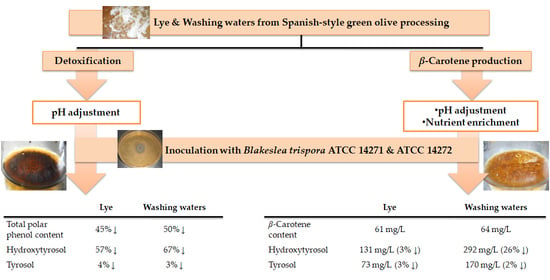
Natural β-Carotene Production by Blakeslea trispora Cultivated in Spanish-Style Green Olive Processing Wastewaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Feedstocks
2.3. Microorganisms
2.4. Submerged Fermentation
2.5. Determination of Total Dry Biomass Content
2.6. Determination of Total Suspended Solids
2.7. Determination of Soluble Sugar Content
2.8. Determination of Total Nitrogen Content
2.9. Determination of Metals
2.10. Determination of Total Carotenoid and β-Carotene Content
2.10.1. Extraction of Carotenoids and Determination of Total Carotenoid Content
2.10.2. RP-HPLC Analysis of Cellular β-Carotene Content
2.11. Determination of Polar Phenolic Compound Content
2.11.1. Extraction of Phenolic Compounds and Determination of Total Polar Phenol Content
2.11.2. RP-HPLC Analysis of Phenolic Compounds
2.12. Determination of Growth and β-Carotene Production Kinetics
2.13. Statistical Analysis
3. Results and Discussion
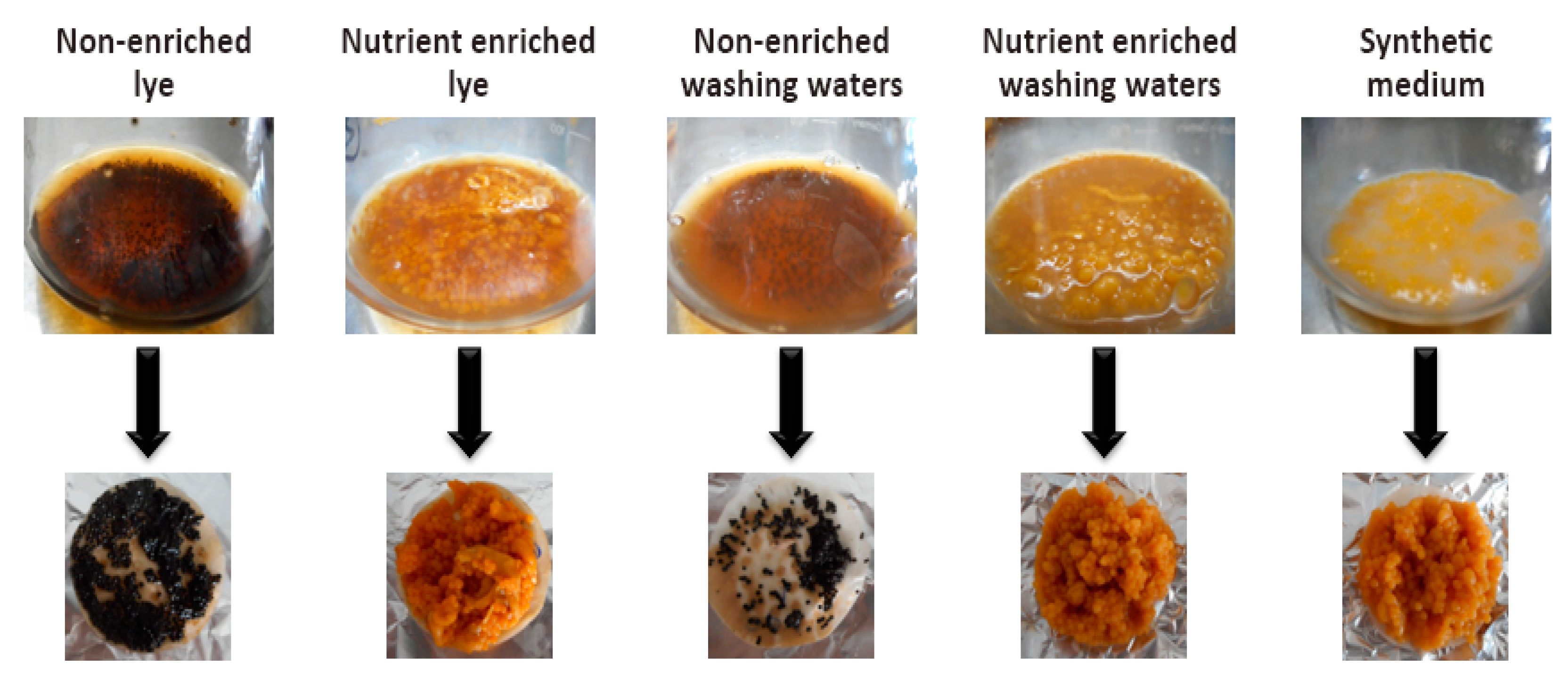
3.1. Kinetics of Microbial Growth
3.2. Kinetics of β-Carotene Production
3.3. Changes in Individual Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Shete, V.; Quadro, L. Mammalian Metabolism of β-Carotene: Gaps in Knowledge. Nutrients 2013, 5, 4849–4868. [Google Scholar] [CrossRef] [PubMed]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Savastano, S.; Colao, A. Nutritional Recommendations for CoVID-19 Quarantine. Eur. J. Clin. Nutr. 2020, 74, 850–851. [Google Scholar] [CrossRef]
- Market Data Forecast Global Beta Carotene Market—Segmented by Application (Pharmaceuticals, Dietary Supplements, Food & Beverage, Animal Feed, Others), By Source (Syntheric, Algae, Fungi, Palm Oil, Others), & By Regional Analysis (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa)—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast (2020–2025); Hyderabad. 2020. Available online: https://www.marketdataforecast.com/market-reports/beta-carotene-market (accessed on 22 December 2020).
- Markets and Markets Carotenoids Market by Type (Astaxanthin, Beta-Carotene, Lutein, Lycopene, Canthaxanthin, and Zeaxanthin), Application (Feed, Food & Beverages, Dietary Supplements, Cosmetics, and Pharmaceuticals), Source, Formulation, and Region—Global Forecast to 2026; Pune. 2020. Available online: https://www.marketsandmarkets.com/Market-Reports/carotenoid-market-158421566.html (accessed on 22 December 2020).
- Bogacz-Radomska, L.; Harasym, J. β-Carotene—Properties and Production Methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the Way Forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission directive 2001/50/EC of 3 July 2001 amending Directive 95/45/EC laying down specific purity criteria concerning colours for use in foodstuffs. Off. J. Eur. Communities 2001, L190, 14–17. [Google Scholar]
- Mantzouridou, F.T.; Roukas, T.; Kotzekidou, P.; Liakopoulou, M. Optimization of β-Carotene Production from Synthetic Medium by Blakeslea trispora: A Mathematical Modeling. Appl. Biochem. Biotechnol. 2002, 101, 153–176. [Google Scholar] [CrossRef]
- Dufossé, L. Microbial Production of Food Grade Pigments. Food Technol. Biotechnol. 2006, 44, 313–321. [Google Scholar]
- Mantzouridou, F.T.; Tsimidou, M.Z. On the Monitoring of Carotenogenesis by Blakeslea trispora using HPLC. Food Chem. 2007, 104, 439–444. [Google Scholar] [CrossRef]
- Papaioannou, E.; Liakopoulou-Kyriakides, M. Substrate Contribution on Carotenoids Production in Blakeslea trispora Cultivations. Food Bioprod. Process. 2010, 88, 305–311. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Roukas, T.; Kotzekidou, P. Effect of the Aeration Rate and Agitation Speed on β-Carotene Production and Morphology of Blakeslea trispora in a Stirred Tank Reactor: Mathematical Modeling. Biochem. Eng. J. 2002, 10, 123–135. [Google Scholar] [CrossRef]
- Roukas, T.; Mantzouridou, F.T.; Boumpa, T.; Vafiadou, A.; Göksungur, Y. Production of β-Carotene from Beet Molasses and Deproteinized Whey by Blakeslea trispora. Food Biotechnol. 2003, 17, 203–215. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Tsimidou, M.Z.; Roukas, T. Performance of Crude Olive Pomace Oil and Soybean Oil during Carotenoid Production by Blakeslea trispora in Submerged Fermentation. J. Agric. Food Chem. 2006, 54, 2575–2581. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Naziri, E.; Tsimidou, M.Z. Industrial Glycerol as a Supplementary Carbon Source in the Production of Beta-Carotene by Blakeslea trispora. J. Agric. Food Chem. 2008, 56, 2668–2675. [Google Scholar] [CrossRef]
- Varzakakou, M.; Roukas, T. Identification of Carotenoids Produced from Cheese Whey by Blakeslea trispora in Submerged Fermentation. Prep. Biochem. Biotechnol. 2009, 40, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M. Agro-food Wastes Utilization by Blakeslea trispora for Carotenoids Production. Acta Biochim. Pol. 2012, 59, 151–153. [Google Scholar] [CrossRef]
- Nanou, K.; Roukas, T.; Papadakis, E.; Kotzekidou, P. Carotene Production from Waste Cooking Oil by Blakeslea trispora in a Bubble Column Reactor: The Role of Oxidative Stress. Eng. Life Sci. 2017, 17, 775–780. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Current Status and Future Challenges of Table Olive Processing Wastewater Valorization. Biochem. Eng. J. 2016, 112, 103–113. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Citric Acid Production from the Integration of Spanish-Style Green Olive Processing Wastewaters with White Grape Pomace by Aspergillus niger. Bioresour. Technol. 2019, 280, 59–69. [Google Scholar] [CrossRef]
- International Olive Oil Council Economic Affairs & Promotion Unit-International Olive Council. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 21 October 2020).
- Rincón-Llorente, B.; De La Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Borja-Padilla, R. Table Olive Wastewater: Problem, Treatments and Future Strategy. A Review. Front. Microbiol. 2018, 9, 1641. [Google Scholar] [CrossRef] [PubMed]
- Campaniello, D.; Carlucci, A.; Speranza, B.; Raimondo, M.L.; Cibelli, F.; Corbo, M.R.; Bevilacqua, A. A Comparative Study on Trichoderma harzianum and a Combination of Candida/Bacillus as Tools for the Bioremediation of Table Olive Processing Water. Microorganisms 2020, 8, 878. [Google Scholar] [CrossRef]
- Papadaki, E.; Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Mantzouridou, F.T. Feasibility of Multi-Hydrolytic Enzymes Production from Optimized Grape Pomace Residues and Wheat Bran Mixture using Aspergillus niger in an Integrated Citric Acid-Enzymes Production Process. Bioresour. Technol. 2020, 309, 123317. [Google Scholar] [CrossRef]
- Papadaki, E.; Tsimidou, M.Z.; Mantzouridou, F.T. Changes in Phenolic Compounds and Phytotoxicity of the Spanish-Style Green Olive Processing Wastewaters by Aspergillus niger B60. J. Agric. Food Chem. 2018, 66, 4891–4901. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Physical and aggregate properties: Solids (Methods 2540). In Standard Methods for the Examination of Water and Wastewater; Baird, R.B., Eaton, A.D., Rice, E.W., Eds.; APHA: Washington, DC, USA, 2017. [Google Scholar]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van’t Riet, K. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Çelekli, A.; Bozkurt, H.; Dönmez, G. Predictive Modeling of β-Carotene Accumulation by Dunaliella salina as a Function of pH, NaCI, and Irradiance. Russ. J. Plant. Physiol. 2014, 61, 215–223. [Google Scholar] [CrossRef]
- Foster, J.W. The Heavy Metal Nutrition of Fungi. Bot. Rev. 1939, 5, 207–239. [Google Scholar] [CrossRef]
- Wehmeier, S.; Morrison, E.; Plato, A.; Raab, A.; Feldmann, J.; Bedekovic, T.; Wilson, D.; Brand, A.C. Multi Trace Element Profiling in Pathogenic and Non-Pathogenic Fungi. Fungal Biol. 2020, 124, 516–524. [Google Scholar] [CrossRef]
- Benito, B.; Garciadeblás, B.; Rodríguez-Navarro, A. Potassium- or Sodium-Efflux ATPase, a Key Enzyme in the Evolution of Fungi the GenBank accession numbers for the sequences reported in this paper are: Pleurotus ostreatus ENA1, AJ420741; Phycomyces blakesleeanus ENA1, AJ420742; Ph. blakesleeanus PCA1, AJ420743; Blakeslea trispora ENA1, AJ420744; B. trispora BCA1, AJ420745; B. trispora BCA2, AJ420746. Microbiology 2002, 148, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Abu-Lafi, S.; Al-Natsheh, M.S.; Yaghmoor, R.; Al-Rimawi, F. Enrichment of Phenolic Compounds from Olive Mill Wastewater and In Vitro Evaluation of Their Antimicrobial Activities. Evid. Based Complement. Altern. Med. 2017, 2017, 3706915. [Google Scholar] [CrossRef]
- Diallinas, G.; Rafailidou, N.; Kalpaktsi, I.; Komianou, A.C.; Tsouvali, V.; Zantza, I.; Mikros, E.; Skaltsounis, A.L.; Besson, T. Hydroxytyrosol (HT) Analogs Act as Potent Antifungals by Direct Disruption of the Fungal Cell Membrane. Front. Microbiol. 2018, 9, 2624. [Google Scholar] [CrossRef]
- Bhosale, P. Environmental and Cultural Stimulants in the Production of Carotenoids from Microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef]
- Ghilardi, C.; Negrete, P.S.; Carelli, A.A.; Borroni, V. Evaluation of Olive Mill Waste as Substrate for Carotenoid Production by Rhodotorula mucilaginosa. Bioresour. Bioprocess. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Extrasynthese. Available online: https://www.extrasynthese.com/ (accessed on 22 December 2020).
- Papadaki, E.; Botsaris, G.; Athanasiadi, E.; Mantzouridou, F.T. Processing Wastewaters from Spanish-Style cv. Chalkidiki Green Olives: A Potential Source of Enterococcus casseliflavus and Hydroxytyrosol. Microorganisms 2020, 8, 1274. [Google Scholar] [CrossRef] [PubMed]
- Imran, B.; Khan, S.J.; Qazi, I.A.; Arshad, M. Removal and Recovery of Sodium Hydroxide (NaOH) from Industrial Wastewater by Two-Stage Diffusion Dialysis (DD) and Electrodialysis (ED) Processes. Desalin. Water Treat. 2016, 57, 7926–7932. [Google Scholar] [CrossRef]
| Fermentation Substrate | Growth Parameters 2 | Goodness of Fit | ||
|---|---|---|---|---|
| Xm (g/L Broth) | μm (1/h) | λB (h) | R2 | |
| Non-enriched lye | 3.123 a ± 0.127 | 0.029 a ± 0.002 | 26.434 a ± 2.566 | 0.982 a ± 0.003 |
| Nutrient-enriched lye | 19.249 b ± 0.809 | 0.234 b ± 0.007 | 37.268 b ± 1.111 | 0.988 b,c ± 0.004 |
| Non-enriched washing waters | 0.830 c ± 0.048 | 0.012 c ± 0.002 | 45.554 c ± 1.350 | 0.989 b,c ± 0.004 |
| Nutrient-enriched washing waters | 13.776 d ± 0.137 | 0.223 b ± 0.010 | 10.435 d ± 1.364 | 0.994 c ± 0.002 |
| Synthetic medium | 10.007 e ± 0.355 | 0.172 d ± 0.008 | 14.319 e ± 0.364 | 0.987 a,b ± 0.001 |
| Fermentation Substrate | Total Carotenoid Content | β-Carotene Content | ||
|---|---|---|---|---|
| mg/L Broth | mg/g Dry Biomass | mg/L Broth | mg/g Dry Biomass | |
| Non-enriched lye | 0.2 a ± 0.0 | 0.07 a ± 0.01 | 0.0 a ± 0.0 | 0.00 a ± 0.00 |
| Nutrient-enriched lye | 83.9 b ± 9.3 | 4.84 b ± 0.44 | 61.2 b ± 5.8 | 3.53 b ± 0.27 |
| Non-enriched washing waters | 0.1 a ± 0.0 | 0.08 a ± 0.01 | 0.0 a ± 0.0 | 0.01 a ± 0.00 |
| Nutrient-enriched washing waters | 83.5 b ± 11.7 | 5.95 c ± 0.95 | 64.1 b ± 8.7 | 4.57 c ± 0.71 |
| Synthetic medium | 56.5 c ± 10.0 | 5.51 b,c ± 0.68 | 44.8 c ± 6.3 | 4.39 c ± 0.37 |
| Fermentation Substrate | % of Total Carotenoid Content | ||
|---|---|---|---|
| β-Carotene | γ-Carotene | Lycopene | |
| Nutrient-enriched lye | 81.6 a ± 0.1 | 9.1 a ± 0.1 | 9.3 a ± 0.1 |
| Nutrient-enriched washing waters | 87.6 b ± 0.3 | 4.8 b ± 0.3 | 7.6 b ± 0.1 |
| Synthetic medium | 89.8 c ± 0.2 | 2.6 c ± 0.1 | 7.6 b ± 0.2 |
| Fermentation Substrate | β-Carotene Production Parameters 2 | Goodness of Fit | ||
|---|---|---|---|---|
| Pm (mg/L Broth) | Rm (1/h) | λCR (h) | R2 | |
| Nutrient-enriched lye | 83.032 a ± 0.798 | 2.018 a ± 0.069 | 47.320 a ± 0.524 | 0.999 a ± 0.001 |
| Nutrient-enriched washing waters | 81.494 a ± 1.765 | 1.418 b ± 0.048 | 31.052 b ± 0.828 | 0.994 b ± 0.001 |
| Synthetic medium | 50.655 b ± 0.182 | 0.842 c ± 0.044 | 26.221 c ± 1.012 | 0.978 c ± 0.005 |
| Compound 1 | Concentration (mg/L) 2 | λmax (nm) 3 | FL 4 | ||
|---|---|---|---|---|---|
| 0 d | 6 d (Non-Enriched) | 6 d (Enriched) | |||
| HTyr | 135.9 a ± 5.5 | 58.0 b ± 8.0 | 131.3 a ± 10.9 | 239, 279 | yes |
| OMeHTyr | 110.5 a ± 3.9 | 69.7 b ± 5.8 | 100.1 a ± 7.5 | 239, 267, 389 | no |
| Tyr | 75.5 a ± 2.8 | 72.6 a ± 8.0 | 73.1 a ± 6.5 | 239, 275 | yes |
| CA | 5.3 a ± 0.3 | 5.1 a ± 0.4 | 5.2 a ± 0.3 | 241, 289, 322 | no |
| LuG | 15.3 a ± 0.6 | 14.9 a ± 1.3 | 15.1 a ± 1.0 | 245, 348 | no |
| CuA | 19.2 a ± 0.8 | not detected | 19.5 a ± 1.6 | 242, 307 | no |
| Compound 1 | Concentration (mg/L) 2 | λmax (nm) 3 | FL 4 | ||
|---|---|---|---|---|---|
| 0 d | 6 d (Non-Enriched) | 6 d (Enriched) | |||
| HTyr | 395.7 a ± 18.4 | 131.3 b ± 15.8 | 291.9 c ± 26.1 | 239, 279 | yes |
| Tyr | 173.9 a ± 6.8 | 167.9 a ± 17.6 | 170.1 a ± 13.0 | 239, 275 | yes |
| OME | 102.5 a ± 4.0 | 99.0 a ± 8.4 | 100.8 a ± 7.4 | 241 | no |
| CA | 7.0 a ± 0.3 | 6.7 a ± 0.6 | 6.9 a ± 0.4 | 241, 289, 322 | no |
| LuG | 13.1 a ± 0.6 | 12.5 a ± 1.0 | 12.8 a ± 1.2 | 245, 348 | no |
| CuA | 20.7 a ± 1.0 | not detected | 19.9 a ± 2.0 | 242, 307 | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadaki, E.; Mantzouridou, F.T. Natural β-Carotene Production by Blakeslea trispora Cultivated in Spanish-Style Green Olive Processing Wastewaters. Foods 2021, 10, 327. https://doi.org/10.3390/foods10020327
Papadaki E, Mantzouridou FT. Natural β-Carotene Production by Blakeslea trispora Cultivated in Spanish-Style Green Olive Processing Wastewaters. Foods. 2021; 10(2):327. https://doi.org/10.3390/foods10020327
Chicago/Turabian StylePapadaki, Eugenia, and Fani Th Mantzouridou. 2021. "Natural β-Carotene Production by Blakeslea trispora Cultivated in Spanish-Style Green Olive Processing Wastewaters" Foods 10, no. 2: 327. https://doi.org/10.3390/foods10020327
APA StylePapadaki, E., & Mantzouridou, F. T. (2021). Natural β-Carotene Production by Blakeslea trispora Cultivated in Spanish-Style Green Olive Processing Wastewaters. Foods, 10(2), 327. https://doi.org/10.3390/foods10020327