1. Introduction
At slaughter, before bleeding, pigs are stunned either electrically, or with high (>80%) CO
2 concentrations. For electrical stunning, two electrodes are placed on either side of the head of the animal and a current of predetermined intensity crosses the brain. This causes a generalized epileptiform seizure; that is, a massive and synchronised depolarisation of the neurons, resulting in a brief period with greatly diminished brain activity causing unconsciousness [
1]. For gas stunning, in EU countries, pigs are introduced into a gondola that is lowered into a 7 to 8 m deep pit containing at least 80% of CO
2 at the bottom of the pit (Council Regulation (EC) No 1099/2009). Following inhalation, the CO
2 causes acidification of the blood and subsequently, the cerebrospinal fluid [
2]. Consequently, the neurons of the brain are acidified and are unable to function correctly, resulting in unconsciousness [
3].
Various studies have compared the two techniques, relative to animal welfare issues and to meat quality. Most modern gas stunning systems allow pigs to stay in groups during the stunning process, considered an advantage for animal welfare, as pigs are gregarious. On the negative side, at a concentration of 80% CO
2, the induction of consciousness needs 21 to 30 s, a period during which pigs exhibit apparent respiratory distress, as well as muscular contractions and convulsions [
4,
5,
6,
7,
8,
9]. Pigs may further express avoidance reactions, also indicative of the aversiveness of high CO
2 concentrations [
10,
11,
12]. The advantage of electrical stunning is its instantaneous induction of unconsciousness. However, automatic systems, which are mostly used in commercial settings, are equipped with a restrainer, which is stressful for the animals [
13]. In addition, the automatic positioning of the electrodes makes it sometimes difficult to ensure that the intensity of the current passing through the brain of the animal is sufficient [
14].
Generally, compared to electrical stunning, gas stunning is described as producing better meat quality. Earlier studies found that electrical stunning resulted in meat with a faster initial post-mortem pH decline, a lighter color, and increased drip loss associated with a higher incidence of Pale Soft Exudative like (PSE-like) meat [
15,
16,
17,
18,
19]. Electrical stunning was further associated with higher incidences of petechiae and ecchymosis, especially in carriers of the n-allele (halothane sensitivity) [
15,
16,
18,
20]. These studies were, however, conducted under commercial or near-commercial conditions, that is, animals were walked to the stunning site. It is, therefore, unknown if the differences in meat quality express more pronounced effects of electrical compared to gas stunning on post-mortem muscle metabolism or whether the electrically stunned pigs were subjected to greater levels of physical activity and/or stress before stunning, which may also influence post-mortem pH decline [
21].
The stun method itself may influence post-mortem muscle metabolism for various reasons. After a successful electrical stun, pigs exhibit a generalized epileptic seizure, first causing muscle contraction (tonic phase), followed by a series of alternating, sometimes violent, contractions and relaxations of the muscles (clonic phase). The seizure induces further hyperactivity of the peripheral nervous system and hypersecretion of hormones, including adrenaline [
22,
23]. The muscle contractions and release of hormones are likely to accelerate early post-mortem energy metabolism leading to a faster acceleration of post-mortem pH decline amongst others [
21]. CO
2 stunning causes stress during the induction period and provokes involuntary muscle contractions, all of which may also cause acceleration of muscle metabolism. Furthermore, CO
2 inhalation causes the acidification of the blood [
23], which could cause additional strain on the buffer system of muscle cells, reducing their capacity to maintain intracellular pH.
The objective of the present study is to investigate whether differences in pre-mortem activity levels contribute to the effects of different stunning methods, gas and electrical, on meat quality traits. The study took place under experimental conditions. To limit pre-mortem physical activity, pigs were conducted from their rearing pen to the stunning site in the adjacent slaughter room using a trolley. Electrical stunning was induced manually without use of a V-restrainer. For gas stunning, the two best performing gas mixtures of a precedent behavioral study [
7], 80% CO
2/air and 70% N
2O/30% CO
2, were used, immerging the pig and the trolley in the mixture as herein described.
2. Materials and Methods
All aspects of the protocol were in accordance with the French legislation relative to animal protection in the context of experimentation, and all necessary official licenses for animal experimentation were up-to-date. The experimental rooms and equipment were the same as in a precedent study [
7].
2.1. Animals and Housing
The study used 30 female pigs (Duroc × (Landrace × Large White)) weighing between 80 and 90 kg. Pigs were bought from a local producer (GAEC Petiot, Liernolles, France) 10 days before the trial and housed in an experimental breeding building located at the INRAE research center. They were kept in groups of 10 pigs in pens (3 × 4.5 m) with straw bedding in a single room. Each pig received 2.5 kg of standard concentrate per day and water was permanently available. Artificial light was on from 8 to 20 h. The experiment was organized in 3 consecutive series balanced for the experimental stunning procedures. The week preceding the trial, the two persons who would manipulate the pigs during the trial entered each pen daily and touched the pigs to habituate them to their presence.
2.2. Gas Equipment
Stunning and slaughter were carried out in the room adjacent to the rearing room described in our previous publication [
7]. The room contained a 1 × 2 × 1.50 m gas chamber made from 1 cm thick Perspex glass and a PVC floor and mounted on wheels (
Figure 1). The lid made from extruded polystyrene consisted of two halves to allow rapid closing and opening of the chamber. Grooves in the lid, both at the level where the lid joined the Perspex glass and where the two halves joined, allowed a relatively airtight closing of the chamber. Each half lid was equipped with a half PVC tube, vertically positioned, that joined each other in the middle to serve as a guide for the chain of the pulley that carried the cage with the pig. This tube was closed airtight between tests if the box contained gas. At a distance of 30 cm above the floor of the chamber, 10 cm from an angle, two connecting sleeves were permanently fitted on, to which gas tubes were attached to fill the chamber with gas. A closed circuit made from 10 cm PVC tubes and equipped with a ventilator (Systemair, Skinnskatteberg, Sweden) turning at a slow speed was fitted externally to the chamber to ensure homogeneous gas mixtures. It drew the gas mixture from the chamber in a corner 15 cm above the floor and returned it to the chamber in the diagonally opposite corner, at 97 cm above the floor. A sensor connected to a CO
2/O
2 analyser (Oxybaby gas analyser, Witt, Morsang sur Orge, France) was attached to the tube, at the level of the opening which the gas mixture was returned. The room was further equipped with manual stunning tongs, a slaughterline and a cold chamber kept at 4 °C.
The chamber was filled with the correct gas mixture before each slaughter session. To fill the chamber with the N2O/CO2 mixture, it was first filled with 100% of N2O, and subsequently, CO2 was added until it reached 30% while releasing the N2O outside the building.
2.3. Procedures
Pigs were either gas (90 s exposure to 80% of CO
2 in air,
n = 10; or 70% of N
2O with 30% of CO
2,
n = 10) or electrically stunned (manual Morphée tongs (Lelong & Cie, Savigny Le Temple, France), 230 V applied for 10 s,
n = 10;
Table 1). The system was not equipped to measure current intensity but was set to be around 1.3 A [
24].
The day before slaughter, five pigs were separated from their rearing group and introduced in an adjacent 1.5 × 3 m pen without food but with access to water. The next day, a few minutes before slaughter, a pig was removed from its pen and introduced into an adjacent waiting pen (1.5 × 1.0 m) with vision on the pen mates. After 10 min, the pig was introduced into a cage (122 × 44 × 88 cm) built with metal bars, mounted on wheels and containing two vertically sliding doors and a removable floor. The pig was transported in the trolley over 6.5 m to the experimental room.
In the case of gas stunning, the chain of the pulley was attached to a hook in the middle of the top of the cage and lifted at a height of 2 m, attached to a slaughter line rail, moved above the chamber containing the correct gas mixture and descended onto the chamber floor (see Figure 1 in [
7]). Duration between the moment of attachment and positioning on the chamber floor was 57 s [
7]. The chamber was only briefly opened, to allow the introduction of the pig. Following the appropriate exposure duration, the cage with the pig was lifted again and descended on the floor of the experimental room. The bottom of the cage was detached, and the top of the cage was removed to have access to the unconscious pig. Recumbent position, and the absence of corneal reflex and breathing were verified to ascertain unconsciousness [
25]. The pig was immediately shackled, hoisted and bled (stun-stick interval of around 40 s) by a trained slaughterman using the normal slaughtering procedure. Each pig was exposed for 95 s to one of the gas mixtures. As the descent and the lifting procedure comprised each time 15 s inside the box, the pigs remained for 75 s on the bottom of the chamber.
In the case of electrical stunning, the pigs were stunned immediately following arrival in the room through a large opening on the top of the cage, then the bottom of the cage was detached and the top removed. Recumbent position and the presence of generalized tonic muscle contraction were indicators of unconsciousness of the pig immediately after the stun; absence of corneal reflex and breathing were following hoisting and during bleeding [
25]. The pig was shackled, hoisted and stuck before the end of the tonic phase (stun–stick interval of 10 to 15 s). The carcass was introduced in the scalding tank (Banss, 65 °C) following the end of bleeding. The remaining hairs were burnt using a gas burner and scraped with a knife to obtain a clean carcass before evisceration and splitting. The carcass was entered in the cold room 45 min post-mortem.
Each day, 5 pigs were slaughtered, on 3 consecutive days, during 2 weeks. Each day a single gas mixture was used on 3 or 4 pigs, and 1 or 2 pigs were electrically stunned. Gas mixtures were alternated over days. For each stunning method, 10 pigs were used, selected from different rearing groups in a balanced design.
2.4. Measurements
Bleeding efficiency. The blood was collected over different intervals: 0–30 s, 30–60 s, 1–2, 2–3 and 3–5 min in five 50 × 30 × 20 cm recipients following the bleeding cut and the contents were weighed (
Table 1).
Carcass weight. 30 h post-mortem, the head and the left half of the carcass were weighed. Carcass weight was estimated as the weight of the head and twice the weight of the half-carcass.
Muscle pH decline, temperature, glycogen and lactate contents. At different moments following slaughter, different variables related to muscle energy metabolism were evaluated in 4 different muscles:
Longissimus lumborum (LL),
Semispinalis capitis (SC),
Adductor femoris (AF) and
Semimembranosus (SM) (
Table 2). Five minutes after bleeding, several grams of the LL muscle at the level of the sixth rib were excised to obtain 2 samples of 2 g and 1 g to determine pH, and at a later stage, glycogen/lactate content, respectively. To determine the pH, samples were immediately homogenised (Polytron, Steinhofhalde, Switzerland) during 20 s in 18 mL of 5 mM iodoacetate, and the pH of the homogenate was measured with a glass electrode (Inlab 427, Mettler Toledo, Greifensee, Switzerland), connected to a portable pH meter (Schött-Geräte, Germany). The samples for glycogen and lactate determination were immediately frozen in liquid N
2 and stored at −80 °C until assaying.
At 30 and 60 min post-mortem, samples were excised from the LL, SC, AF and SM to determine pH as described above. At 30 h post-mortem, pH was measured directly on the carcass using a pH meter (WTW 340-B, Weilheim, Germany) equipped with a probe (Sentix SP, WTW, Weilheim, Germany). Temperature of the LL muscle was measured 5, 30 and 60 min and 30 h post-mortem, and of the SC, AF and SM muscles 30 and 60 min and 30 h post-mortem, directly on the carcass using an electronic thermometer equipped with a probe (TFK 150/E Weilheim, Germany).
Fractures and petechiae. The slaughterman verified the possible presence of bone fractures during splitting of the carcass and one of the experimenters was assigned to verify the presence of petechiae on a slice of the LL and SM muscles of similar size 30 h port-mortem.
Drip loss (DL). A total of thirty hours post-mortem, two slices about 1 cm thick were excised of each of the LL and SM muscles, weighed and suspended in a plastic bag and maintained at 4 °C. One (DL1), 2 (DL2) and 5 days later (DL5), after removing the water on the surface, each slice was weighed and suspended again on days 1 and 2). For the different time points, water loss was calculated as the percentage of the preceding weight [
12]. Total DL refers to the weight loss on day 5 relative to the initial weight of the slice.
Rendement Napole (RN). This measurement is a standardized laboratory method for estimating yield of cured and cooked ham [
26]. A total of thirty hours post-mortem, 100 g of the LL and SM muscles were excised, ground and mixed with 20 g of brine (136 g of nitrate salt/l water) in glass beakers. After closing them with a lid, the beaker remained at 4 °C for 24 h, and was subsequently placed in a water bath. The water bath was heated up to boiling temperature and maintained boiling for 10 min. The beakers were then removed from the water and the cooked meat placed on a draining rack at room temperature for 2.5 h before weighing. The Rendement Napole was calculated as the final weight relative to the initial weight expressed as a percentage.
Meat color. The color coordinates L*, a* and b* were measured 30 h post-mortem using a Minolta chromameter (CR-300, Minolta Corp. Osaka, Japan, no protective glass) equipped with a 0° viewing angle and using illuminant C on the surfaces of the LL, SM, and AF, after 1 h of blooming. Before measurement, humidity was gently removed from the surface using a clean tissue. For the SM color measurements, in order to standardize, the more internal, whiter part of the muscle was chosen [
13].
2.5. Glycogen and Lactate Assays
Approximately 200 mg of lyophilized LL was ground and suspended in 10 mL of 0.5 M perchloric acid for 15 s with a homogenating device (Polytron, Steinhofhalde, Switzerland). Lactate and glycogen content were determined spectrophotometrically as described in an earlier paper [
21], following methodologies described by [
27,
28]. Glycolytic potential (GP), the sum of compounds likely to produce lactic acid post-mortem, was calculated using the formula proposed by [
29], to estimate muscle glycogen reserves at the moment of slaughter. In the calculation glucose and glucose-6-phosphate were excluded as they had not been determined but they have a minor contribution (between 4.5 and 7.5%; [
29] to the GP. Values are expressed as µmol lactate equivalents per g of fresh tissue. GP of the LL and SM were based on samples collected 5 and 30 min post-mortem, respectively.
2.6. Statistical Analysis
XLStat (version 2018.1.1; Addinsoft 2020; XLSTAT statistical and data analysis solution, Paris, France) was used for comparisons between stunning methods and muscles. Averages of the different stunning methods were compared using ANOVA for mixed methods with “stunning method” as fixed and “slaughter day” as random effect. Where relevant, lactate or glycogen levels or pH were introduced as covariables into the ANOVA of certain meat quality indicators to evaluate whether effects of stunning method could be statistically explained by these quantitative explanatory variables. ANOVA for repeated analyses was used to compare muscles (repeated factor “muscle”; fixed effect “stunning method”).
3. Results and Discussion
All pigs were unconscious after stunning. A total of four pigs after immersion in N
2O/CO
2 and one after immersion in the CO
2/air mixture showed leg movements at the end of bleeding, but no respiration. These movements are a sign of a risk of return of consciousness, which is obviously unacceptable, both in terms of animal welfare and security [
25]. This observation is consistent with our earlier observation that pigs stunned with CO
2/N
2O stood sooner than when stunned with the CO
2/air mixture [
7]. This problem may be solved by longer dwelling durations or sooner bleeding than the 40 s delay practiced in the present study.
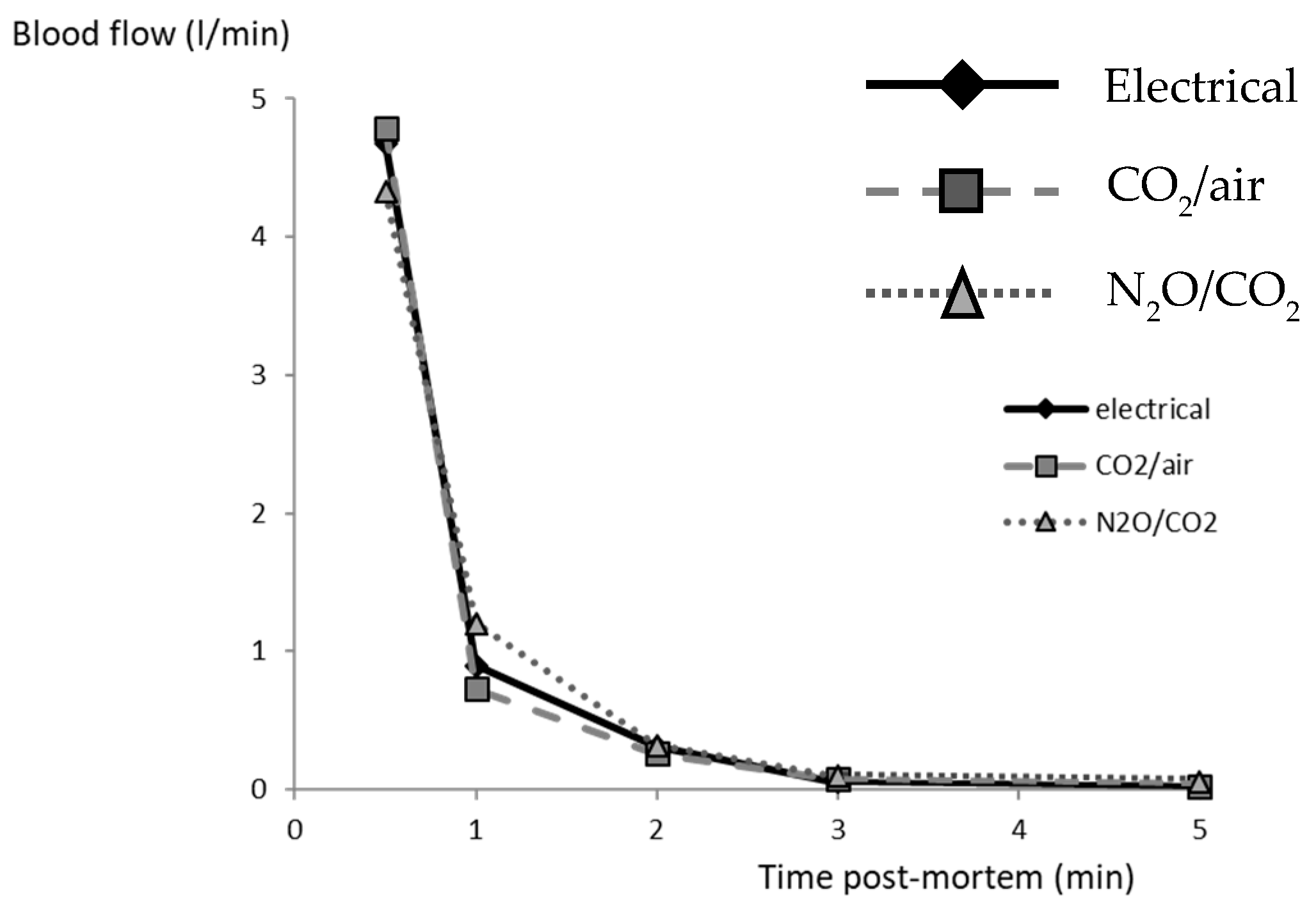
Neither the quantities of blood collected over 5 min, nor the calculated flow rates, were influenced by the stunning method, with 2288 ± 230, 461 ± 80, 329 ± 80, 86 ± 30 and 103 ± 50 g for the successive intervals (total of 3163 ± 160 g;
Figure 2). The total amount of blood collected correlated with carcass weight (r = 0.59,
p < 0.001) and with the amount collected during the first 30 s (r = 0.69,
p < 0.0001). Hence, the bleeding rates and total blood loss were similar and satisfactory for all pigs, irrespective of the stunning method.
In total, 3 of the 10 electrically stunned pigs and a pig stunned with the CO
2/air mixture had 3 or 4 blood spots on the SM slice (Chi square: not significant). The latter pig also had a fractured pelvis. The lack of significance is probably due to the reduced number of pigs used. However, the trend is consistent with literature data [
15,
16,
17,
18,
20], which report higher incidences of petechiae after electrical than after CO
2 stunning.
An electrically stunned pig and another pig stunned with the N2O/CO2 mixture had shown much physical resistance when introducing them in the metal cage for transport. Probably as a consequence of this, they had a low pH and low GP 5 min post-mortem. These pigs were excluded from the ANOVA analysis for meat quality aspects to avoid bias, but not for the correlations.
At 5 and 60 min, electrically stunned pigs had a higher LL pH than pigs stunned with the CO
2/air mixture and at 5 min a higher LL pH than pigs stunned with the N
2O/CO
2 mixture (
Table 2). LL lactate was lower (
p < 0.01) 5 min after electrical stunning (22.0 ± 2.8 μmol/g) than after CO
2/air (33.4 ± 1.7 μmol/g) or N
2O/CO
2 stunning (31.3 ± 2.2 μmol/g). Some other minor effects were observed. Thus, LL drip loss on day 5 was greater (
p < 0.03) after CO
2/air stunning (2.87 ± 0.17%) than after electrical (2.20 ± 0.21%) or N
2O/CO
2 stunning (2.32 ± 0.09%). Following N
2O/CO
2 stunning SM drip loss on days 1 (
p = 0.08) and 2 (
p < 0.05) was greater (total of 4.26 ± 0.37;
p < 0.05) than following electrical (2.97 ± 0.16%) or CO
2 stunning (3.42 ± 0.34%).
p-values for the effects of stun method on GPs, and glycogen levels of the LL and SM were greater than 0.15. In summary, the effects of stunning method on LL and SM technological meat quality indicators concerned only a few indicators. Particularly, early post-mortem pH decline in the LL was faster following gas stunning than following electrical stunning, while the pH of other muscles were not influenced. Following CO
2/air stunning, LL drip loss between days 2 and 5 post-mortem was higher, and following CO
2/N
2O stunning SM drip loss was higher the first two days, compared to the other methods, but overall drip loss over 6 days was not influenced by stunning method.
Finally, AF meat had lower lightness (
p < 0.01) after CO
2/air stunning (42.1 ± 0.8) compared to electrical (46.5 ± 1.0) or N
2O/CO
2 stunning (45.9 ± 0.7). No other effects of stunning method were found. Light meat color is generally associated with faster early post-mortem pH decline and/or lower ultimate pH [
30], but electrical stunning had no effect on AF post-mortem pH decline. Possibly, the stunning method has influenced other mechanisms involved in meat color determinism [
30].
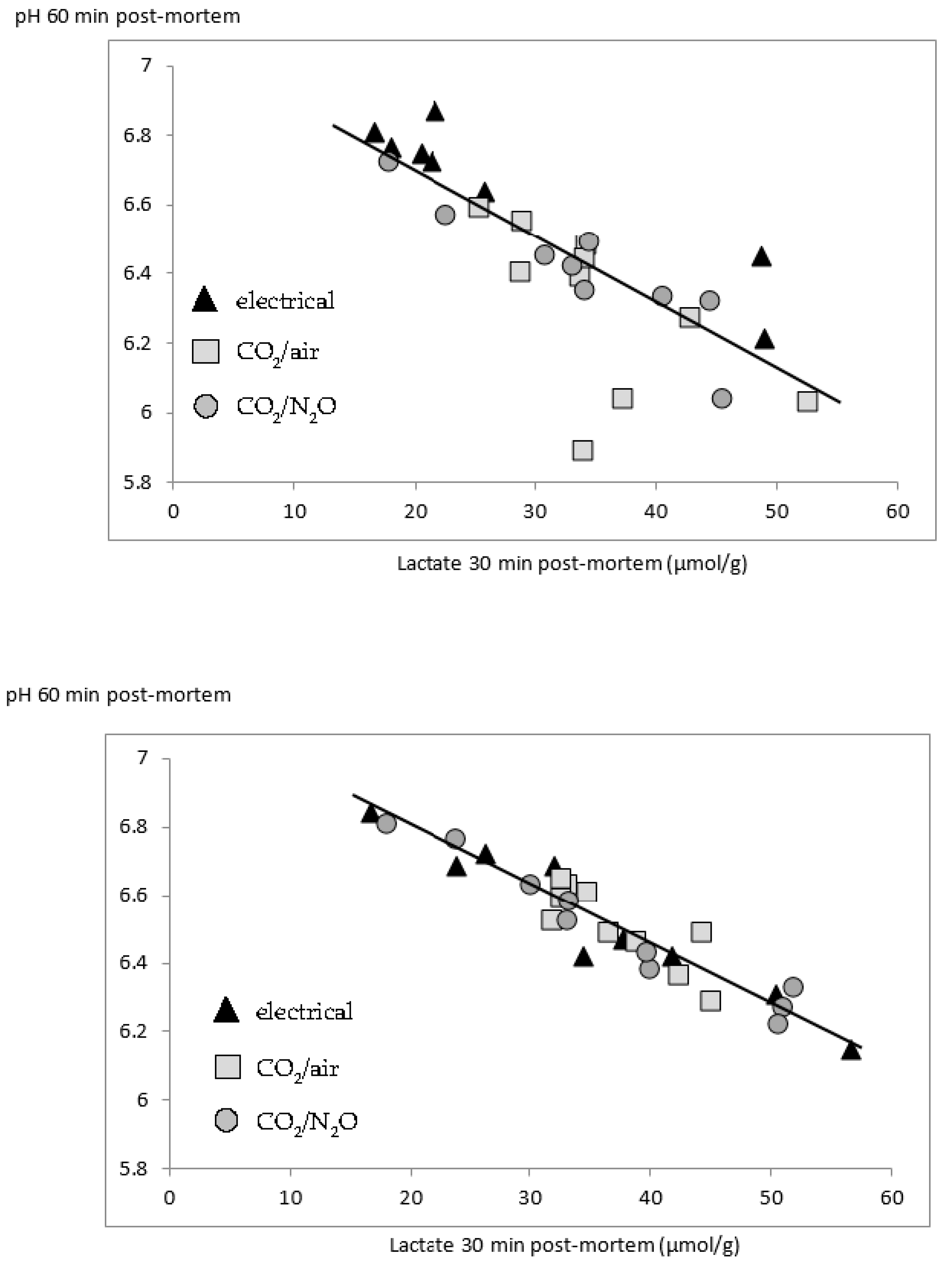
Correlation analysis on the overall LL data set found strong correlations between early post-mortem pH, and early glycogen and lactate contents, while correlations with temperature were weaker (
Table 3;
Figure 3). Ultimate pH was weakly correlated with GP, and more strongly with RN and DL (
Table 3). RN was also positively correlated with muscle temperature 30 h post-mortem (
Table 3). In a regression analysis on RN, both ultimate pH (
p = 0.03), and temperature 30 h post-mortem (
p = 0.02) were significant, but this effect was due to 3 pigs with relatively high RN values.
Total DL was correlated with ultimate pH (r = −0.46; p = 0.01) and lactate 5 min post-mortem (r = 0.50; p < 0.01). In a regression analysis on total DL, ultimate pH was significant (p = 0.002), but not lactate content 5 min post-mortem (p = 0.35). Fitting ultimate pH as explanatory variable in the ANOVA on DL5 (ultimate pH: p < 0.0001) did not remove the effect of stunning method (p < 0.03). The introduction of the LL pH 5 min post-mortem in the analysis of variance cancelled the effect of the stunning on the lactate content measured 30 min post-mortem (pH effect: p < 0.0001, stun effect: p > 0.38). Glycogen content 5 min post-mortem was significant as quantitative variable (p < 0.05) but did not remove the significance of the effect of the stunning method on the pH 5 (p = 0.01) or 60 min post-mortem (p = 0.05). Glycogen content 30 min post-mortem was not significant (p > 0.22) as quantitative variable in the ANOVA of pH 5 or 60 min post-mortem.
As for the LL, correlation analysis on the overall SM data set found correlations between early post-mortem pH, glycogen and lactate contents, but correlations with temperature were weak or absent (
Table 3;
Figure 3). Red index was weakly correlated with early post-mortem pH. Additional correlations for the SM were found between RN and lactate contents 30 h post-mortem (r = −0.49;
p < 0.01); b* and L* (r = 0.37;
p < 0.05); temperature 30 min post-mortem and glycogen content 30 h post-mortem (r = −0.37;
p < 0.05); temperature 60 min and 30 h post-mortem (r = 0.52;
p < 0.01) and DL2 and DL5. For the AF, early post-mortem pH was correlated with red index and temperature (
Table 3). For the SC, the pH 30 min post-mortem was correlated with both the pH (r = 0.66;
p < 0.001) and temperature 60 min post-mortem (r = −0.43;
p < 0.05). Slaughter live weight was not correlated with any of the measured variables.
These correlations show that higher lactate contents early post-mortem explain, at least partly, the higher drip losses following gas stunning. This relationship is well known [
31]. Post-mortem, muscle glycogen is degraded, producing energy, but also heat, hydrogen ions, and lactate. During the early post-mortem period, certain steps of the glycolysis produce hydrogen ions, while lactate production occurs once pyruvate has been formed [
32]. Under the anaerobic conditions of the post-mortem muscle, the process cannot sustain the energy needs and progressively ATP levels lower. The glycolysis itself and the net hydrolysis of ATP result in the production of hydrogen ions leading to a pH decline [
26,
32,
33]. The associations between higher muscle temperature, faster early post-mortem pH decline, higher lactate content and lower remaining glycogen reserves observed in the present study are coherent with a faster post-mortem metabolism. The fast pH decline while the muscle is still warm causes increased protein denaturation, which may explain the increased drip loss observed at certain time points following gas stunning [
31]. The greater RN yield in meat with lower glycolytic potential and higher ultimate pH is coherent with existing knowledge [
29]; none was influenced by stunning method. The effect on pH on meat color is also well known and related to its effects on myoglobin, among others [
30].
Irrespective of stunning method, the SC had higher (
p < 0.001) ultimate pH (5.68 ± 0.02) compared to the other muscles. AF had higher (
p < 0.01) ultimate pH (5.45 ± 0.01) than LL (5.38 ± 0.01) and SM (5.39 ± 0.01). Muscles differed also in temperature at different times post-mortem (
Table 4). Irrespective of stunning method, the AF muscle had lower lightness (
p < 0.01), and higher yellow (
p < 0.0001) and red index (
p < 0.05) than the LL and SM muscles (
Table 3). The higher pH of the SC and the AF and the different color of the AF muscle are at least partly explained by their different fiber composition [
21,
30].
The results obtained contrast with those earlier reported which were indicative of faster early post-mortem metabolism following electrical compared to gas stunning. Thus, in two field studies, Velarde et al. [
15,
16] found that abattoirs using electrical stunning produced lighter
Longissimus thoracis (LT) meat with a higher risk of PSE compared to abattoirs using CO
2 stunning, both indicative of a faster early post-mortem pH decline. Similarly, using an experimental abattoir to compare electrical and gas stunning, Channon et al. [
17,
18,
20] found overall, faster early post-mortem pH decline after electrical stunning in the
Longissimus thoracis et lumborum (LTL), and
Biceps femoris (BF) muscles. Following electrical stunning, the LTL presented also greater drip loss [
17,
18,
20]. Similar results were obtained by Marcon et al. [
19].
The different results obtained in the earlier studies compared to the present study may be caused by higher and uncontrolled pre-slaughter stress levels in the former. Increased physical efforts of the animal just before slaughter accelerates ante-mortem muscle metabolism causing faster post-mortem glycogen breakdown and an increased rate of hydrogen ion, lactate and heat production [
34,
35,
36]. Psychological stress due to fear of unfamiliar circumstances increases the secretion of adrenaline; this exacerbates the effects of physical activity on ante-mortem muscle metabolism and further accelerates early post-mortem energy metabolism [
21,
37,
38,
39]. The earlier studies by Velarde et al. [
15,
16], Marcon et al. [
19] and Channon et al. [
17,
18,
20] were conducted in commercial or pilot abattoirs. In these earlier studies, before stunning, pigs were more active and more psychologically stressed than in the present study, as the studies involved transport and driving of the walking animals and for the electrical stunning treatment, often the use of V-restrainers.
In the present study, pigs performed very little physical effort before slaughter, pre-slaughter psychological stress was low, as pigs did not walk but were wheeled to the stunning spot, and the delay between leaving the home pen and stunning was very short. During gas stunning, loss of consciousness is not immediate and during the induction, pigs are in respiratory distress and present uncontrolled muscle contractions [
7]. Therefore, the faster pH decline and greater lactate production in the LL muscle in the gas compared to electrically stunned pigs probably results from the greater physical effort and/or psychological stress during the induction period, leading to a faster glycolytic rate. An earlier report suggests also that the involuntary muscle contractions during gas stunning may accelerate pH decline: when comparing different CO
2/N
2 mixtures, mixtures with lower CO
2 and higher N
2 concentrations were associated with increased involuntary muscular excitation, a faster pH decline and increased drip loss [
40]. Other factors, such as gas concentration (a progressive increase in the earlier studies), the exact site of application of the stunning electrodes on the pig, or the genetic background of the animals were also different between the present and earlier studies [
17,
18] and may have contributed to their different results.
In both the LL and SM muscles, greater hydrogen ion content (lower pH) was strongly associated with greater lactate contents and greater lactate and hydrogen ion contents were associated with lower glycogen contents (faster glycogen breakdown). The latter correlations were weaker than the correlations between hydrogen ion and lactate contents, probably because animals differed in their initial muscle glycogen contents at the moment of slaughter. The correlation between the hydrogen ion and lactate production during the early post-mortem period explains up to 90% (based on the r2 for pH/lactate correlation) of the individual variability in the early post-mortem rate of pH decline in the SM. This suggests that glycolytic rate is the main cause of the effect of stunning method on pH decline: if ever the absorption of CO2 contributes to the acidification of the muscles because of its acidifying effects on blood, the effect is likely minor.
Like gas stunning, electrical stunning influences post-mortem metabolic rate, due to its effect on muscle contraction. Particularly, higher intensities, longer, or head-brisket (rather than head-only) current applications increased rate of pH decline and drip loss compared to controls [
18,
20], probably because of the increased contractions of the muscles. For a given muscle, its sensitivity to the electrical parameters may partly depend on their position on the body. For example, sites of the LTL, further removed from the vertebrae, were not influenced by the electrical parameters [
17,
18,
20].