Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Quality Assessment
3.3. Effect of Polyphenols on Food/Energy Intake, Obesity-Related Parameters, and Markers-Associated with Inflammation
3.3.1. Effect of Polyphenols on Energy/Food Intake and Bodyweight
3.3.2. Effect of Polyphenols on Adiposity
3.3.3. Effect of Polyphenols on Lipid Profile
3.3.4. Effect of Polyphenols on Glucose Homeostasis
3.3.5. Effect of Polyphenols on Adipocytokines, CRP, and LPS/LBP
3.3.6. The Overall Effect of Polyphenols on Obesity-Related Parameters and Inflammation
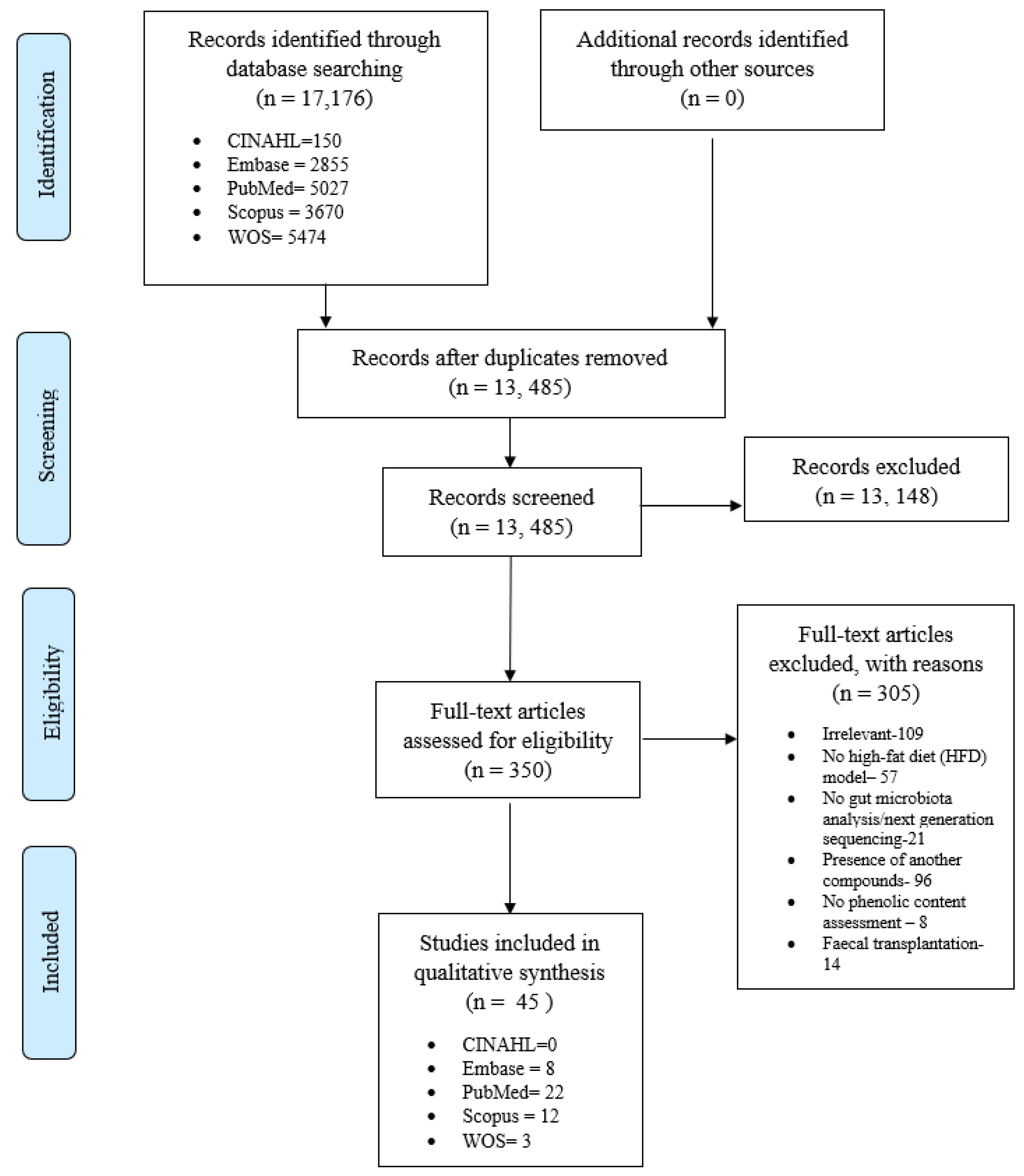
3.4. Effect of Polyphenols on Gut Microbiota
3.4.1. Alpha and Beta-Diversity
3.4.2. Modulation of Firmicutes:Bacteroidetes Ratio (F:B Ratio), Phyla, and Family/Genus
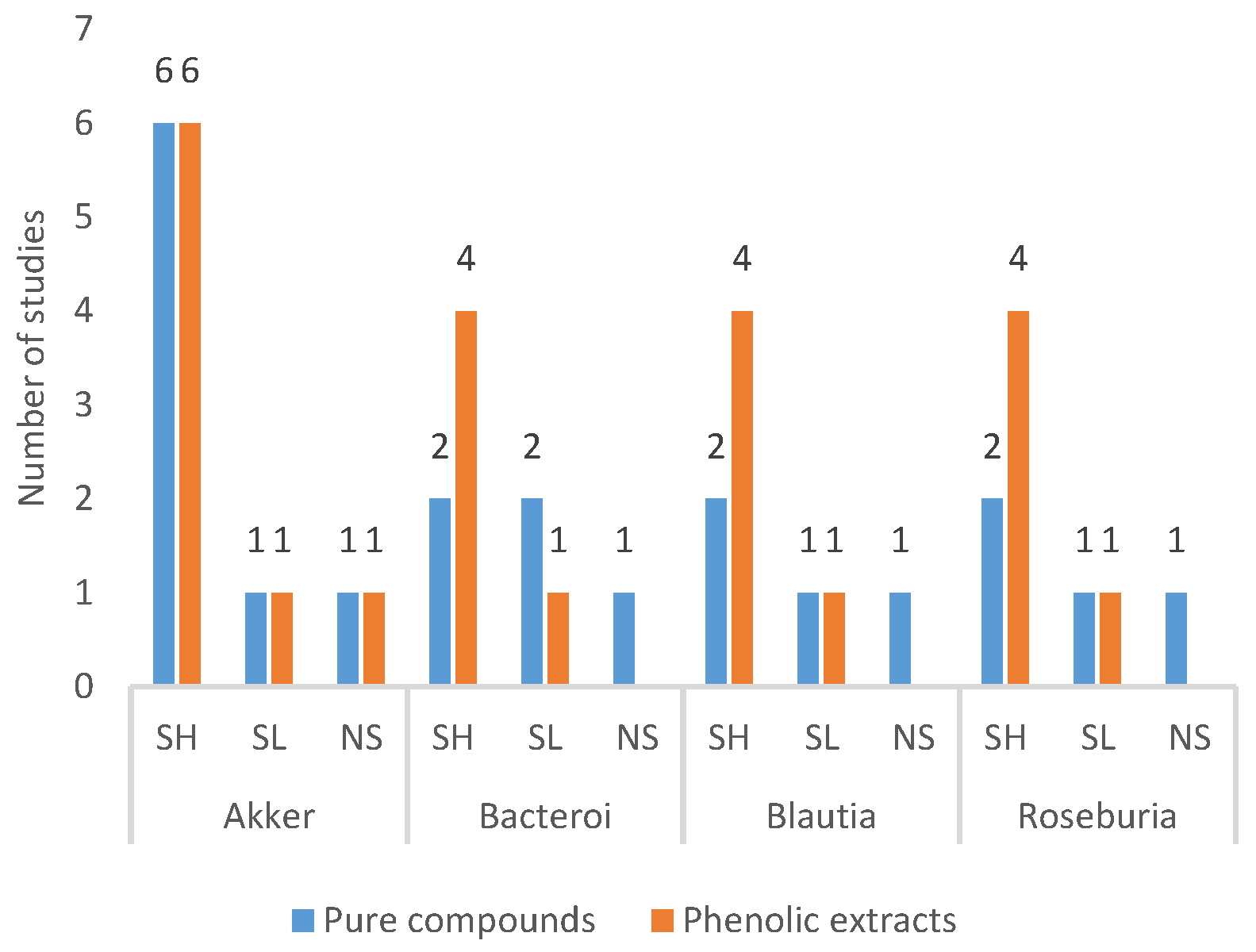
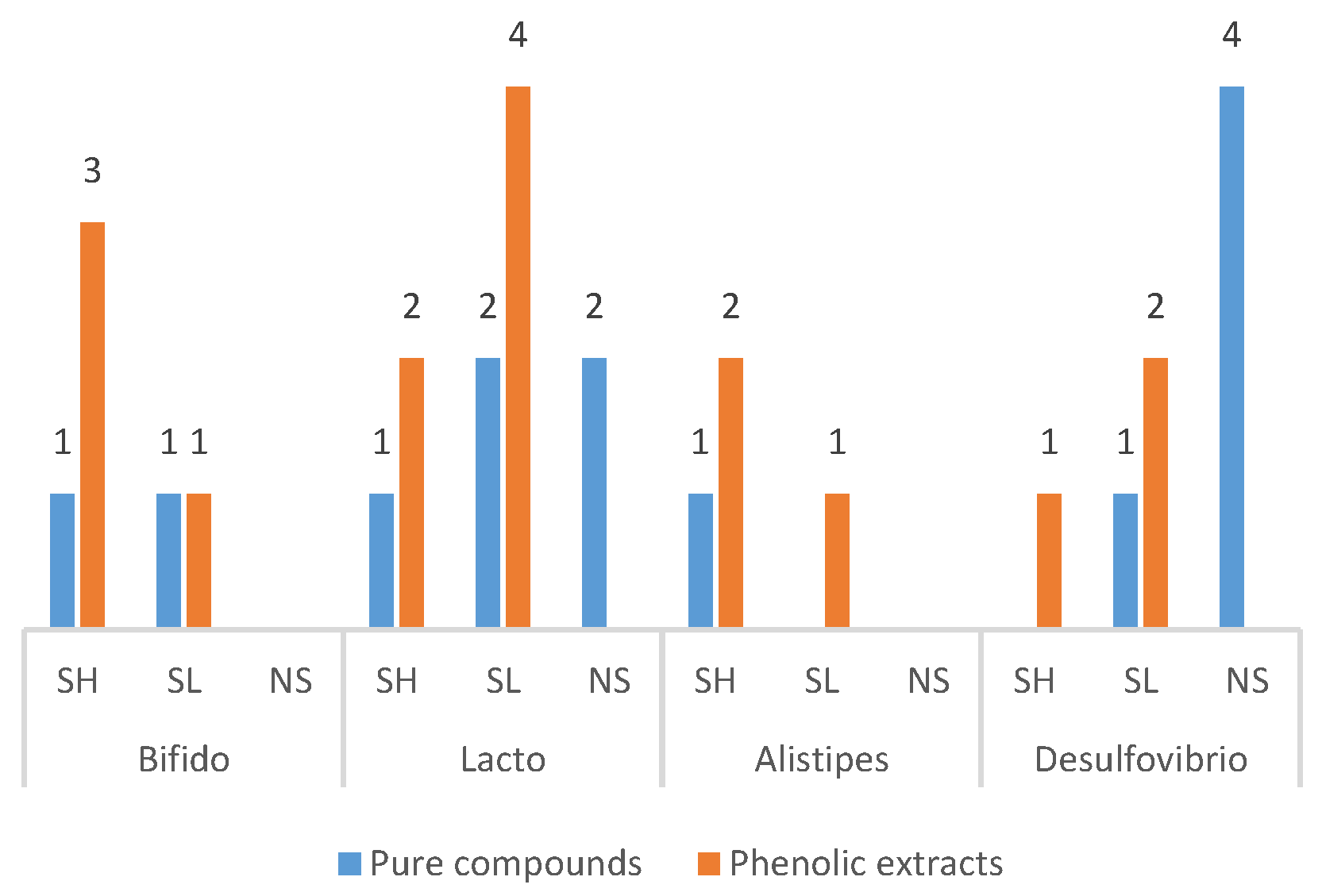
3.4.3. Frequently Modulated Gut Microbes
4. Discussion
4.1. Effect of Polyphenols on Obesity-Associated Parameters, Adipocytokines, and LPS/LBP
4.2. Effect of Polyphenols on Glucose Homeostasis
4.3. Effect of Polyphenols on Gut Microbiota
4.4. Is LPS the Only Linking Factor between Metabolic Derangements and Gut Dysbiosis?
5. Limitations
5.1. Experimental Animals and Their Environment
5.2. Types of Intervention
5.3. Methodology to Assess GM
5.4. Outcomes Reported
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- CDC. The Health Effects of Overweight and Obesity 2020. Available online: https://www.cdc.gov/healthyweight/effects/index.html (accessed on 11 March 2020).
- Pereira, M.A. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: A review of the evidence. Nutr. Rev. 2013, 71, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C. Committee obotWKDS. Obesity and Kidney Disease: Hidden Consequences of the Epidemic. Am. J. Hypertens. 2017, 30, 328–336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dye, L.; Boyle, N.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Alsalhe, T.A.; Chalghaf, N.; Riccò, M.; Bragazzi, N.L.; Wu, J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17, e1003198. [Google Scholar] [CrossRef]
- Schnurr, T.M.; Jakupović, H.; Carrasquilla, G.D.; Ängquist, L.; Grarup, N.; Sørensen, T.I.A.; Tjønneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef]
- Ghosh, S.; Bouchard, C. Convergence between biological, behavioural and genetic determinants of obesity. Nat. Rev. Genet. 2017, 18, 731–748. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Gérard, P. Gut microbiota and obesity. Cell. Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Justino, A.B.; Pereira, M.N.; Peixoto, L.G.; Vilela, D.D.; Caixeta, D.C.; De Souza, A.V.; Teixeira, R.R.; Silva, H.C.G.; de Moura, F.B.R.; Moraes, I.B.; et al. Hepatoprotective Properties of a Polyphenol-Enriched Fraction fromAnnona crassifloraMart. Fruit Peel against Diabetes-Induced Oxidative and Nitrosative Stress. J. Agric. Food Chem. 2017, 65, 4428–4438. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, B.O.; Ojo, O.A.; Akuboh, O.S.; Abiola, O.M.; Idowu, O.; Amuzat, A.O. Anti-Hyperglycemic and Anti-Inflammatory Activities of Polyphenolic-Rich Extract of Syzygium cumini Linn Leaves in Alloxan-Induced Diabetic Rats. J. Evid. Based Integr. Med. 2018, 23. [Google Scholar] [CrossRef]
- Palanisamy, U.D.; Ling, L.T.; Manaharan, T.; Appleton, D. Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem. 2011, 127, 21–27. [Google Scholar] [CrossRef]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-Y.; Lee, Y.-J.; Hong, J.T.; Lee, H.-J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, J.; Rogers, J.; Xie, J. Brain Iron Metabolism Dysfunction in Parkinson’s Disease. Mol. Neurobiol. 2016, 54, 3078–3101. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Fahmy, H.; Hegazi, N.M.; El-Shamy, S.; Farag, M.A. Pomegranate juice as a functional food: A comprehensive review of its polyphenols, therapeutic merits, and recent patents. Food Funct. 2020, 11, 5768–5781. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Lacueva, M.C.A.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Moorthy, M.; Chaiyakunapruk, N.; Jacob, S.A.; Palanisamy, U.D. Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: A systematic review of randomised controlled trials. Trends Food Sci. Technol. 2020, 99, 634–649. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Leenaars, M.; Ritskes-Hoitinga, M. A Gold Standard Publication Checklist to Improve the Quality of Animal Studies, to Fully Integrate the Three Rs, and to Make Systematic Reviews More Feasible. Altern. Lab. Anim. 2010, 38, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Bule, M.H.; Abdurahman, A.; Nikfar, S.; Abdollahi, M.; Amini, M. Antidiabetic effect of quercetin: A systematic review and meta-analysis of animal studies. Food Chem. Toxicol. 2019, 125, 494–502. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Sánchez, M.; Perez-Cruz, C.; Velázquez-Villegas, L.A.; Syeda, T.; Aguilar-López, M.; Rocha-Viggiano, A.K.; Del Carmen Silva-Lucero, M.; Torre-Villalvazo, I.; Noriega, L.G. Long-Term Genistein Consumption Modifies Gut Microbiota, Improving Glucose Metabolism, Metabolic Endotoxemia, and Cognitive Function in Mice Fed a High-Fat Diet. Mol. Nutr. Food Res. 2018, 62, e1800313. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tang, R.; Yang, S.; Lu, Y.; Luo, J.; Liu, Z. Rutin and Its Combination With Inulin Attenuate Gut Dysbiosis, the Inflammatory Status and Endoplasmic Reticulum Stress in Paneth Cells of Obese Mice Induced by High-Fat Diet. Front. Microbiol. 2018, 9, 2651. [Google Scholar] [CrossRef]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Lin, Y.-H.; Chen, H.-J.; Chou, S.-C.; Cheng, A.-C.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. Piceatannol Exerts Anti-Obesity Effects in C57BL/6 Mice through Modulating Adipogenic Proteins and Gut Microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Florez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free. Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Brandt, N.; Kotowska, D.; Kristensen, C.M.; Olesen, J.; Lützhøft, D.O.; Halling, J.F.; Hansen, M.; Al-Soud, W.A.; Hansen, L.; Kiilerich, P.; et al. The impact of exercise training and resveratrol supplementation on gut microbiota composition in high-fat diet fed mice. Physiol. Rep. 2018, 6, e13881. [Google Scholar] [CrossRef]
- Guo, J.; Han, X.; Zhan, J.; You, Y.; Huang, W. Vanillin Alleviates High Fat Diet-Induced Obesity and Improves the Gut Microbiota Composition. Front. Microbiol. 2018, 9, 2733. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef] [PubMed]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.L.; Yu, R.; Li, F.; Zhou, Q.; Chen, D.; Qi, C.; Yin, Y.; Sun, J. Modulation of fat metabolism and gut microbiota by resveratrol on high-fat diet-induced obese mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Huang, K.; Zhao, C.; Xu, W.; Sheng, Y.; Luo, Y.; He, X. Procyanidin attenuates weight gain and modifies the gut microbiota in high fat diet induced obese mice. J. Funct. Foods 2018, 49, 362–368. [Google Scholar] [CrossRef]
- Xie, M.; Chen, G.; Wan, P.; Dai, Z.; Zeng, X.; Sun, Y. Effects of Dicaffeoylquinic Acids from Ilex kudingcha on Lipid Metabolism and Intestinal Microbiota in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2018, 67, 171–183. [Google Scholar] [CrossRef]
- Sung, M.M.; Kim, T.; Denou, E.; Soltys, C.-L.M.; Hamza, S.M.; Byrne, N.J.; Masson, G.; Park, H.; Wishart, D.S.; Madsen, K.L.; et al. Improved Glucose Homeostasis in Obese Mice Treated With Resveratrol Is Associated With Alterations in the Gut Microbiome. Diabetes 2016, 66, 418–425. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front. Microbiol. 2019, 10, 390. [Google Scholar] [CrossRef]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A.; et al. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [CrossRef]
- Sheng, L.; Jena, P.K.; Liu, H.X.; Hu, Y.; Nagar, N.; Bronner, D.N.; Settles, M.L.; Bäumler, A.J.; Yvonne Wan, Y.-J. Obesity treatment by epigallocatechin-3-gallate—Regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J. 2018, 32, 6371–6384. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61, 1601082. [Google Scholar] [CrossRef]
- Wang, Z.; Lam, K.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.-X.; Lin, S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Yin, X.; Li, Q.; Zhang, H.; Liu, Z.; Zheng, X.; Zheng, L.; Feng, X. Resveratrol-Induced White Adipose Tissue Browning in Obese Mice by Remodeling Fecal Microbiota. Molecules 2018, 23, 3356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F.-H. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef]
- Luo, Q.; Cheng, D.; Huang, C.; Li, Y.; Lao, C.; Xia, Y.; Liu, W.; Gong, X.; Hu, D.; Li, B.; et al. Improvement of Colonic Immune Function with Soy Isoflavones in High-Fat Diet-Induced Obese Rats. Molecules 2019, 24, 1139. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, S.; Wang, X.; Weng, Y.; Fan, X.; Sheng, H.; Zhu, X.; Lou, L.; Zhang, F. The flavonoid-rich Quzhou Fructus Aurantii extract modulates gut microbiota and prevents obesity in high-fat diet-fed mice. Nutr. Diabetes 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Li, C.; Lin, K.; Li, K.; Deng, X.; Li, C. Reshaped fecal gut microbiota composition by the intake of high molecular weight persimmon tannin in normal and high-cholesterol diet-fed rats. Food Funct. 2017, 9, 541–551. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; An, J.; Lee, S.; Kong, H.; Song, Y.; Choi, H.R.; Lee, S.J.; Chae, K.S.; Kwon, J.W.; et al. Amelioration of hyperglycemia by Rubus occidentalis (black raspberry) and increase in short-chain fatty acids producing bacteria. J. Funct. Foods 2019, 54, 433–439. [Google Scholar] [CrossRef]
- Collins, B.; Hoffman, J.; Martinez, K.; Grace, M.; Lila, M.A.; Cockrell, C.; Nadimpalli, A.; Chang, E.; Chuang, C.-C.; Zhong, W.; et al. A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. J. Nutr. Biochem. 2016, 31, 150–165. [Google Scholar] [CrossRef]
- Guo, J.; Han, X.; Tan, H.; Huang, W.; You, Y.; Zhan, J. Blueberry Extract Improves Obesity through Regulation of the Gut Microbiota and Bile Acids via Pathways Involving FXR and TGR5. iScience 2019, 19, 676–690. [Google Scholar] [CrossRef]
- Cheng, D.M.; Roopchand, D.E.; Poulev, A.; Kuhn, P.; Armas, I.; Johnson, W.D.; Oren, A.; Ribnicky, D.; Zelzion, E.; Bhattacharya, D.; et al. High phenolics Rutgers Scarlet Lettuce improves glucose metabolism in high fat diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, H.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice. Food Funct. 2016, 7, 4869–4879. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.E.; Witrick, K.A.; Klotz, C.; Dorenkott, M.R.; Goodrich, K.M.; Fundaro, G.; McMillan, R.P.; Hulver, M.W.; Ponder, M.A.; Neilson, A.P. Alterations to metabolically active bacteria in the mucosa of the small intestine predict anti-obesity and anti-diabetic activities of grape seed extract in mice. Food Funct. 2017, 8, 3510–3522. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-J.; Li, J.; Su, Q.; Liu, Y. Sinapine reduces non-alcoholic fatty liver disease in mice by modulating the composition of the gut microbiota. Food Funct. 2019, 10, 3637–3649. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, B.; Hu, Y.; Wang, J.; Liu, J.-M.; Qin, R.; Lv, S.; Wang, S. Correlation Analysis of Intestinal Redox State with the Gut Microbiota Reveals the Positive Intervention of Tea Polyphenols on Hyperlipidemia in High Fat Diet Fed Mice. J. Agric. Food Chem. 2019, 67, 7325–7335. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, M.; Geurts, L.; Plovier, H.; Druart, C.; Everard, A.; Ståhlman, M.; Rhimi, M.; Chira, K.; Teissedre, P.-L.; Delzenne, N.M.; et al. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am. J. Physiol. 2018, 314, E334. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Vilela, V.R.; Stephanie, D.; Pilon, G.; Fournier, M.; Lecours, M.-A.; Desjardins, Y.; Roy, D.; et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol. Metab. 2017, 6, 1563–1573. [Google Scholar] [CrossRef]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Pilon, G.; Dudonné, S.; Trottier, J.; St-Pierre, P.; Harris, C.S.; Lucas, M.; Lemire, M.; et al. Arctic berry extracts target the gut–liver axis to alleviate metabolic endotoxaemia, insulin resistance and hepatic steatosis in diet-induced obese mice. Diabetologia 2018, 61, 919–931. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Dai, Z.; Wan, P.; Ye, H.; Zeng, X.; Sun, Y. Kudingcha and Fuzhuan Brick Tea Prevent Obesity and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, e1700485. [Google Scholar] [CrossRef]
- Xu, J.; Liu, T.; Li, Y.; Liu, W.; Ding, Z.; Ma, H.; Seeram, N.P.; Mu, Y.; Huang, X.; Li, L. Jamun (Eugenia jambolana Lam.) Fruit Extract Prevents Obesity by Modulating the Gut Microbiome in High-Fat-Diet-Fed Mice. Mol. Nutr. Food Res. 2019, 63, e1801307. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Sasaki, G.Y.; Wei, P.; Li, J.; Wang, L.; Zhu, J.; McTigue, D.; Yu, Z.; Bruno, R.S. Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin translocation and adipose inflammation. J. Nutr. Biochem. 2019, 67, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hu, R.; Nakano, H.; Chen, K.; Liu, M.; He, X.; Zhang, H.; He, J.; Hou, D.-X. Modulation of Gut Microbiota by Lonicera caerulea L. Berry Polyphenols in a Mouse Model of Fatty Liver Induced by High Fat Diet. Molecules 2018, 23, 3213. [Google Scholar] [CrossRef]
- Liu, M.; Tan, J.; He, Z.; He, X.; Hou, D.-X.; He, J.; Wu, S. Inhibitory effect of blue honeysuckle extract on high-fat-diet-induced fatty liver in mice. Anim. Nutr. 2018, 4, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tian, Y.; Yu, J.; Zhang, R.; Zhang, X.; Guo, P. The pandanus tectorius fruit extract (PTF) modulates the gut microbiota and exerts anti-hyperlipidaemic effects. Phytomedicine 2019, 58, 152863. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sánchez, M.; Toral, M.; Martín-García, B.; Gómez-Caravaca, A.M.; Arráez-Román, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef]
- Henning, S.M.; Yang, J.; Hsu, M.; Lee, R.-P.; Grojean, E.M.; Ly, A.; Tseng, C.-H.; Heber, D.; Li, Z. Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur. J. Nutr. 2018, 57, 2759–2769. [Google Scholar] [CrossRef]
- Zhao, R.; Long, X.; Yang, J.; Du, L.; Zhang, X.; Li, J.; Hou, C. Pomegranate peel polyphenols reduce chronic low-grade inflammatory responses by modulating gut microbiota and decreasing colonic tissue damage in rats fed a high-fat diet. Food Funct. 2019, 10, 8273–8285. [Google Scholar] [CrossRef]
- Lee, S.; I Keirsey, K.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; De La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet–Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, C.; Li, Y.; Liu, R.; Ao, M.; Li, F.; Yao, Y.; Tao, Z.; Yu, L.-J. The ameliorative effect of the Pyracantha fortuneana (Maxim.) H. L. Li extract on intestinal barrier dysfunction through modulating glycolipid digestion and gut microbiota in high fat diet-fed rats. Food Funct. 2019, 10, 6517–6532. [Google Scholar] [CrossRef]
- Ding, Y.; Song, Z.; Li, H.; Chang, L.; Pan, T.; Gu, X.; He, X.; Fan, Z. Honokiol Ameliorates High-Fat-Diet-Induced Obesity of Different Sexes of Mice by Modulating the Composition of the Gut Microbiota. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Liao, Z.-L.; Zeng, B.-H.; Wang, W.; Li, G.-H.; Wu, F.; Wang, L.; Zhong, Q.-P.; Wei, H.; Fang, X. Impact of the Consumption of Tea Polyphenols on Early Atherosclerotic Lesion Formation and Intestinal Bifidobacteria in High-Fat-Fed ApoE−/− Mice. Front. Nutr. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef]
- Lee, Y.S.; Li, P.; Huh, J.Y.; Hwang, I.J.; Lu, M.; Kim, J.I.; Ham, M.; Talukdar, S.; Chen, A.; Lu, W.J.; et al. Inflammation Is Necessary for Long-Term but Not Short-Term High-Fat Diet-Induced Insulin Resistance. Diabetes 2011, 60, 2474–2483. [Google Scholar] [CrossRef]
- Cheng, H.S.; Goh, B.H.; Phang, S.C.W.; Amanullah, M.M.; Ton, S.H.; Palanisamy, U.D.; Kadir, K.A.; Tan, J.B.L. Pleiotrosepic ameliorative effects of ellagitannin geraniin against metabolic syndrome induced by high-fat diet in rats. Nutrition 2020, 79–80, 110973. [Google Scholar] [CrossRef]
- Vernochet, C.; Mourier, A.; Bezy, O.; Macotela, Y.; Boucher, J.; Rardin, M.J.; An, D.; Lee, K.Y.; Ilkayeva, O.R.; Zingaretti, C.M.; et al. Adipose-Specific Deletion of TFAM Increases Mitochondrial Oxidation and Protects Mice against Obesity and Insulin Resistance. Cell Metab. 2012, 16, 765–776. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Henning, S.; Chan, B.; Long, J.; Zhong, J.; Acin-Perez, R.; Petcherski, A.; Shirihai, O.; Heber, D.; et al. Ellagic Acid and Its Microbial Metabolite Urolithin a Alleviate Diet-induced Insulin Resistance in Mice (OR24-03-19). Curr. Dev. Nutr. 2019, 3 (Suppl. 1), nzz031-OR24. [Google Scholar] [CrossRef]
- Perera, A.; Ton, S.H.; Moorthy, M.; Palanisamy, U.D. The insulin-sensitising properties of the ellagitannin geraniin and its metabolites from Nephelium lappaceum rind in 3T3-L1 cells. Int. J. Food Sci. Nutr. 2020, 2020, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran. 2017, 31, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Sheedy, K. Effects of Polyphenols on Insulin Resistance. Nutrients 2020, 12, 3135. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Kerimi, A.; Nyambe-Silavwe, H.; Gauer, J.S.; Tomás-Barberán, F.A.; Williamson, G. Pomegranate juice, but not an extract, confers a lower glycemic response on a high–glycemic index food: Randomized, crossover, controlled trials in healthy subjects. Am. J. Clin. Nutr. 2017, 106, 1384–1393. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal α-glucosidase. J. Enzym. Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef]
- Song, J.; Kwon, O.; Chen, S.; Daruwala, R.; Eck, P.; Park, J.B.; Levine, M. Flavonoid Inhibition of Sodium-dependent Vitamin C Transporter 1 (SVCT1) and Glucose Transporter Isoform 2 (GLUT2), Intestinal Transporters for Vitamin C and Glucose. J. Biol. Chem. 2002, 277, 15252–15260. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Aydin, E.; Gauer, J.S.; Pyner, A.; Williamson, G.; Kerimi, A. Green and Chamomile Teas, but not Acarbose, Attenuate Glucose and Fructose Transport via Inhibition of GLUT2 and GLUT. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Houghton, M.J.; Kerimi, A.; Mouly, V.; Tumova, S.; Williamson, G. Gut microbiome catabolites as novel modulators of muscle cell glucose metabolism. FASEB J. 2018, 33, 1887–1898. [Google Scholar] [CrossRef]
- Ueda-Wakagi, M.; Mukai, R.; Fuse, N.; Mizushina, Y.; Ashida, H. 3-O-Acyl-epicatechins Increase Glucose Uptake Activity and GLUT4 Translocation through Activation of PI3K Signaling in Skeletal Muscle Cells. Int. J. Mol. Sci. 2015, 16, 16288–16299. [Google Scholar] [CrossRef]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.J.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, T.; Beasley, D.E.; Heděnec, P.; Xiao, Z.; Zhang, S.; Li, J.; Lin, Q.; Li, X. Diet Diversity Is Associated with Beta but not Alpha Diversity of Pika Gut Microbiota. Front. Microbiol. 2016, 7, 1169. [Google Scholar] [CrossRef] [PubMed]
- Castañer, O.; Goday, A.; Park, Y.-M.; Lee, S.; Magkos, F.; Shiow, S.-A.T.E.; Schröder, H. The Gut Microbiome Profile in Obesity: A Systematic Review. Int. J. Endocrinol. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; van der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill–Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716–1724.e2. [Google Scholar] [CrossRef] [PubMed]
- Tims, S.; Derom, C.; Jonkers, D.; Vlietinck, R.; Saris, W.H.; Kleerebezem, M.; De Vos, W.M.; Zoetendal, E.G. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013, 7, 707–717. [Google Scholar] [CrossRef]
- Patil, D.P.; Dhotre, D.P.; Chavan, S.G.; Sultan, A.; Jain, D.S.; Lanjekar, V.B.; Gangawani, J.; Shah, P.S.; Todkar, J.S.; Shah, S.; et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J. Biosci. 2012, 37, 647–657. [Google Scholar] [CrossRef]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef]
- Sze, M.A.; Schloss, P.D. Looking for a Signal in the Noise: Revisiting Obesity and the Microbiome. mBio 2016, 7, e01018-16. [Google Scholar] [CrossRef]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef]
- Finucane, M.M.; Sharpton, T.J.; Laurent, T.J.; Pollard, K.S. A Taxonomic Signature of Obesity in the Microbiome? Getting to the Guts of the Matter. PLoS ONE 2014, 9, e84689. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions with Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y. Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl. Environ. Microbiol. 2020, 86, e03004-19. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.K.; Renuka; Dangi, A.K.; Shandilya, U.K.; Puniya, A.K.; Shukla, P. Chapter 44—New-Generation Probiotics: Perspectives and Applications. In Microbiome and Metabolome in Diagnosis, Therapy, and other Strategic Applications; Faintuch, J., Faintuch, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 417–424. [Google Scholar]
- Sears, C.L.; Franco, A.A.; Wu, S. CHAPTER 28—Bacteroides fragilis toxins. In The Comprehensive Sourcebook of Bacterial Protein Toxins, 3rd ed.; Alouf, J.E., Popoff, M.R., Eds.; Academic Press: London, UK, 2006; pp. 535–546. [Google Scholar]
- Benítez-Páez, A.; Del Pugar, E.M.G.; López-Almela, I.; Moya-Pérez, Á.; Codoñer-Franch, P.; Sanz, Y.; Jenq, R. Depletion of Blautia Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening. mSystems 2020, 5. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Smecuol, E.; Hwang, H.J.; Sugai, E.; Corso, L.; Cherñavsky, A.C.; Bellavite, F.P.; González, A.; Vodánovich, F.; Moreno, M.L.; Vázquez, H.; et al. Exploratory, Randomized, Double-blind, Placebo-controlled Study on the Effects of Bifidobacterium infantis Natren Life Start Strain Super Strain in Active Celiac Disease. J. Clin. Gastroenterol. 2013, 47, 139–147. [Google Scholar] [CrossRef]
- Agrawal, A.; Houghton, L.A.; Morris, J.; Reilly, B.; Guyonnet, D.; Feuillerat, N.G.; Schlumberger, A.; Jakob, S.; Whorwell, P.J. Clinical trial: The effects of a fermented milk product containingBifidobacterium lactisDN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. 2009, 29, 104–114. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Yu, J.G.; Lee, I.; Liu, M.; Shen, Y.; Sharma, S.P.; Jamal, M.A.H.M.; Yoo, J.; Kim, H.; Hong, S. Intestinal removal of free fatty acids from hosts by Lactobacilli for the treatment of obesity. FEBS Open Biol. 2016, 6, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Angelakis, E.; Paul, M.; Armougom, F.; Leibovici, L.; Raoult, D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb. Pathog. 2012, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Ling, Z.; Chen, D.; Liu, Y.; Yang, F.; Li, L. Disorganized Gut Microbiome Contributed to Liver Cirrhosis Progression: A Meta-Omics-Based Study. Front. Microbiol. 2018, 9, 3166. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Gerner, R.R.; Wang, J.; Klepsch, V.; Adolph, T.E.; Reider, S.J.; Hackl, H.; Pfister, A.; Schilling, J.; Moser, P.L.; et al. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Microbe 2016, 19, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Rowan, F.; Docherty, N.G.; Murphy, M.; Murphy, B.; Coffey, J.C.; O‘Connell, P.R. Desulfovibrio Bacterial Species Are Increased in Ulcerative Colitis. Dis. Colon Rectum 2010, 53, 1530–1536. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.; Kootte, R.S.; Van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]


| Polyphenol | Gut Microbiota |
|---|---|
| Dietary polyphenol Polyphenol Flavonoid Fruit Vegetable Plant extract Herbal drug Medicinal plant Antioxidant Anthocyanin Chalcones Catechin Flavanone Proanthocyanidin Ellagitannin Functional food Green tea Puerh tea Cocoa Chocolate Myo-inositol Soy isoflavone Blueberries Berries Grape Quercetin Citrus Cinnamon Red wine Resveratrol Natural s-equol | Microbiota Gut microbiota Colonic microbiota Gastrointestinal microbiota Intestinal microbiota Gut organism Microbial consortia Gut bacterium Gut flora Gastrointestinal flora Intestinal flora |
| No. | Author, Yr, [Reference] | Species, Sex, Age (w)/ Weight (g) | Number of Animals, n/ Groups (Grp) | Prevention (p)/Treatment (T) | Duration of Intervention (d/wks/mths) | HFD (%Fat) | Dosage | MOA | Sample/Method/ Hypervariable Region/GM Composition | Energy/Food Intake | Weight | VAT/ SAT | Glucose (FG/GTT/ITT) | Hormones | HOMA-IR | Adipocytokine | Lipid Profile | Endotoxins |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PURE COMPOUNDS-MICE | ||||||||||||||||||
| 1 | López et al., 2018 [35] | C57BL6 mice, M, 9 w | 8/grp ND HFD + genistein(G) | p | 24 w | NA | Genistein −0.2% | Within diet | Feces, 16s rRNA, V3-V4 regions, Illumina MiSeq: Alpha diversity−high Phyla: Firmicutes-H Bacteroidetes−L Verrucomicrobia-H Genera: Bacteroides-L Prevotella and Akkermansia-H Species: Prevotella copri, Prevotella stercorea, Akkermansia muciniphila-H Bacteroides acidifaciens and Bacteroides uniformis-L | SL | SL | SAT:SL | FG−NS IpGTT(AUC)−SL | TC-NS TAG-SL LDL-NS | LPS-SL | |||
| 2 | X. Guo et al., 2018 [36] | C57BL/6J mice, M, 4 w | 12/grp ND HFD HFD + R HFD + R + Inulin * only results for rutin reported in this review | p | 20 w | 60% | Rutin (R)-6.4 mg/g diet | Within diet | small intestine content, 16s rRNA, V4 region, Illumina HiSeq: Beta-diveristy (PCoA)-cluster near ND Phyla: Firmicutes-SL Bacteroidetes-NS Deferribacteres-NS Actinobacteria-NS Proteobacteria-SH F:B ratio-NS Families: Rikenellaceae-NS Porphyromonadaceae-NS Bacteroidaceae-SH Bacteroidales_S24-7 group-SH Deferribacteraceae-NS Erysipelotrichaceae-SH Ruminococcaceae-NS Desulfovibrionaceae-SH Helicobacteraceae-NS Lachnospiraceae-SL LDA > 3 (genus): Desulfovibrio | NS | SL | Leptin-SL IL-6-SL TNFa-SL IFNy-SL IL-2-SL IL4-SL | TC-NS TAG-NS | LPS-SL | ||||
| 3 | Masumoto et al., 2016 [37] | C57BL/6Jmice, M, 9 w | 10/grp ND HFHS HFHS + OP HFHS + PP | NA | 20 w | NA | Oligomeric procyanidins (OP)-NA Polymeric procyanidins (PPs)-0.5% | NA | Cecal content, 16S rRNA, V3–V4 regions, Illumina MiSeq: Beta-diversity (PCoA)-Distinct cluster for OP and PP Phyla: Firmicutes-SL (PP) Bacteroidetes-NS Verrucomicrobia-NS Proteobacteria-NS Tenericutes-NS Deferribacteres-NS Actinobacteria-NS Significance observed with PP only for the following genera: Bifidobacterium-SL Adlercreutzia-SH Bacteroides-SH Rikenellaceae-SH S24-7-SH Lachnospiraceae-SL Roseburia-SH Ruminococcus-SL Peptococcaceae-SL rc4-4-SH Ruminococcus-SL Anaerovorax-SH Anaeroplasma-SH Akkermansia-SH | OP, PP-NS | OP-NS, PP-SL | VAT: OP, PP-SLSAT: OP, PP-SL | OP, PP-SL | TNFa: PP-SL IL-6:PP-SL | TC: OP, PP-SLTAG:OP, PP-SL | LPS:PP-SL | ||
| 4 | Tung et al., 2016 [38] | C57BL/6 mice, M, 5 w | 8/grp ND HFD HFD + 0.1%R HF+ 0.1% Pic (LP)HFD+ 0.25% Pic(HP) | p | 18 w | 45% | 0.1% Resveratrol (R), 0.1% Piceatannol (Pic), 0.25% Pic(1 kg of HFD contained 1 or 2.5 g Pic powde) | Withi n diet | Feces, 16S rRNA, V4 region, Illumina MiSeq:* No statistics Phyla: Firmicutes: LP, HP-H Bacteroidetes: LP, HP-L Genera:Bifidobacterium: LP, HP-L Lactobacillus: LP, HP-HPedobacter: LP-L, HP-HBlautia:LP-H, HP-L Dysgonomonas: LP, HP-L | R, LP, HP-NS | LP, HP-SL | Perigonadal: R, LP, HP-SL RP: R, LP, HP-SL Mesen: R, LP, HP-NS | FG:LP, HP-SL | TC: LP, HP-SL TAG:LP, HP, R-NS LDL:HP-SL HDL: LP, HP-SL | ||||
| 5 | Porras et al., 2017 [39] | C57BL/6J mice, M, 7 w | 10/grp:NDND + QHFDHFD + Q | p | 16w | 60% | Quercetin (Q)-0.05% (w/w) aglycone quercetin | Within diet | Cecal content, 16 srRNA, V3–V4regions, Illumina MiSeq: Beta-diversity (PCoA)-cluster with HFD Phyla: Firmicutes-NS Bacteroidetes-NS Proteobacteria-SL F:B ratio-SL Genera: Desulfovibrio-SL Helicobacter-SL Flavobacterium-SHAllobaculum-SH Sutturella-SH Blautia, Akkermansia, Oscillospira, Parabacteroides, Alkaliphilus, Lactobacillus-NS | NS | SL | Epid-SL | FG-SL | FI-SL | SL | IL-6-SL | Plasma:TAG-SLLiver:TAG, FFA-SL | LPS-SL |
| 6 | Brandt et al., 2018 [40] | C57BL/6N with loxP insertions in the Ppargc1a gene, M, 8–10 w | n = NANDHFDHFD + R HFD + exercise (Ex) * only results for resveratrol reported in this review | p | 16 w | 60% | Resveratrol (R)-4 g/kg HFD | Within diet | Colon sample, 16s rRNA, V3–V4 region, GS FLX titanium pyrosequencing: Alpha diversity (Shannon index)-NSBeta-diversity (PCoA)-distinct centroids were observed for each treatment, SL Phyla affected by R: Proteobacteria, Verrucomicrobia Genera: Allobaculum-SHAlistipes-NS Dorea-NS | SL | SL | VAT: NSSAT: NS | Serum Amyloid A-NS | |||||
| 7 | J. Guo et al., 2018 [41] | C57BL/6J mice, M, 3 w | 7–8/grp ND HFDHFD + V | p | 14 w | 60% | 0.1% vanillin | Within diet | Pooled content of colon, rectum and cecum, 16r rRNA, Illumina HiSeq: Ace index, Chao1, OTU (richness)-SHShannon and Simpson (homogeneity)-NSBeta-diversity(PCoA)-similar to NDPhyla: Firmicutes-L Proteobacteria-L Verrucomicrobia-SH Actinobacteria-H Genera: (LDA > 3) Akkermansia, Romboutsia, Peptoclostridium | SH | SL | Epid-SL Inguinal-SL | FG-SL OGTT(AUC)-SL ITT(AUC)-SL | TNFa-SL IL-6-SL | TC-SL TAG-SL LDL-SL HDL-NS | LPS-SL | ||
| 8 | Cremonini et al., 2019 [42], Daveri et al., 2018 [43] | C57BL/6J mice, M, (20–25 g) | 10/grp NDND + AC HFDHFD + AC | p | 14 w | 60% | Anthocyanins(AC)-40 mg/kg | Within diet | Cecal content, 16s rRNA, V4 region, Illumina MiSeq: Beta-diversity(NMDS)-closer to ND F:B ratio-SL Akkermansia-SH | SL | SL | VAT: SL SAT: SL | FG-SL OGTT(AUC)-SL ITT(AUC)-SL | FI-SL GIP, GLP-1-SL GLP-2-SH | Leptin-SL Adiponection-NS | Plasma: TC-SL TAG-SL Liver:TAG-SL | Endotoxin-SL FITC-dextran-SL | |
| 9 | Campbell et al., 2019 [44] | C57BL/6J mice, M, 4 w | 12/grp ND HFD HFD + L HFD + M HFD + H | T | 12 w | 35% | HFD + L − 50 mg/kg/day HFD + M − 75 mg/kg/day HFD + H − 100 mg/kg/day Resveratrol were dissolved in 0.4 mL of absolute ethanol and added to 100 mL of drinking water daily | Drinking water | Cecal content, 16s rRNA, V4 region, Illumina MiSeq: Alpha diversity (Chao1)-MD, HD-SHPCoA-RSV improved the GM shift caused by HFD but not completely Family: Desulfovibrionaceae-LD, MD, HD-NS Prevotellaceae-LD, MD, HD-NS Verrucomicrobiaceae-LD, MD, HD-NS Deferribacteraceae-MD-SH, LD, HD-NS | LD, MD, HD-NS | LD-NS MD-SL HD-SL | Epid, perinephric, mesen:LD-NS MD-SL HD-SL | FG: LD, MD, HD-SL | IL-1: MD, HD-SLIL-10: LD, MD, HD-SL TNFa: MD, HD-SL | TAG: LD, MD, HD-SL HDL:HD-SH LDL:LD, MD, HD-SL | LPS:LD, MD, HD-SL LBP: LD, MD HD-SL | ||
| 10 | Zheng et al., 2018 [45] | C57BL/6J mice, M, 3 w | 6/grp: LFHFD HFD + p | T | 12 | 60% | Procyanidin(p) 100 mg/kg | Oral gavage | Feces, 16s rRNA, V3–V4 regions, Illumina MiSeq:Alpha diversity:Simpson: NSBeta diversity (Bray curtis): SH Phyla: Firmicutes: NS Bacteroidetes: SH F:B ratio-SL Genera: Rikenellaceae RC9 gut group-H Blautia-H Anaerotruncus colihominis-H Helicobacter hepaticus-H Rikenella-L Lachnospiraceae_FCS020_group-L Clostridiales_bacterium_CIEAF_020-L Lachnospiraceae_UCG-006-L Peptococcus-L Ruminococcaceae-L [Eubacterium] _coprostanoligenes_group-L Ruminiclostridium-L Ruminiclostridium_5-L Ruminococcaceae_UCG-004-L Ruminococcaceae_UCG-014-L Desulfovibrio-L | SL | VAT: NS | TC-SH TAG-NS HDL-SH LDL-NS | ||||||
| 11 | Xie et al., 2018 [46] | C57BL/6 mice, M, 6 w | 8/grp ND ND + LD HFD HFD + LD HFD + HD | p | 9 w | 45% | kudingcha dicaffeoylquinic acids Low does (LD)−3.3 mg/mouse high dose (HD)-10.0 mg/mouse | Oral gavage | Feces, 16S rRNA, V4 region, Illumina MiSeq:Alpha diversity:Shannon: LD-SH, HD-NS Simpson: LD-SL, HD-NS Beta diversity: PCoA, NMD-LD cluster near HFD, HD cluster relatively far from HFD Phyla:Firmicutes-LD, HD-NS Bacteroidetes: LD, HD-NS Proteobacteria: LD, HD-NS Actinobacteria: LD-SL, HD-NS Verrucomicrobia: LD-NS, HD-SH F:B ratio-NS Genera:Akkermansia, Bifidobacterium, Anaerobacterium-LD, HD-SH Coprobacter, Olsenella-LD, HD-L | NS | Perirenal: LD, HD-SL Epid:HD-SL | CRP:LD, HD-SL TNFa:LD, HD-SL Il-6:LD, HD-SL | TC:LD-NS, HD-SL TAG:LD, HD-NS LDL: LD, HD-SL HDL: LD-NS, HD-SL | LPS:LD, HD-SL | ||||
| 12 | Sung et al., 2017 [47] | C57BL/6N mice, M, 8 w | 10/grp NDND + RHFHSHFHs + R | p | 8 w | 45% | Resveratrol (R)-0.4% | Within diet | Cecal content, 16s rRNA, V3 region, Illumina MiSeq: PCA-distinct cluster F:B ratio: SL Genera:Moryella-SL Akkermansia-SL Bacteroides-SH Parabacteroides-SH LDA > 3: Anaerostipes, Adlercreutzia, Parabacteroides, Coprobacillus | NS | Total body fat: SL | OGTT (AUC): SL | ||||||
| 13 | Zhuoqun Liu et al., 2019 [48] | C57BL/6J mice, M, 3 w | 7/grp ND HFD HFD + HTHFD + Fecal transplantation (FT) * only results for HT reported in this review | p | 8 w | 45% | Hydroxytyrosol (HT)−50 mg/kg/day | Oral gavage | Feces, 16s rRNA, V3–V4, Ilumina MiSeq: Alpha diversity: Simpson Index-NSBeta-diversity: PCoA-the HT clusters are not distinct from HFD Phyla:Firmicutes-NS Bacteroidetes-NS Proteobacteria-NS Deferribacteres-NS F:B-NS Genera:Lactobacillus-SH Rikenella-SL Desulfovibrio-NS Ruminiclostridium-NS Species: Lactobacillus johnsonii-SHAnaerotruncus sp. G3 [2012]-SL | NS | NS | RP-SL Epid-SL SAT: NS | FG-SL OGTT(AUC)-NS ITT(AUC)-NS | FI-SL | SL | LPS-SL | ||
| 14 | Ushiroda et al., 2019 [49] | C57BL/6N mice, M, 5 w | 8/grp NDHFDHFD + EGCG | p | 8 w | 32% | Epigallocatechin gallate (EGCG)-0.32% within diet | Within diet | Cecal content, 16 s rRNA, V3–V4 region, Illumina MiSeq: Alpha-diversity: Chao 1 index-NSShannon index-SLBeta-diversity: PCoA-distinct cluster Phyla: Firmicutes-SL Bacteroidetes-NS Actinobacteria-SH Deferribacteres-SL Proteobacteria-SL Verrucomicrobia-SH F:B ratio-SL Genera: Adlercreutzia, Akkermansia, Allobaculum, Parabacteroides, f_Erysipelotrichaceae; g_Clostridium-SH Mucispirillum, [Ruminococcus], f_Lachnospiraceae; g_Unclassified, f_Desulfovibrionaceae; g_Unclassified, and Anaerotruncus-SL | NS | NS | Epid-NS | Serum:TC, TAG, HDL, LDL, NEFA-NS Liver:TAG-SL | |||||
| 15 | Sheng et al., 2018 [50] | C57BL/6 wild-type (WT) mice, M, 3 w | ND WDWD + EGCG WD + vancomycin + polymyxin B + AbxWD + Akkermansia muciniphila supplementation * only results for EGCG reported in this review | T | 8 w | 21% | Epigallocatechin gallate (EGCG) −100 ug/d/gram body weight | Oral gavage | Cecal content, 16 s rRNA, V4 region, Illumina MiSeq: Phyla: Firmicutes-NS Bacteroidetes-NS Proteobacteria-SL Verrucomicrobia-SH Deferribacteres-SL Actinobacteria-NS Family: Enterococcaceae-SH Verrucomicrobiaceae-SH Lachnospiraceae-SL Desulfovibrionaceae-SL Bacteroidaceae-SL Prevotellaceae-SL Rikenellaceae-SL Deferribacteraceae-SL | SH | SL | SL | FG-NSITT (AUC)-SL | PYY-SHGLP-1-SH | TC-SL TAG-SL | LPS-SL | ||
| 16 | W. Liu et al., 2017 [51] | C57BL/6 mice, M, 8 w | 10–12/grp ND HFDHFD + GSPE HFD + antibiotics + GSPEHFD + antibiotics * only results for GSPE reported in this review | p | 7 w | 60% | 300 mg/kg body weight grape seed proanthocyanidin extract (GSPE) | Oral gavage | Feces, 16s rRNA, V3–V4 region, Illumina MiSeq:Alpha diversity(chao1)-NSBeta-diversity (PCoA)-distinct cluster Phyla: Firmicutes-NS Proteobacteria-SH Actinobacteria-H Genera: Prevotella-H Clostridium XIVa-SH Escherichia/Shigella-SH Blautia-SH Flavonifractor-SH Arthrobacter-SH Roseburia spp-SH Roseburia inulinivorans-SH Lactococcus-SLBacteroides-SL | NS | Epid-SLInguinal-NS | ipGTT(AUC)-NSITT(AUC)-SL | TNFa, IL-6, MCP-1-SL | |||||
| 17 | Wang et al., 2019 [52] | ICR mice, M, 5–6 w (29–31g) | 6/grp NDHFDHFD + CA | p | 6 w | 18.40% | Chlorogenic acid(CA)-150 mg/kg/day | Oral gavage | Cecal content, 16s rRNA, V3–V4, Ilumina MiSeq:Alpha diversity-NS Phyla: Firmicutes-NSBacteroidetes-NS Proteobacteria-NS Verrucomicrobia-NS Actinobacteria-NS F:B-NS Family:Desulfovibrionaceae, Ruminococcaceae, Lachnospiraceae, Erysipeiotrichaceae-SL Bacteroidaceae, Lactobacillaceae-SH Genera:Oscillospira, Coprococcus, Anaerotruncus, Allobacterium, Bifidobacterium, Turicibacte-LBacteroides and Ruminococcus-H | SL | Epid-SL | TC-SL TAG-SL LDL-SL HDL-SH | ||||||
| 18 | W. Liao et al., 2018 [53] | C57BL/6J mice, M, 8 w | 7–8/grp NDND+RHFD HFD + R HFD + FT * only results for HFD+R included in this review | p | 4 w | 60% | Resveratrol (R) − 400 mg/kg in diet | Within diet | Feces, 16s rRNA, V4–V5 regions, Illumina HiSeq:Alpha-diversity (Shannon)-SL Beta-diversity(PCoA)-distinct cluster Phyla:Firmicutes-L Bacteroidetes-H Proteobacteria-HLDA > 3.5: Bacteroidaceae, lachnospiraceae, Bacteroides | NS | SL | Perigonadal-SL Inguinal-SL | OGTT(AUC): SL IpITT (AUC)-SL | |||||
| PURE COMPOUNDS-RATS | ||||||||||||||||||
| 19 | L. Zhao et al., 2017 [54] | Wistar rats, M, (160–180 g) | 8/grp NDHFD HFD + QR | T | 8 w | 45% | Combination of Quercetin(Q) − 30 mg/kg body weight/day Resveratrol (R)-15 mg/kg body weight/day | Oral gavage | Feces, 16s rRNA, V4–V5 regions, Illumina MiSeq: Alpha-diversity (Shannon index)-SH Beta-diversity (PCoA)-distinct cluster Phyla:Firmicutes–SL Bacteroidetes-NS Proteobacteria–SH Verrucomicrobia-NS Actinobacteria-NS F:B ratio-NS Genera: Ruminococcaceae_UCG-014-SH Bacteroidales_S247_group_norank-SHRuminococcaceae_UCG-005-SH [Eubacterium]_coprostanoligenes_group-SH Akkermansia-SH Lachnoclostridium–SL Bilophila-SL | NS | SL | Perinephric-SLEpid-SLSAT: NS | SL | Leptin-NSAdiponectin-SHTNFa-SL IL-6-SL MCP-1-SL | TC-SL TAG-SL HDL-SH LDL-SL | |||
| 20 | C. Yang et al., 2019 [55] | Wistar rats, M, 5 w | 6/grp NDHFDHFD + RHFD + SHFD + C | p | 8 w | 45% | Resveratrol(R) − 400 mg/kg of diet Sinapic acid (S) − 200 mg/kg of dietR-S(C) − 400 mg/kg resveratrol and 200 mg/kg sinapic acid | Within diet | Feces, 16s rRNA, V3–V4 regions, Illumina MiSeq: Alpha-diversity (ACE, Chao1): R, S, C-SHNon-metric multi-dimensional scaling (NMDS)-distinct cluster for R, S Phyla: Firmicutes; R, S-NS, C-SH Bacteroidetes: R, S, C-NS Proteobacteria: R, S, C-NS Tenericutes: S, C-SL, R-NS Actinobacteria: R-NS, S, C-SL Genera: Unclassified Peptostreptococcaceae: R, S, C-NSRF-39: S, C-SH Blautia: R, S-SHDorea: R, S-SH Ruminococcaceae:R-SH, C-SLRoseburia: C-SH Clostridiales, Ruminococcus, Oscillospira, Lachnospiraceae, S24_7, Bacteroides, Desulfovibrionaceae: R, S, C-NS | R, S, C-NS | R, S, C-NS | FG: R-SL OGTT(AUC): R, S, C-NS | FI-R, S, C-NS | R, C-SL | TC: R, S, C-NS TAG:S-SL HDL:R-SH LDL:R, S, C-NS NEFA:R, S, C-NS | |||
| 21 | Luo et al., 2019 [56] | Sprague Dawley rats, M, 5 w | 16/grp ND HFDHFD + LSIFHFD + HSIF | T | 4 w | ND + 15%pork fat | LSIF = 150 mg/kg soy isoflavone (SIF)HSIF = 450 mg/kg(SIF) | Oral gavage | Cecal content, 16S rRNA, V3–V4 region, Illumina HiSeq:Beta-diversity:PCA-no distinct pattern in treatment grps Phyla: Firmicutes:LSIF, HSIF-SL Bacteroidetes:LSIF, HSIF-SH Fusobacteria:LSIF, HSIF-NS Actinobacteria:LSIF, HSIF-NS Proteobacteria:LSIF, HSIF-SH F:B ratio:LSIF, HSIF-SL Genera: Coprococcus_1:LSIF, HSIF-SH Faecalibacterium:LSIF, HSIF-SH [Eubacterium]_oxidoreducens group:HSIF-SHRuminococcaceae UCG-005:HSIF-SH Phascolarctobacterium:LSIF, HSIF-SH Prevotella_9:LSIF, HSIF-SH Lachnospira:LSIF, HSIF-SH Bacteroides:LSIF, HSIF-SH Ruminiclostridium_9:HSIF)-SH, LSIF-NS [Eubacterium]_ruminantium group: LSIF-SH, HSIF-SL Morganella:LSIF, HSIF-SL Lactobacillus:LSIF, HSIF-SL Oscillibacter:HSIF-SL Ruminococcaceae_NK4A214:LSIF, HSIF-SL Dorea:LSIF, HSIF-SL Pasteurella:LSIF, HSIF-SL Blautia:LSIF, HSIF-SL Roseburia:LSIF, HSIF-NS Candidatus Saccharimonas:LSIF, HSIF-NS Ruminococcus_1:LSIF, HSIF-NS | LSIF, HSIF-NS | LSIF, HSIF-SL | TC:LSIF-NS, HSIF- SL TAG:LSIF, HSIF-NS HDL:LSIF-NS, HSIF-SL LDL:LSIF-NS, HSIF-SL | LPS: LSIF, HSIF-SL | |||||
| COMBINATION OF PURE COMPOUNDS | ||||||||||||||||||
| 22 | Yong-Feng et al., 2019 [57] | C57BL/6J mice, M, 8 w | 12/grp NDHFDHFD + TFQ | p | 12 w | 60% | Total flavonoids of Quzhou (TFQ)-300 mg/kg/day | Oral gavage | Colonic content, 16S rRNA, V3–V4 regions, Illumina HiSeq: Alpha diveristy-Chao1 index and the Shannon index-SH Beta-diversity (PCoA)-TFQ group was closer to HFD Phyla:Firmicutes-NSVerrucomicrobia-SH Bacteroidetes–SH Actinobacteria-SL F:B ratio-SL Genera:Akkermansia-SH Alistipes-SH Dubosiella-SL Faecallbaculum-SL Lactobacillus-SL LDA > 4 Blautia | SL | Epid-SL | FG-SL OGTT(AUC)-SL ITT(AUC)-NS | FI-NS | SL | Serum: TC-SL TAG-SL LDL-SL HDL-NS NEFA-SL Liver:TC-NS TAG-SL | LPS-SL | ||
| 23 | Zhu et al., 2018 [58] | Sprague-Dawley rats, M (120–140 g) | 6/grp NDND+LPT ND + MPT ND + HPTHCHC + LPTHC + MPT HC + HPT | T | 2wks | HC diet-81.8% basic diet, 6% dried egg yolk, 5% full cream milk powder, 5% lard, 2% cholesterol, and 0.2% sodium cholate | Low persimmon tannin (LPT)-50 mg/kg bwMedium persimmon tannin (MPT)-100 mg/kg bwHigh persimmon tannin (HPT)-200 mg/kg bw | Oral gavage | Cecal content, 16s rRNA, v4 region, Illumina MiSeq: Phyla:Firmicutes, Bacteroidetes, Proteobacteria-NS F:B ratio-MPT, HPT-SL Genera:Roseburia: LPT-SL Helicobacter: LPT-SL Bacteroides: LPT-SL Oscillospira: LPT-SH Phascolarctobacterium: LPT, MPT, HPT-NS Ruminococcus: LPT, MPT, HPT-NS Sutterella: LPT, MPT, HPT-NS Desulfovibrio: LPT, MPT, HPT-NS Prevotella: LPT, MPT, HPT-NS | LPT, MPT, HPT-NS | LPT, MPT, HPT-NS | Serum:TC: LPT, HPT-SL TAG:MPT- SL LDL: LPT-SL Liver:TC: LPT, HPT, MPT-NS TAG:LP, HPT, MPT-NS | ||||||
| PHENOLIC EXTRACTS-MICE | ||||||||||||||||||
| 24 | H. Lee et al., 2019 [59] | C57BL/6Nmice, M, 5 w | n = NANDHFDHFD + RO125HFD + RO250 | T | 16 w | 45% | Rubus occidentalis(RO) 125: 125 mg/kg/day; RO250: 250 mg/kg/day | Oral gavage | Cecal content, 16s rRNA, V4 region, Illumina MiSeq, Alpha diversity (Chao1, Shannon)-NS Beta diversity PCoA-clear separation between groups Phyla: Bacteroidetes:RO125, RO250-H Deferribacteres:RO125, RO250-H F:B ratio:RO125, RO250-L Genera:Butyricimonas: RO250-SH Bacteroides:RO250-SH Mucispirillum:RO250-SH Ruminococcus:RO250-SH Oscillospira:RO125-SH Species: Mucispirillum schaedleri:RO250-SH | NS | iPGTT (AUC)RO250-SL | |||||||
| 25 | Collins et al. 2016 [60] | C57BL/6J mice, M, 4 w | 10/grp ND HFDHFD + EPHFD + NEPHFD + EP-NEPHFD + GP | p | 16 w | 44% | Extractable polyphenol (EP)-1.1 g/kg of dietNon-extractable polyphenol (NEP)-3.5 g/kg of dietEp-NEP-1.1 g.kg EP 3.5 g/kg NEP of dietGrape powder (GP)-50 g/kg of diet | Within diet | Cecum mucosa, 16s rRNA, V4–V5 regions, Illumina MiSeq: Observed species (AUC):EP-NSNEP-NS EP-NEP-NSGP-SHBeta-diversity (PCoA)-No distinct cluster Genera: Coprococcus: NEP, EP + NEP-SH Ruminococcus: EP, NEP, EP-NEP, GP-NS Rc4-4: NEP, EP-NEP, GP-SL S24-7: EP-NEP-SL Adlercreutzia; EP, NEP, EP-NEP, GP-NSHRF-39: NEP-SH | EP-SLNEP-SL EP-NEP-SL GP-NS | % body fat (wk 15): EP-SL NEP-NS EP-NEP-SL GP-NS WAT:EP–SL NEP-SL EP-NEP-SL GP-NS | IpGTT (AUC):EP-SL NEP-SL EP-NEP-SLGP-NS | EP-SLNEP-NS EP-NEP-SLGP-NS | Plasma MCP-1: NEP-NS EP-NEP-SLGP-NS | Plasma TAG:EP-SL NEP –NS EP-NEP-SL GP-NS Liver TAG:EP-Ns NEP-NS EP-NEP-SL GP-NS | Plasma LBP:EP –NS NEP-SL EP-NEP-SL GP-NS | ||
| 26 | Guo et al., 2019 [61] | C57BL/6 mice, M, 3 w | Study 1, n = 9-12/grp NDND + BEHFDHFD + BE The results of study 2 and 3 are not included in this review | p | 14 w | 60% | ND+BE-5 g/L blueberry extract (BE) in drinking waterHFD + BE-0.5% (m/v) BE in drinking water | Drinking water | Feces, 16s rRNA, Illumina HiSeq: Alpha-diversity:Shannon index-SH Beta diversity (PCoA)-pattern similar to ND Phyla:F:B ratio-SL Genera:Akkermansia-SH Bifidobacterium-SH Desulfovibrio-SL Bilophila-SL | NS | SL | Epid-SL Inguinal-SL | OGTT(AUC)-SL ITT(AUC)-SL | TNF-a-SLIL-6-SL Leptin-SL | Plasma:TAG-SL Liver:TAG-SL | LPS-SL | ||
| 27 | D. M. Cheng et al., 2016 [62] | C57BL/6J mice, M, 5 w | 15/grp ND HFDHFD + RSL HFD + GL | p | 13 w | 60% | Rutgers Scarlet Lettuce (RSL), Green lettuce (GL)-6.4% (w/w) RSL or GL powder | Within diet | Feces, cecal content, 16s rRNA, V3–V4 regions, Illumina Miseq:PCA-same diet clustered together, HFDs, ND and treatments groups formed distinct cluster Phyla: Firmicutes: RSL-L Bacteroidetes: RSL-H Verrucomicrobia: RSL, GL-NS Proteobacteria: RSL, GL-NS Actinobacteria: RSL, GL-NS Tenericute: RSL, GL-NS F:B ratio: RSL, GL-NS Genera (fecal and cecal content):Roseburia spp: RSL-SH Ruminococcus spp:RSL-SH rc4_4:RSL-SH Coprococcus:GL-SH Blautia:GL-SH Moryella spp:GL-SL Clostridium spp:GL-SH | RSL, GL-NS | Fat mass:RSL, GL-NS | FG: RSL, GL-NS OGTT(AUC)-RSL-SL, GL-NS(wk9) ITT(AUC)-RSL, GL-NS | FI: RSL, GL-NS | TAG:RSL, GL-NS FFA:RSL, GL-NS | LPS:RSL, GL-NS | |||
| 28 | Zhibin Liu et al., 2016 [63] | C57BL/6J mice, M, 10 w | 10/grp ND HFD HFD + GTHFD + OT HFD + BT | p | 13 w | 45% | Green tea (GT), oolong tea (OT), black tea (BT)-dosage:NA | Drinking water | Cecal content, 16s rRNA, V3–V4 regions, Illumina MiSeq:Alpha diversity: ACE, Chao1: GT, OT, BT-SH Shannon: GT, BT-SH Beta-diversity (PCA)-distinct clusters between ND, HFD, HFD + tea (all tea) Taxa (LDA > 3.5): Family/Genera:S24-7, Blautia sp., Helicobacter ganmani, Oscillibacter sp., Anaerotruncus sp.-SH Alistipes sp., Lachnospiraceae (OTU173), Lachnospiraceae (OTU45), S24-7, Akkermansia sp., Rikenella microfusus-Hllobaculum sp., Bacteroides acidifaciens, S24-7(OTU319), S24-7(OTU192), Lachnospiraceae, S24-7 (OTU535), Clostridium leptum (OTU450), Parabacteroides goldsteinii-L | GT, OT, BT-SL | VAT: GT, OT, BT-SL | FG: GT, OT, BT-SL | TC: GT, OT, BT-SL TAG: GT, OT, BT-SL LDL: GT, OT, BT-SL HDL: GT, OT, BT-SL | LBP: GT, OT, BT-SL | ||||
| 29 | Griffin et al., 2017 [64] | C57BL/6J mice, M, 9 w | 8/grp NDHFDHFD10HFD100 | T | 12 w | 45% | HFD10 ∼10 grape seed extract (GSE) mg/kg/day HFD100 ∼100 mg/kg/day | Within diet | Mucosal adherent microbiota-small intestine, cecum, colon), 16S rRNA, V4, Illumina MiSeq (only for HFD10): Phyla:Firmicutes–NS Bacteroidetes-NS Proteobacteria –NS Deferribacteres-NS F:B ratio-NS Genera:Cecum:Allobaculum-SL Lactococcus–SL Colon:Turicibacter spp.-SH Phascolarctobacterium-SH Roseburia-SH Peptoniphilus-SH Desulfovibrionaceae spp-SH | HFD10-NS HFD100-NS | HFD10-NS HFD100-NS | Total body fat:HFD10-NS HFD100-NS | FG:HFD10-SL HFD100-NS OGTT(AUC): HFD10-SL HFD100-NS ITT (AUC):HFD10-NS HFD100-NS | |||||
| 30 | Y. Li et al., 2019 [65] | C57BL/6J (B6) mice, M, (22 ± 2 g) | 10/grp NDHFDHFD + CROHFD + SRO | p | 12w | 24.50% | Common rapeseed oil (CRO)-10%in the dietRapeseed oil with sinapine (SRO)-CRO with 100 mg sinapine in the diet | Within diet | Feces, 16S rRNA, V3–V4 region, Illumina MiSeq:Alpha diversity:Chao1 and Shannon index: CRO-NS, SRO-SL Simpson diversity: CRO-NS, SRO-SL Beta-diversity: PCoA-distinct clustering of each SRO, CRO overlap with HFD Phyla:Firmicutes, Bacteroidetes: CRO-NS, SRO-L Proteobacteria: CRO-NS, SRO-L Genera:Muribaculaceae, Desulfovibrio, Lachnospiraceae-CRO-H Mucispirillum: CRO-L Lactobacillus, Bifidobacterium: SRO-H Blautia:SRO-SH Mucispirillum: SRO-L(LDA) effect size (LEfSe) > 3: CRO-RuminiclostridiumSRO-Blautia, Desulfovibrio | NS | CRO-NS SRO-SL | Epid: CRO-NS SRO-SL Perirenal:CRO-NS SRO-SL | FG:CRO-NS SRO-SL | FI:CRO-NS SRO-SL | CRO-NS SRO-SL | Serum: TAG:CRO-NS, SRO-SL LDL: CRO, SRO-SL Liver: VLDL: CRO-NS, SRO-SL | ||
| 31 | Ma et al., 2019 [66] | C57BL/6 mice, M, 6 w | 8/grp ND HFDHFD + TPLHFD + TPMHFD + TPH | p | 12 w | 36.71% | Tea polyphenol lowdose (TPL)-100 mg/kg/day Tea polyphenol medium dose (TPM)-200 mg/kg/day Tea polyphenol high dose (TPH)-400 mg/kg/day | Oral gavage | Cecal content, 16S rRNA, V3–V4 region, Ion S5 XL platform: Phyla:Firmicutes:TPL, TPM, TPH-NS Bacteroidetes:TPL, TPM, TPH-L Proteobacteria:TPL, TPM, TPH-SL Actinobacteria:TPL, TPM, TPH-H Verrucomicrobia:TPL, TPM, TPH-H Genera:TPL: Butyrivibrio, Anaerostipes, Alloprevotella-SHTPM: Paraprevotella-SHTPH: Alitipes, Bacteroides, Faecalibaculum, Erysipelatoclostridium, Flavonifractor, Coprobacillus, Fusicatenibacter, Parasutterella, Bifidobacterium, Akkermansia, Ruminococcaceae, Lachnoclostridium, Clostridiales, Roseburia, Blautia-SHLDA > 4: TPL: unidentified_Lachnospiraceae, Alloprevetella, Anaerostipe TPM: Family Atopobiaceae TPH:Lachnoclostridium, Akkermansia, Bifidobacterium, Erysipelatoclostridium, and unidentified Clostridiales | TPL, TPM, TPH-SL | FG:TPL, TPM, TPH-NS | FI:TPL, TPM-SL, TPH-NS | TPL.TPM-SL, TPH-NS | TNF-α: TPL, TPM, TPH-SLIL-6: TPL, TPM, TPH-SL | TC:TPL, TPM, TPH-NS TAG:TPL, TPM, TPH-NS HDL:TPL, TPM, TPH-SH LDL:TPL-NS, TPM, TPH- SL | LPS:TPL, TPM-NS, TPH-SL | ||
| 32 | Van Hul et al., 2018 [67] | C57BL/6J mice, M, 9 w | 14/grp NDHFDHFD + CBE HFD + GPE | p | 8 w | 60% | Cinnamon bark extract (CBE)-2 g/kg grape pomace extract (GPE)-8.2 g/kg | Within diet | Feces, 16s rRNA, V3–V4 regions, Illumina MiSeq: Beta-diversity (PCoA): Most CBE and GPE-fed mice were separated from the untreated HFD mice according to the axis 2. Phyla (CBE and GP): Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria-NS Genera: CBE:Peptococcus-SL GPE: Desulfovibrio-SL Clostridium sensu stricto-SL Lactococcus-SL Allobaculum-SH Roseburia-SH | CBE, GPE-NS | CBE, GPE-NS | VAT: CBE, GPE-NS SAT: CBE, GPE-NS | OGTT (AUC): CBE-SL GPE-NS | FI:CBE, GPE-NS | IR-index: CBE-NS GPE-SL | Leptin:CBE, GPE-NSResistin: CBE, GPE-NS IL-1B:CBE- SH GPE-NS IGNy: CBE, GPE-NS MCP1:CBE- SH GPE-NS MIP1A:CBE, GPE-NS PAI1: CBE, GPE-NS | Plasma:TC, TAG, NEFA:CBE, GPE-NS Liver:TC:CBE, GPE-NS TAG-CBE-NS, GPE-SL | |
| 33 | Anhê et al., 2015 [68] | C57Bl/6J mice, M, 8 w | 12/grp NDHFHS HFHS + CE | p | 8 w | 65% | Cranberry powdered extract (CE)-200 mg/kg | Oral gavage | Feces, 16s rRNA, V6–V8 regions, 454 pyrosequencing:Beta diversity-PCA-distinct cluster at week 5 and 9 Phyla: Firmicutes–NS Bacteroidetes-SL at wk 9 compared to wk1(within CE)Verrucomicrobia-SH at wk 9 compared to wk 1 (within CE) Genera (wk 9 vs. wk 1-within CE): Akkermansia–SH Oscillibacter–SH Ruminococcus-SH Pseudoflavonifractor-SH unclassified Ruminococcaceae-SH unclassified Porphyromonadaceae-SH Barnesiella-SL unclassified lachnospiraceae-SL Turicibacter-SLEubacterium-SL Clostridium-SLLactobacillus-SL | SL | SL | VAT: SL SAT: NS | FG-NS OGTT(AUC)-NS ITT(AUC):SL | FI-SL C-peptide AUC-SL | SL | Plasma:TC-SL TAG-SL Liver:TAG-SL Jejunum:TAG-SL | SL | |
| 34 | Anhê et al., 2017 [69] | C57Bl/6J mice, M, 8 w | 8–11/grp ND ND + CEHFHSHFHS + CE | T | 8 w | 65% | Cranberry extract (CE)-200 mg/kg | Oral gavage | Feces, 16s rRNA, V3–V4, Illumina MiSeq:Beta-diversity (PCoA)-distinct cluster for CE F:B ratio-SL Genera LDA > 2.5: A. muciniphila, Coprobacillus, and Barnesiella-H | NS | NS | VAT: NS SAT: NS | OGTT (AUC): NS ipITT (AUC):SL | Plasma: TAG-NS Liver:TAG-SL | ||||
| 35 | Anhê et al., 2018 [70] | C57BL/6 J mice, M, 8 w | 12/grp NDHFHSHFHS + BBEHFHS + CLEHFHS + CRE HFHS + ABEHFHS + LGE | p | 8 w | 65% | Bog blueberries (BBE), cloudberries (CLE), crowberries (CRE), alpine bearberries (ABE) and lingonberries (LGE)-200 mg powdered extract/kg body weight | Oral gavage | Feces, 16s rRNA, V3–V4 regions, Illumina MiSeq:Beta-diversity (PCA)-distinct cluster for CLE and ABE Phyla: Firmicutes-All-NS Bacteroidetes-All-NS Actinobacteria-All-NS Proteobacteria: CLE-SH, ABE, LGE-NS Tenericutes-All-NS Verrucomicrobia-All-NS F:B ratio-NS Genera (LDA > 2):CLE:Turicibacter, Akkermansia (SH), Bifidobacterium Lactobacillus (SL) ABE: Oscillibacter, A. muciniphila (SH) LGE: Oscillibacter, Turicibacter (SH) | All grps-NS | All grps-NS | VAT: All grps-NS SAT: All grps-NS | FG-NS (all) OGTT (AUC)-NS (all) ITT(AUC): CLE-SL C | FI:CLE, ABE, LGE-SLpeptide: All-NSAll (AUC)-NS | Plasma:TAG: CLE, ABE, LGE-SL Liver: TAG: CLE, ABE, LGE-SL | LPS:CLE, ABE, LGE-SL | ||
| 36 | Chen et al., 2018 [71] | C57BL/6 mice, M, 6 w | 8/grp ND HFDHFD + KDCHFD + FBT | p | 8 w | 45% | Kudingcha (KDC) and Fuzhuan Brick Tea (FBT)-400 mg/kg/d | Intragastric gavage | Feces, 16s rRNA, V4 region, Illumina MiSeq:Alpha-diversity: Shannon: KDC, FBT-SHSimpson: KDC, FBT-SLInvSimpson: KDS-SH, FBT-NS Beta-diversity-PCoA-distinct clusters of treatment groups Phyla:Firmicutes: KDC-NS, FBT-SL Bacteroidetes: KDC-NS, FBT-SHF:B ratio: KDC-NS, FBT-SL Genera:Pseudoflavonifractor: FBT-SL Coprobacter:FBT-SL Olsenella: KDC, FBT-SL Oscillibacter:FBT-SL Anaerobacterium: KDC-SH, FBT-SL Anaerotruncus:FBT-SL Bilophila:FBT-SL Clostridium_IV:FBT-SL, Streptococcus: KDC, FBT-SL Lactobacillus:FBT-SL, Lactonifacto:-FBT-SL Streptococcus: KDC, FBT-SL Leuconostoc: KDC, FBT-SL Clostridium_XlVb:FBT-SL Anaerotruncus:FBT-SL Catabacter: KDC, FBT-SH Barnesiella: KDC, FBT-SH Alistipes: KDC, FBT-SH, Odoribacter-KDC, FBT-SH Bacteroides: KDC, FBT-SH | NS | KDC, FBT-SL | Epid: KDC, FBT-SL Perirenal: KDC, FBT-SL | TNFa:KDC, FBT-SL IL6:KDC, FBT-SL CRP: KDC, FBT-SL | Serum:TC: KDC-NS FBT-SL TAG: KDC, FBT-NS LDL: KDC-NS, FBT-SL HDL: KDC, FBT-NS Liver:TAG: KDC-NS, FBT-SL | LPS:KDC, FBT-SL | |||
| 37 | J. Xu et al., 2019 [72] | C57BL/6 mice, M, 7 w | 12/grp ND HFDHFD + J | p | 8 w | 60% | Jamun extract (J)-100 mg/kg | Oral gavage | Feces, 16s rRNA, V3–V4regions, Illumina HiSeq: Alpha-diversity (Shannon, chao1)-NSBeta-diversity (PCoA)-distinct cluster Phyla: Firmicutes-LBacteroidetes-H F:B ratio-SL Genera:Bacteroides, Alistipes, Prevotella, Alloprevotella-H ClostridiumXlVb-L | NS | SL | VAT:SL SAT:SL | FG-SL OGTT (AUC): SL ITT (AUC)-SL | FI-SL | SL | Plasma:TC-NS TAG-SL FFA-SH Liver: TC-SL TAG-SL FFA-SL | ||
| 38 | Dey et al., 2019 [73] | C57BL/6J mice, M, 5 w | 10/grp ND ND + GTEHFDHFD + GTE | p | 8 w | 60% | Green tea extract (GTE)-2% (w/w) | Within diet | Cecal content, 16s rRNA, V4–V5, Ilumina MiSeq:Alpha-diversity: Shannon index and the Chao1-SH Beta-diversity:PCA-cluster closer to ND Phyla (significance not mentioned):Firmicutes-LBacteroidetes-H Actinobacteria-H Verrucomicrobia-H Proteobacteria-unaffected Tenericutes-unaffected F:B ratio-NS Genera:Bifidobacterium-HBlautia-H Dorea-H Lactobacillus-H Ruminococcus-H SMB53-L Akkermansia-SH Species:Akkermansia muciniphila-SH Ruminococcus gnavus–H Bifidobacterium pseudolongum-SH Bifidobacterium adolescentis-SH | SH | SL | Epid-SL RP-SL SAT: SL | FG-NS | FI-NS | SL | TC-SL TG-SL NEFA-SL | LPS-NS FITC-dextran-SL | |
| 39 | S. Wu et al., 2018 [74], M. Liu et al., 2018 [75] | C57BL/6N mice, M, 5 w | 4/grp NDND + 1% LCBPHFDHFD + 0.5% LCBPHFD + 1% LCBP | p | 45 d | 40% | Lonicera caerulea L. Berry Polyphenols (LCBP)-0.5% and 1% | Within diet | Feces, 16S rRNA, V3–V4 regions, Illumina MiSeq:Alpha-diversity: Chao1, Shannon index, PD-NS Phyla (both doses):Firmicutes-LBacteroidetes-H Proteobacteria-H F:B ratio-L Genera:Both doses:Bacteroides-H Parabacteroides–H Staphylococcus, Lactobacillus, Oscillospira. Ruminococcus-L | 0.5%, 1% LCBP-SL | FG: 0.5%, 1% LCBP-SL | FI 0.5%, 1% LCBP-SL | 0.5%, 1% LCBP-SL | IL-2: 0.5%, 1% LCBP–SL IL-6: 0.5%, 1% LCBP-SL MCP1: 0.5% LCBP –NS 1% LCBP-SL TNFa: 0.5%, 1% LCBP-SL | TAGSerum: 0.5% LCBP-SL 1% LCBP-SL Liver: 0.5% LCBP –SL 1% LCBP-SL | Endotoxin: Serum: SL (0.5%, 1% LCBP) Liver: SL (0.5%, 1% LCBP) | ||
| 40 | C. Wu et al. 2019 [76] | C57BL/6J mice, M, 8 w | 8/grp NDHFDHFD + PTF HFD + AbHFD + Ab + PTF * only results for PTF reported in this review | T | 6w | 60% | Pandanus tectorius fruit extract (PTF)-200 mg/kg bw | Oral gavage | Feces, 16S rRNA, V4–V5 regions, 454 FLX pyrosequencing platform: Alpha-diversity(Shannon, Choa1)-SHBeta-diversity (PCA)-distinct cluster Phyla:Firmicutes-SH Bacteroidetes-SL Actinobacteria-SH Verrucomicrobia-NS Proteobacteria-NS Tenericutes-NS Genera:Lactobacillus-SH Lactococcus-SH Streptococcus-SH Enterococcus-SH Clostridium sensu stricto-SHBacteroides-SL Alistipes-SL Akkermansia-SL Clostridium XIVa group-SL | NS | SL | Epid–NS SAT: NS | FG-SL OGTT (AUC): SL | TC-SL TG-SL LDL-SL | ||||
| 41 | Vezza et al., 2019 [77] | C57BL/6 J mice, M, 5w | 9/grp NDND + OL HFDHFD + LDHFD + MDHFD + HD FT * only results for LD, MD, HD reported in this review | NA | 5 w | 60% | ND + olive leaf (OL)-25 mg/kgHFD + LD-1 mg/kgHFD + MD-10 mg/kgHFD + HD-25 mg/kg | Oral gavage | Feces, 16S rRNA, V4–V5, Illumina MiSeq:Beta-diversity: PCA-distinct pattern Phyla:Firmicutes: LD, MD-NS, HD-SL Bacteriodetes: LD-SL, MD-NS, HD-SH Proteobacteria: LD-SH, MD-SL, HD-NS Actinobacteria: LD, MD, HD-NS Verrumicrobioa: LD-SH, MD-SL, HD-NS Tenericutes: LD-NS, MD, HD-SHF:B ratio: LD-NS, MD, HD-SL Genera: Cytophaga: LD, MD-NS, HD-SH Akkermansia: LD-NS, MD, HD-SH | LD, MD, HD-NS | LD, MD, HD-SL | Epid:LD, MD, HD-SL | FG:LD, MD-NS, HD-SL OGTT(AUC):LD, MD, HD-SL | LD, MD, HD-SL | LD, MD, HD-SL | LDL:LD-NS, MD, HD-SL HDL: LD, MD, HD-NS | ||
| 42 | Henning et al., 2018 [78] | C57BL/6J mice (strain JAX 000664), M, 6–7 w | 12/grp NDHFHSHFHS + GTPHFHS + BTP | p | 4 w | NA | 0.5 g/100 g of diet providing 0.25 g polyphenols/100 g diet of green tea polyphenol (GTP) or black tea polyphenol (BTP) | Within diet | Cecal content, 16s rRNA, v4 region: Beta-diversity (PCoA)-distinct cluster for GTP, BTP Phyla:Firmicutes: GTP, BTP-SL Bacteroidetes: GTP, BTP-SH Actinobacter: GTP, BTP-SL Genera:Parabacteroides, Bacteroides, Prevotella: GTP, BTP-SH Roseburia, Lactobacillus, Blautia, Anaerostipes, Shuttleworthia, Bryantella, Lactococcus, Acetitomaculum, Collinsella: GTP, BTP-SL Clostridium Coprococcus: GTP-SH Turicibacter, Marvinbryantia: GTP-SL Oscillibacter, Anaerotruncus, Pseudobutyrivibrio: BTP-SH | GTP-SL, BTP-NS | GTP, BTP-SL | Mesen:GTP, BTP-SL Epid: GTP, BTP-SL SAT: GTP, BTP-NS | ||||||
| PHENOLIC EXTRACTS-RATS | ||||||||||||||||||
| 43 | R. Zhao et al., 2019 [79] | Sprague-Dawley rats, M, (250–270 g) | 12/grp NDHFD HFD +P PPLHFD + PPPH | p | 12 w | 45% | PPPL-150 mg/kg of Pomegranate polyphenols (PPP) PPPH-300 mg/kg of PPP | Oral gavage | Feces, 16s rRNA, V4–V5 regions, Illumina HiSeq:Beta diversity (PCoA)-some PPPL and PPPH separated from HFD but some not. Phyla: Firmicutes, Bacteroidetes, Proteobacteria, Tenericutes, Actinobacteria-no significance were given F/B ratio:PPPL-SL Genera: HFD vs. PPL:Bacteroidales S24-7 group_norank-SH Paraprevotella-SH Lactobacillus-SH Family XII AD3011 group-SL Lachnospiraceae_uncultured-SL Ruminococcaceae_uncultured-SL Ruminococcaceae UCG-009-SL Ruminococcus 1-SHHFD vs. PPPH: Lactobacillus-SH Family XII AD3011 group-SL Lachnospiraceae_uncultured-SL Prevotellaceae UCG-001-SH | PPPL, PPP-NS | PPPL, PPP-SL | TNFa:PPPL, PPPH-SL IL-6: PPPL-NS, PPPH-SL IL-1B:PPPL-SL, PPPH-NS | TC:PPPL-SL, PPPH-NS TAG:PPPL-NS, PPPH-SL HDL:PPPL, PPPH-NS LDL:PPPL, PPPH-SL | LPS:PPPL-SL, PPPH-NS | ||||
| 44 | S. Lee et al., 2018 [80] | Wistar rats, M, (200-220g) | 8/grp NDHFDHFD + BB | p | 8 w | 45% | HFD with 10 g freeze-dried blueberry powder (BB)/100 g | Within diet | Cecal content, 16s rRNA, V4 region, Illumina MiSeq:Phyla: Firmicutes-SLBacteriodetes-SL Proteobacteria–SH Fusobacteria–SH Genera:Actinobacillus-SH Aggregatibacter-SH | NS | VAT: NS | OGTT(AUC):NS | Serum LBP-SL | |||||
| 45 | H. Xu et al., 2019 [81] | Sprague-Dawley rats, M, 5 w | 6/grp NDHFDHFD + PFELHFD + PFEHHFD + CAE(Positive control) * only results for PFEL, PFEH reported in this review | p | 8 w | ND-76% + fat-12% | PFEL-0.4% Pyracantha fortuneana extract (PFE) PFEH-1% PFECAE-0.4% Citrus aurantium extract (CAE) | Within diet | Feces, 16s rRNA, V4–V5 region, Illumina MiSeq:PFEL and PFEH-combinedAlpha-diversity: Chao1 index-Shannon, and Simpson-NS Phyla:Firmicutes –SL Bacteroidetes-NS Actinobacteria-SH F:B ratio-SL Genera:Bacteroides, Corynebacterium, Lactobacillus, Blautia-HR uminococcus, Oscillospira, Flexispira-SL | PFEL, PFEH-NS | PFEL, PFEH-SL | Epid/bw:PFEL-NS PFEH-SL | FG:PFEL, PFEH-SL | TC:PFEL-NS, PFEH-SL TAG:PFEL-NS, PFEH-SL LDL:PFEL-NS, PFEH-SL HDL:PFEL-NS, PFEH-SH | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moorthy, M.; Sundralingam, U.; Palanisamy, U.D. Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies. Foods 2021, 10, 299. https://doi.org/10.3390/foods10020299
Moorthy M, Sundralingam U, Palanisamy UD. Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies. Foods. 2021; 10(2):299. https://doi.org/10.3390/foods10020299
Chicago/Turabian StyleMoorthy, Mohanambal, Usha Sundralingam, and Uma D. Palanisamy. 2021. "Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies" Foods 10, no. 2: 299. https://doi.org/10.3390/foods10020299
APA StyleMoorthy, M., Sundralingam, U., & Palanisamy, U. D. (2021). Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies. Foods, 10(2), 299. https://doi.org/10.3390/foods10020299








