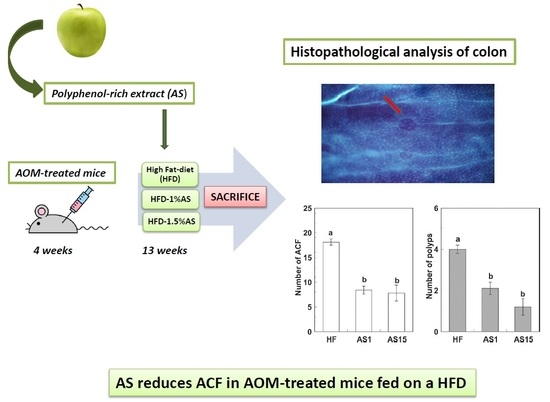
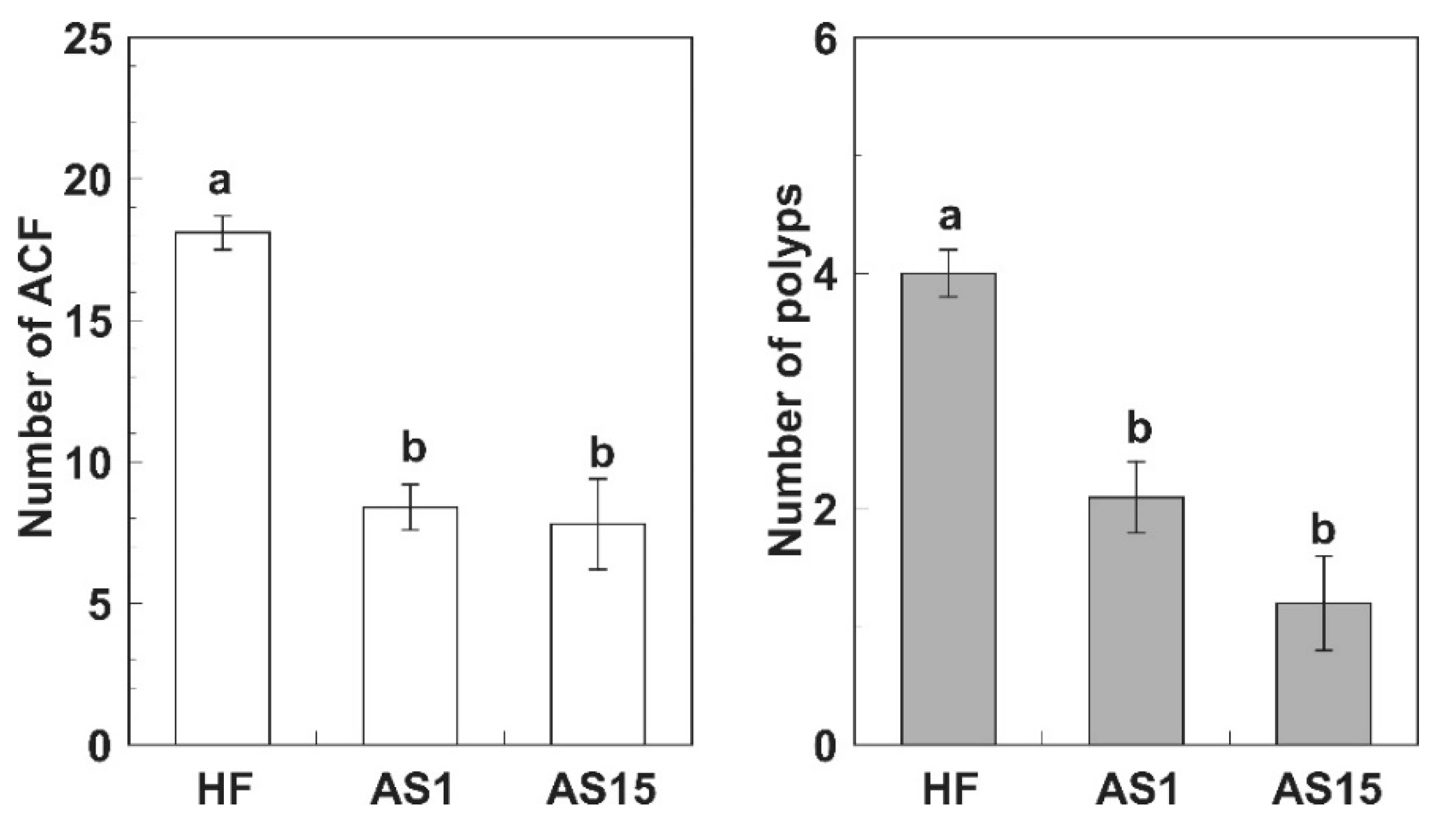
Azoxymethane-Induced Colorectal Cancer Mice Treated with a Polyphenol-Rich Apple Extract Show Less Neoplastic Lesions and Signs of Cachexia
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Phenolic Compounds by HPLC
2.2. Animals and Treatments
2.3. Indirect Calorimetry
2.4. Enzyme Activities
2.5. Histopathological Assessment
2.6. Fatty Acid Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tárrega López, P.J.T.; Solera Albero, J.S.; Rodríguez-Montes, J.A. Primary and secondary prevention of colorectal cancer. Clin. Med. Insights Gastroenterol. 2014, 7, 33–46. [Google Scholar]
- Corpet, D.E.; Pierre, F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur. J. Cancer. 2005, 41, 1911–1922. [Google Scholar] [CrossRef]
- Nandan, M.O.; Yang, V.W. Genetic and chemical models of colorectal cancer in mice. Curr. Colorectal. Cancer Rep. 2010, 6, 51–59. [Google Scholar] [CrossRef]
- Perse, M.; Cerar, A. Morphological and molecular alterations in 1,2 Dimethylhidrazine and Azoxymethane induced colon carcinogenesis in rats. J. Biochem. Biotech. 2010, 2011. [Google Scholar] [CrossRef]
- Corpet, D.E.; Tache, S. Most effective colon cancer chemopreventive agents in rats: A systematic review of aberrant crypt foci and tumor data, ranked by potency. Nutr. Cancer 2002, 43, 1–21. [Google Scholar] [CrossRef] [PubMed]
- O´Neill, A.M.; Burrington, C.M.; Gillaspie, E.A.; Lynch, D.T.; Horsman, M.J.; Greene, M.W. Higf-fat Western diet-induced obesity contributes to increased tumor growth in mouse models of human colon cancer. Nutr. Res. 2016, 36, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Snider, A.J. Diet, Lipids and colon cancer. Int. Rev. Cell Mol. Biol. 2019, 347, 106–135, ISSN 1937-6448. [Google Scholar]
- Chen, J.; Huang, X.F. High fat diet-induced obesity increases the formation of colon polyps induced by azoxymethane in mice. Ann. Transl. Med. 2015, 3, 79. [Google Scholar]
- Lin, J.; Zhang, S.M.; Cook, N.R.; Lee, I.-M.; Buring, J.E. Dietary fat and fatty acids and risk of colorectal cancer in women. Am. J. Epidemiol. 2004, 160, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Matusiewicz, M.; Baczek, K.B.; Kosieradzka, I.; Niemiec, T.; Grodzik, M.; Szczepaniak, J.; Orlińska, S.; Węglarz, Z. Effect of juice and extracts of saposhnikovia divaricata root on the colon cancer Caco-2 cells. Int. J. Mol. Sci. 2019, 20, 4526. [Google Scholar] [CrossRef] [PubMed]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimentale vidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef]
- Rijken, P.J.; Timmer, W.G.; van de Kooij, A.J.; van Benschop, I.M.; Wiseman, S.A.; Meijers, M.; Tijburg, L.B. Effect of vegetable and carotenoid consumption on aberrant crypt multiplicity, a surrogate end-point marker for colorectal cancer in azoxymethane-induced rats. Carcinogenesis 1999, 20, 2267–2272. [Google Scholar] [CrossRef][Green Version]
- Gonzales, G.F.; Miranda, S.; Nieto, J.; Fernández, G.; Yucra, S.; Rubio, J.; Yi, P.; Gasco, M. Red maca (Lepidium meyenii) reduced prostate size in rats. Reprod. Biol. Endocrinol. 2005, 3, 5. [Google Scholar] [CrossRef][Green Version]
- Kim, H.-A.; Jeong, K.-S.; Kim, Y.K. Soy extract is more potent than genistein on tumor growth inhibition. Anticancer Res. 2008, 28, 2837–2841. [Google Scholar] [PubMed]
- Niedzwiecki, A.; Waheed Roomi, M.; Kalinovsky, T.; Matthias, R. Aticancer effects of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Lambert, J.D.; Hong, J.; Yang, G.; Liao, J.; Yang, C.S. Inhibition of carcinogenesis by polyphenols: Evidence from laboratory investigations. Am. J. Clin. Nutr. 2005, 81, 284S–291S. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCg): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Bellion, P.; Olk, M.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Formation of hydrogen peroxide in cell culture media by apple polyphenols and its effect on antioxidant biomarkers in the colon cell line HT-29. Mol. Nutr. Food Res. 2009, 53, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Speisky, H.; Brunser, O.; Pastene, E.; Gotteland, M. Apple peel polyphenols protect against gastrointestinal mucosa alterations induced by indomethacin in rats. J. Agric. Food Chem. 2011, 59, 6459–6466. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, G.; Mazzone, G.; Tuccillo, C.; Ribecco, M.T.; Graziani, G.; Gravina, A.G.; Caserta, S.; Guido, S.; Fogliano, V.; Caporaso, N.; et al. Apple polyphenols extract (APE) improves colon damage in a rat model of colitis. Digest. Liver Dis. 2012, 44, 555–562. [Google Scholar] [CrossRef]
- Femia, A.P.; Luceri, C.; Bianchini, F.; Salvadori, M.; Salvianti, F.; Pinzani, P.; Dolara, P.; Calorini, L.; Caderni, G. Marie Ménard apples with high polyphenol content and a low-fat diet reduce 1,2-dimethylhydrazine-induced colon carcinogenesis in rats: Effects on inflammation and apoptosis. Mol. Nutr. Food Res. 2012, 56, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Annema, N.; Heyworth, J.S.; McNaughton, S.A.; Iacopetta, B.; Fritschi, L. Fruit and vegetable consumption and the risk of proximal colon, distal colon, and rectal cancers in a case-control study in western Australia. J. Am. Diet Assoc. 2011, 111, 1479–1490. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Maugeri, U.; Popiela, T.; Kulig, J.; Sochacka-Tatara, E.; Pac, A.; Sowa, A.; Musial, A. Case–control study on beneficial effect of regular consumption of apples on colorectal cancer risk in a population with relatively low intake of fruits and vegetables. Eur. J. Cancer Prev. 2010, 19, 42–47. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.J.; Gill, C.I.R.; O’ Brien, G.; Rao, J.R.; McRoberts, W.C.; Hughes, P.; McEntee, R.; Rowland, I.R. Anti-cancer properties of phenolics from apple waste on colon carcinogenesis in vitro. Food Chem. Toxicol. 2007, 45, 1224–1230. [Google Scholar] [CrossRef]
- Fini, L.; Piazzi, G.; Daoud, Y.; Selgrad, M.; Maegawa, S.; Garcia, M.; Fogliano, V.; Romano, M.; Graziani, G.; Vitaglione, P.; et al. Chemoprevention of intestinal polyps in ApcMin/+ mice fed with western or balanced diets by drinking annurca apple polyphenol extract. Cancer Prev. Res. 2011, 4, 907–915. [Google Scholar] [CrossRef]
- Canadian Council on Animal Care, E. Guide to the Care and Use of Experimental Animals, 2nd ed.; Olfert, E., Cross, B., McWilliam, A., Eds.; Canadian Council on Animal Care: Ottawa, ON, Canada, 1993. [Google Scholar]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- García-Díaz, D.; Campion, J.; Milagro, F.; Lomba, A.; Marzo, F.; Martínez, J. Chronic mild stress induces variations in locomotive behavior and metabolic rates in high fat fed rats. J. Physiol. Biochem. 2007, 63, 337–346. [Google Scholar] [CrossRef]
- Shirazi-Beechey, S.P.; Davies, A.G.; Tebbutt, K.; Dyer, J.; Ellis, A.; Taylor, C.J.; Fairclough, P.; Beechey, R.B. Preparation and properties of brush-border membrane vesicles from human small intestine. Gastroenterology 1990, 98, 676–685. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Barrenetxe, J.; Aranguren, P.; Grijalba, A.; Martinez-Peñuela, J.M.; Marzo, F.; Urdaneta, E. Effect of dietary quercetin and sphingomyelin on intestinal nutrient absorption and animal growth. Br. J. Nutr. 2006, 95, 455–461. [Google Scholar] [CrossRef]
- Bird, R.P. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995, 93, 55–71. [Google Scholar] [CrossRef]
- Bird, R.P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: Preliminary findings. Cancer Lett. 1987, 37, 147–151. [Google Scholar] [CrossRef]
- Paulsen, J.E.; Løberg, E.M.; Ølstørn, H.B.; Knutsen, H.; Steffensen, I.-L.; Alexander, J. Flat dysplastic aberrant crypt foci are related to tumorigenesis in the colon of azoxymethane-treated rat. Cancer Res. 2005, 65, 121–129. [Google Scholar]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Tweed, J.K.S.; Kim, E.J.; Scollan, N.D. Beef, chicken and lamb fatty acid analysis—A simplified direct bimethylation procedure using freeze-dried material. Meat Sci. 2012, 92, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Pretlow, T.P.; Barrow, B.J.; Ashton, W.S.; O’Riordan, M.A.; Pretlow, T.G.; Jurcisek, J.A.; Stellato, T.A. Aberrant crypts: Putative preneoplastic foci in human colonic mucosa. Cancer Res. 1991, 51, 1564–1567. [Google Scholar] [PubMed]
- Roncucci, L.; Medline, A.; Bruce, W.R. Classification of aberrant crypt foci and microadenomas in human colon. Cancer Epidemiol. Biomark. Prev. 1991, 1, 57–60. [Google Scholar]
- Thorup, I. Histomorphological and immunohistochemical characterization of colonic aberrant crypt foci in rats: Relationship to growth factor expression. Carcinogenesis 1997, 18, 465–472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Femia, A.P.; Dolara, P.; Luceri, C.; Salvadori, M.; Caderni, G. Mucin-depleted foci show strong activation of inflammatory markers in 1,2-dimethylhydrazine-induced carcinogenesis and are promoted by the inflammatory agent sodium dextran sulfate. Int. J. Cancer 2009, 125, 541–547. [Google Scholar] [CrossRef]
- Ashokkumar, P.; Sudhandiran, G. Protective role of luteolin on the status of lipid peroxidation and antioxidant defense against azoxymethane-induced experimental colon carcinogenesis. Biomed Pharmacother. 2008, 62, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Lai, C.-S.; Wu, J.-C.; Ho, C.-T. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol. Nutr. Food Res. 2011, 55, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Nagaprashantha, L.D.; Singhai, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals; role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E. The theoretical bases of indirect calorimetry: A review. Metabolism 1988, 37, 287–301. [Google Scholar] [CrossRef]
- Stewart, L.K.; Soileau, J.L.; Ribnicky, D.; Wang, Z.Q.; Raskin, I.; Poulev, A.; Majewski, M.; Cefalu, W.T.; Gettys, T.W. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism 2008, 57 (Suppl. S1), 39–46. [Google Scholar] [CrossRef]
- Klaus, S.; Pultz, S.; Thone-Reineke, C.; Wolfram, S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. Relat. Metab. Disord. 2005, 29, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, R.; Shetty, A.K.; Salimath, P.V. Quercetin alleviates activities of intestinal and renal disaccharidases in streptozotocin-induced diabetic rats. Mol. Nutr. Food Res. 2005, 49, 355–360. [Google Scholar] [CrossRef]
- Shahkhalili, Y.; Finot, P.A.; Hurrell, R.; Fern, E. Effects of foods rich in polyphenols on nitrogen excretion in rats. J. Nutr. 1990, 120, 346–352. [Google Scholar] [CrossRef]
- Beck, S.A.; Tisdale, M.J. Nitrogen excretion in cancer cachexia and its modification by a high fat diet in mice. Cancer Res. 1989, 49, 3800–3804. [Google Scholar] [PubMed]
- Tisdale, M.J.; Brennan, R.A.; Fearon, K.C. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br. J. Cancer 1987, 56, 39–43. [Google Scholar] [CrossRef]
- Padidar, S.; Farquharson, A.; Williams, L.; Kearney, R.; Arthur, J.; Drew, J. High-fat diet alters gene expression in the liver and colon: Links to increased development of aberrant crypt foci. Dig. Dis. Sci. 2012, 57, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.W.; Faehndrich, C.; Bub, A.; Watzl, B.; Will, F.; Dietrich, H.; Rechkemmer, G.; Briviba, K. Cloudy apple juice is more effective than apple polyphenols and an apple juice derived cloud fraction in a rat model of colon carcinogenesis. J. Agric. Food Chem. 2007, 55, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Katayama, M.; Sakata, K.; Kuno, T.; Yoshida, K.; Yamada, Y.; Hirose, Y.; Yoshimi, N.; Mori, H. Inhibitory effects of chlorogenic acid on azoxymethane-induced colon carcinogenesis in male F344 rats. Asian Pac. J. Cancer Prev. 2002, 3, 163–166. [Google Scholar]
- Fang, M.; Chen, D.; Yang, C.S. Dietary polyphenols may affect dna methylation. J. Nutr. 2007, 137, 223S–228S. [Google Scholar] [CrossRef]
- Xiao, H.; Hao, X.; Simi, B.; Ju, J.; Jiang, H.; Reddy, B.S.; Yang, C.S. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis 2008, 29, 113–119. [Google Scholar] [CrossRef]
- Hao, X.; Bose, M.; Lambert, J.D.; Ju, J.; Lu, G.; Lee, M.-J.; Park, S.; Husain, A.; Wang, S.; Sun, Y.; et al. Inhibition of intestinal tumorigenesis in ApcMin/+ mice by green tea polyphenols (polyphenon e) and individual catechins. Nutr. Cancer 2007, 59, 62–69. [Google Scholar] [CrossRef]
- Tan, C.W.; Hirokawa, Y.; Gardiner, B.S.; Smith, D.W.; Burgess, A.W. Colon cryptogenesis: Asymmetric budding. PLoS ONE 2013, 8, e78519. [Google Scholar]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green tea polyphenol EGCG suppresses Wnt/β-catenin signaling by promoting GSK-3β- and PP2A-independent β-catenin phosphorylation/degradation. BioFactors 2014, 40, 586–595. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Joe, A.K.; Mckoy, J.F.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of HER3 and expression of cyclooxygenase-2 in human colon cancer cells. J. Exp. Ther. Oncol. 2005, 5, 69–78. [Google Scholar] [PubMed]
- Goldstein, N.S.; Armin, M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: Implications for a standardized scoring system. Cancer 2001, 92, 1331–1346. [Google Scholar] [CrossRef]
- Kotronen, A.; Seppänen-Laakso, T.; Westerbacka, J.; Kiviluoto, T.; Arola, J.; Ruskeepää, A.L.; Yki-Järvinen, H.; Oresic, M. Comparison of lipid and fatty acid composition of the liver, subcutaneous and intra-abdominal adipose tissue, and serum. Obesity 2010, 18, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Wachira, A.M.; Sinclair, L.A.; Wilkinson, R.G.; Enser, M.; Wood, J.D.; Fisher, A.V. Effects of dietary fat source and breed on the carcass composition, n−3 polyunsaturated fatty acid and conjugated linoleic acid content of sheep meat and adipose tissue. Br. J. Nutr. 2002, 88, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Hodson, L. The influence of dietary fat on liver fat accumulation. Nutrients 2014, 6, 5018–5033. [Google Scholar] [CrossRef] [PubMed]
| Component | Content (g kg−1 Diet) |
|---|---|
| Casein * | 200.0 |
| Cornstarch * | 130.0 |
| Sucrose * | 293.0 |
| Cellulose * | 50.0 |
| Maltodrextrin * | 22.0 |
| Lard * | 200.0 |
| Soy oil * | 25.0 |
| Mineral additive (SAFE mineral 205B) * | 70.0 |
| Vitamin additive (SAFE vitamin 200) * | 10.0 |
| Fatty acid profile ** | |
| Lauric acid (C12:0) | 27.4 |
| Myristic acid (C14:0) | 5.9 |
| Pentadecanoic acid (C15:0) | 0.6 |
| Palmitic acid (C16:0) | 107.0 |
| Heptadecanoic acid (C17:0) | 1.4 |
| Stearic acid (C18:0) | 56.7 |
| Palmitoleic acid (C16:1) | 1.4 |
| Oleic cid (C18:1) | 22.9 |
| Linoleic cid (C18:2) | 1.4 |
| α-Linolenic acid (C18:3) | 0.3 |
| Compound | Content (g kg−1) |
|---|---|
| Phenolic acids | |
| Chlorogenic acid | 165.3 ± 2.4 |
| p-Coumaric acid | 30.7 ± 1.7 |
| Caffeic acid | 58.7 ± 20.0 |
| Ferulic acid | 49.2 ± 2.4 |
| Gallic acid | nd |
| Dihydrochalcones | |
| Phloridzin | 122.2 ± 3.5 |
| Phloretin | 4.5 ± 0.1 |
| Phloretin derivative | 38.7 ± 1.1 |
| Flavanols | |
| Epicatechin | 35.9 ± 1.3 |
| Catechin | 14.2 ± 0.2 |
| Procyanidin B1 | 13.8 ± 0.2 |
| Procyanidin B2 | 25.2 ± 0.5 |
| Flavonols | |
| Quercetin | 4.9 ± 0.3 |
| Quercetin-3-glucoside | 2.8 ± 0.2 |
| AOM-HF | AOM-HF- AS1 | AOM-HF- AS1.5 | |
|---|---|---|---|
| Initial body weight (week 0) | 26.8 ± 1.3 | 26.8 ± 1.2 | 26.8 ± 1.2 |
| Final body weight (week 12) | 40.0 ± 4.5 | 38.9 ± 2.3 | 39.0 ± 3.4 |
| Liver | 1.454 ± 0.229 | 1.384 ± 0.191 | 1.483 ± 0.152 |
| Spleen | 0.145 ± 0.071 | 0.135 ± 0.079 | 0.155 ± 0.075 |
| Kidneys | 0.562 ± 0.057 | 0.525 ± 0.079 | 0.542 ± 0.047 |
| Gastrocnemius muscle | 0.187 ± 0.024 | 0.187 ± 0.021 | 0.194 ± 0.024 |
| Jejunum | 0.311 ± 0.040 | 0.314 ± 0.043 | 0.318 ± 0.044 |
| Colon | 0.191 ± 0.050 | 0.190 ± 0.043 | 0.179 ± 0.028 |
| Epididimal fat depot | 0.943 ± 0.449 | 1.111 ± 0.414 | 0.994 ± 0.472 |
| Suprarenal fat depot | 0.217 ± 0.090 b | 0.313 ± 0.093 a | 0.314 ± 0.086 a |
| Abdominal fat | 0.294 ± 0.092 | 0.365 ± 0.113 | 0.296 ± 0.170 |
| Enzyme Activity (nmol Substrate mg−1 Protein min−1) | AOM-HF | AOM-HF-AS1 | AOM-HF-AS1.5 |
|---|---|---|---|
| Sucrase | 1082 ± 102 a | 856 ± 91 b | 758 ± 79 c |
| Maltase | 1089 ± 74 | 1077 ± 96 | 1040 ± 62 |
| Dipeptidyl-peptidase IV | 619 ± 28 b | 704 ± 42 a | 453 ± 17 c |
| Aminopeptidase N | 6091 ± 925 a | 5879 ± 937 a | 4794 ± 815 b |
| Faeces | Urine | |||||
|---|---|---|---|---|---|---|
| Compound | AOM-HF | AOM-HF-AS1 | AOM-HF-AS1.5 | AOM-HF | AOM-HF-AS1 | AOM-HF-AS1.5 |
| Chlorogenic acid | nd | 5.18 ± 2.23 | 9.23 ± 3.53 * | nd | 4.64 ± 2.94 | 5.33 ± 2.21 |
| p-Coumaric acid | nd | 2.90 ± 1.71 | 4.60 ± 3.18 | nd | 1.81 ± 0.55 | 3.99 ± 0.21 * |
| Caffeic acid | nd | 4.79 ± 3.66 | 6.50 ± 3.75 | nd | 1.81 ± 0.30 | 3.33 ± 1.06 |
| Ferulic acid | nd | 0.57 ± 0.43 | 1.00 ± 0.81 | nd | 0.63 ± 0.10 | 1.22 ± 0.21 * |
| Gallic acid | nd | nd | nd | nd | nd | nd |
| Phloridzin | nd | 4.05 ± 1.39 | 5.50 ± 1.82 | nd | 1.57 ± 0.62 | 2.92 ± 1.33 |
| Phloretin | nd | 0.32 ± 0.25 | 0.38 ± 0.25 | nd | 0.30 ± 0.30 | 1.97 ± 0.45 * |
| Phloretin derivative | nd | 1.55 ± 0.25 | 2.63 ± 0.97 * | nd | nd | nd |
| Epicatechin | nd | 14.47 ± 8.30 | 28.39 ± 14.10 | nd | nd | nd |
| Catechin | nd | 7.91 ± 3.59 | 9.62 ± 3.63 | nd | nd | nd |
| Procyanidin B1 | nd | 3.50 ± 1.25 | 6.49 ± 2.95 * | nd | nd | nd |
| Procyanidin B2 | nd | 1.43 ± 0.99 | 1.93 ± 0.89 | nd | nd | nd |
| Quercetin | nd | nd | 0.26 ± 0.30 | nd | 0.12 ± 0.01 | 0.15 ± 0.05 |
| Quercetin-3-glucoside | nd | nd | nd | nd | nd | nd |
| Liver | Faeces | |||||
|---|---|---|---|---|---|---|
| AOM-HF | AOM-HF-AS1 | AOM-HF-AS1.5 | AOM-HF | AOM-HF-AS1 | AOM-HF-AS1.5 | |
| Lauric acid C12:0 | nd | nd | nd | 0.11 ± 0.03 | 0,08 ± 0.01 | 0.07 ± 0.04 |
| Myristic acid C14:0 | 0.25 ± 0.09 | 0.31 ± 0.06 | 0.28 ± 0.11 | 0.74 ± 0.08 | 0.71 ± 0.12 | 0.63 ± 0.07 |
| C15:0 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.03 | 0.37 ± 0.04 | 0.28 ± 0.12 | 0.25 ± 0.05 |
| C16:0 | 20.29 ± 1.89 | 19.82 ± 1.06 | 20.02 ± 2.78 | 20.69 ± 0.35 | 21.10 ± 0.65 | 21.54 ±0.13 |
| C17:0 | 0.25 ± 0.03 | 0.24 ± 0.03 | 0.27 ± 0.02 | 0.66 ± 0.03 | 0.64 ± 0.04 | 0.68 ± 0.02 |
| C18:0 | 9.20 ± 2.47 | 8.71 ± 2.91 | 10.63 ± 2.29 | 46.63 ± 4.31 | 45.73 ± 4.74 | 49.28 ± 2.82 |
| C20:0 | 0.18 ± 0.05 | 0.19 ± 0.11 | 0.28 ± 0.11 | 2.76 ± 0.22 | 2.54 ± 014 | 2.45 ± 0.09 |
| C22:0 | nd | nd | nd | 1.31 ± 0.11 | 1.21 ± 0.12 | 1.17 ± 0.10 |
| C23:0 | nd | nd | nd | 0.25 ± 0.01 | 0.18 ± 0.04 | 0.15 ± 0.03 |
| C24:0 | nd | nd | nd | 0.53 ± 0.05 | 0.39 ± 0.14 | 0.50 ± 0.04 |
| C16:1 | 1.82 ± 0.66 | 1.99 ± 0.78 | 1.44 ± 0.56 | 0.80 ± 0.19 | 0.78 ± 0.25 | 0.58 ± 0.14 |
| C17:1 | 0.18 ± 0.06 | 0.18 ± 0.04 | 0.16 ± 0.05 | 0.11 ± 0.01 | 0.09 ± 0.01 | 0.08 ±0.01 |
| C18:1 | 40.04 ± 8.71 | 41.76 ± 9.01 | 38.16 ± 5.12 | 18.77 ± 2.52 | 19.64 ± 2.75 | 17.25 ± 1.84 |
| C20:1n9 | 0.86 ± 0.30 | 1.10 ± 0.36 | 1.23 ± 0.28 | 1.41 ± 0.36 | 1.30 ± 0.20 | 1.19 ± 0.07 |
| C22:1n9 | nd | nd | nd | 0.40 ±0.09 | 0.36 ± 0.04 | 0.37 ± 0.04 |
| C18:2 | 14.17 ± 1.46 | 14.84 ± 1.48 | 15.33 ± 2.00 | 3.54 ± 0.96 | 3.98 ± 1.13 | 2.93 ± 0.79 |
| C18:3 | nd | nd | nd | 0.40 ± 0.05 | 0.45 ± 0.09 | 0.38 ± 0.05 |
| C20:2 | nd | nd | nd | 0.16 ± 0.02 | 0.20 ± 0.04 | 0.20 ± 0.01 |
| C20:3 | 0.99 ± 0.33 | 0.92 ± 0.40 | 1.11 ± 0.29 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.10 ± 0.03 |
| C20:4 | 7.38 ± 3.34 | 6.24 ± 3.74 | 7.06 ± 2.26 | 0.24 ± 0.05 | 0.23 ± 0.02 | 0.22 ± 0.09 |
| C22:5 | 0.33 ± 0.13 | 0.30 ± 0.13 | 0.32 ± 0.12 | nd | nd | nd |
| C22:6 | 3.72 ± 1.85 | 3.14 ± 2.00 | 3.37 ± 1.15 | nd | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzo, F.; Milagro, F.I.; Barrenetxe, J.; Díaz, M.T.; Martínez, J.A. Azoxymethane-Induced Colorectal Cancer Mice Treated with a Polyphenol-Rich Apple Extract Show Less Neoplastic Lesions and Signs of Cachexia. Foods 2021, 10, 863. https://doi.org/10.3390/foods10040863
Marzo F, Milagro FI, Barrenetxe J, Díaz MT, Martínez JA. Azoxymethane-Induced Colorectal Cancer Mice Treated with a Polyphenol-Rich Apple Extract Show Less Neoplastic Lesions and Signs of Cachexia. Foods. 2021; 10(4):863. https://doi.org/10.3390/foods10040863
Chicago/Turabian StyleMarzo, Florencio, Fermin I. Milagro, Jaione Barrenetxe, María Teresa Díaz, and J. Alfredo Martínez. 2021. "Azoxymethane-Induced Colorectal Cancer Mice Treated with a Polyphenol-Rich Apple Extract Show Less Neoplastic Lesions and Signs of Cachexia" Foods 10, no. 4: 863. https://doi.org/10.3390/foods10040863
APA StyleMarzo, F., Milagro, F. I., Barrenetxe, J., Díaz, M. T., & Martínez, J. A. (2021). Azoxymethane-Induced Colorectal Cancer Mice Treated with a Polyphenol-Rich Apple Extract Show Less Neoplastic Lesions and Signs of Cachexia. Foods, 10(4), 863. https://doi.org/10.3390/foods10040863