- Yang, H.X.; Zhang, M.; Long, S.Y.; Tuo, Q.H.; Tian, Y.; Chen, J.X.; Zhang, C.P.; Liao, D.F. Cholesterol in LDL receptor recycling and degradation. Clin. Chim. Acta. 2020, 500, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Lee, H.S.; Kim, K.S.; Kim, Y.K.; Yoon, D.; Park, S.W. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J. Lipid Res. 2008, 49, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997, 89, 331–340. [Google Scholar] [CrossRef]
- Costet, P.; Cariou, B.; Lambert, G.; Lalanne, F.; Lardeux, B.; Jarnoux, A.L.; Grefhorst, A.; Staels, B.; Krempf, M. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J. Biol. Chem. 2006, 281, 6211–6218. [Google Scholar] [CrossRef]
- Li, H.; Dong, B.; Park, S.W.; Lee, H.S.; Chen, W.; Liu, J. Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 2009, 284, 28885–28895. [Google Scholar] [CrossRef]
- Dong, B.; Wu, M.; Li, H.; Kraemer, F.B.; Adeli, K.; Seidah, N.G.; Park, S.W.; Liu, J. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: Mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J. Lipid Res. 2010, 51, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, M.J.; Cirillo, A.; Orsatti, L.; Ruggeri, L.; Fisher, T.S.; Santoro, J.C.; Cummings, R.T.; Cubbon, R.M.; Lo Surdo, P.; Calzetta, A.; et al. Structural and biochemical characterization of the wild type PCSK9-EGF(AB) complex and natural familial hypercholesterolemia mutants. J. Biol. Chem. 2009, 284, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Yu, L.; Yu, X.; Qian, Y.W.; Cao, G.; Wang, J. Role of ubiquitination in PCSK9-mediated low-density lipoprotein receptor degradation. Biochem. Biophys. Res. Commun. 2011, 415, 515–518. [Google Scholar] [CrossRef]
- Zhang, D.W.; Lagace, T.A.; Garuti, R.; Zhao, Z.; McDonald, M.; Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J. Biol. Chem. 2007, 282, 18602–18612. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.A.; Korhonen, L.; Mercer, E.A.; Lindholm, D. MIR is a novel ERM-like protein that interacts with myosin regulatory light chain and inhibits neurite outgrowth. J. Biol. Chem. 1999, 274, 36288–36292. [Google Scholar] [CrossRef] [PubMed]
- Zelcer, N.; Hong, C.; Boyadjian, R.; Tontonoz, P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 2009, 325, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Scotti, E.; Hong, C.; Yoshinaga, Y.; Tu, Y.; Hu, Y.; Zelcer, N.; Boyadjian, R.; de Jong, P.J.; Young, S.G.; Fong, L.G.; et al. Targeted disruption of the idol gene alters cellular regulation of the low-density lipoprotein receptor by sterols and liver x receptor agonists. Mol. Cell Biol. 2011, 31, 1885–1893. [Google Scholar] [CrossRef]
- van Loon, N.M.; Ottenhoff, R.; Kooijman, S.; Moeton, M.; Scheij, S.; Roscam Abbing, R.L.P.; Gijbels, M.J.J.; Levels, J.H.M.; Sorrentino, V.; Berbee, J.F.P.; et al. Inactivation of the E3 Ubiquitin Ligase IDOL Attenuates Diet-Induced Obesity and Metabolic Dysfunction in Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1785–1795. [Google Scholar] [CrossRef]
- Tai, M.H.; Chen, P.K.; Chen, P.Y.; Wu, M.J.; Ho, C.T.; Yen, J.H. Curcumin enhances cell-surface LDLR level and promotes LDL uptake through downregulation of PCSK9 gene expression in HepG2 cells. Mol. Nutr. Food Res. 2014, 58, 2133–2145. [Google Scholar] [CrossRef]
- Chang, H.Y.; Wu, J.R.; Gao, W.Y.; Lin, H.R.; Chen, P.Y.; Chen, C.I.; Wu, M.J.; Yen, J.H. The Cholesterol-Modulating Effect of Methanol Extract of Pigeon Pea (Cajanus cajan (L.) Millsp.) Leaves on Regulating LDLR and PCSK9 Expression in HepG2 Cells. Molecules 2019, 24, 493. [Google Scholar] [CrossRef]
- Dong, B.; Li, H.; Singh, A.B.; Cao, A.; Liu, J. Inhibition of PCSK9 transcription by berberine involves down-regulation of hepatic HNF1alpha protein expression through the ubiquitin-proteasome degradation pathway. J. Biol. Chem. 2015, 290, 4047–4058. [Google Scholar] [CrossRef]
- Chittur, S.V.; Sangster-Guity, N.; McCormick, P.J. Histone deacetylase inhibitors: A new mode for inhibition of cholesterol metabolism. BMC Genom. 2008, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.S.; Silva, G.D.B.; Pavan, A.R.; Chiba, D.E.; Chin, C.M.; Dos Santos, J.L. Epigenetic Regulatory Mechanisms Induced by Resveratrol. Nutrients 2017, 9, 1201. [Google Scholar] [CrossRef]
- Benassi, B.; Santi, C.; Santangeli, S.; Grollino, M.G.; Raschellà, G.; Bacchetta, L.; Pacchierotti, F. Modulation of LDL receptor expression and promoter methylation in HepG2 cells treated with a Corylus avellana L. extract. J. Funct. Foods. 2019, 53, 208–218. [Google Scholar] [CrossRef]
- Canuel, M.; Sun, X.; Asselin, M.C.; Paramithiotis, E.; Prat, A.; Seidah, N.G. Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1). PLoS ONE 2013, 8, e64145. [Google Scholar] [CrossRef] [PubMed]
| LD | WD | WD+1%CBE | |
|---|---|---|---|
| Total Cholesterol (mg/dL) | 94.85 ± 4.06 | 195.70 ± 7.24 ### | 145.50 ± 3.23 *** |
| LDL Cholesterol (mg/dL) | 12.69 ± 2.22 | 48.03 ± 6.77 ### | 24.00 ± 3.54 ** |
| LD | WD | WD+1%CBE | |
|---|---|---|---|
| PCSK9 | 2.45 ± 0.47 | 2.72 ± 0.57 | 0.94 ± 0.25 ** |
| LDLR | 1.37 ± 0.20 | 1.14 ± 0.20 | 1.35 ± 0.21 |
| SREBP2 | 2.27 ± 0.40 | 2.13 ± 0.35 | 1.38 ± 0.24 |
| HMGCR | 2.02 ± 0.24 | 2.04 ± 0.27 | 1.19 ± 0.23 * |
| Polyphenolic Compounds | Concentration |
|---|---|
| Acacetin | 24.4 ± 1.2 |
| Sinensetin | 106.6 ± 8.6 |
| Icaritin | 17.5 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-T.; Choi, E.; Choi, H.-K.; Park, J.-H.; Chung, M.-Y. The Cholesterol-Lowering Effect of Capsella Bursa-Pastoris Is Mediated via SREBP2 and HNF-1α-Regulated PCSK9 Inhibition in Obese Mice and HepG2 Cells. Foods 2021, 10, 408. https://doi.org/10.3390/foods10020408
Hwang J-T, Choi E, Choi H-K, Park J-H, Chung M-Y. The Cholesterol-Lowering Effect of Capsella Bursa-Pastoris Is Mediated via SREBP2 and HNF-1α-Regulated PCSK9 Inhibition in Obese Mice and HepG2 Cells. Foods. 2021; 10(2):408. https://doi.org/10.3390/foods10020408
Chicago/Turabian StyleHwang, Jin-Taek, Eunji Choi, Hyo-Kyoung Choi, Jae-Ho Park, and Min-Yu Chung. 2021. "The Cholesterol-Lowering Effect of Capsella Bursa-Pastoris Is Mediated via SREBP2 and HNF-1α-Regulated PCSK9 Inhibition in Obese Mice and HepG2 Cells" Foods 10, no. 2: 408. https://doi.org/10.3390/foods10020408
APA StyleHwang, J.-T., Choi, E., Choi, H.-K., Park, J.-H., & Chung, M.-Y. (2021). The Cholesterol-Lowering Effect of Capsella Bursa-Pastoris Is Mediated via SREBP2 and HNF-1α-Regulated PCSK9 Inhibition in Obese Mice and HepG2 Cells. Foods, 10(2), 408. https://doi.org/10.3390/foods10020408







